Abstract
Purpose: Inflammatory indexes, including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic immune-inflammation index (SII) and lymphocyte-to-monocyte ratio (LMR), have been confirmed as prognostic factors in multiple manigances. However, the prognostic value of these parameters in bevacizumab-treated non-small-cell lung cancer (NSCLC) is still not clear. Methods: We retrospectively studied 119 patients with advanced NSCLC who received bevacizumab treatment. The associations of pretreatment NLR, PLR, SII and LMR with progression-free survival (PFS) and overall survival (OS) were analyzed. Results & Conclusion: The median PFS and OS of patients with high baseline NLR, PLR and SII and low LMR were significantly decreased than those of patients with low baseline NLR, PLR and SII and high LMR. Multivariable analysis indicated that high baseline SII was independently related with inferior prognosis, and baseline LMR was an independent predictor for OS.
Plain Language Summary
In this study we retrospectively studied 119 patients with advanced non-small-cell lung cancer receiving bevacizumab treatment. We found that the prognosis of the patients with high baseline neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and systemic immune-inflammation index (SII) and low lymphocyte-to-monocyte ratio (LMR) were significantly poorer than those in patients with low baseline NLR, PLR and SII and high LMR. Multivariable analysis indicated that high baseline SII was independently related with inferior progression-free survival and overall survival, and that baseline LMR was an independent predictor for overall survival. This study suggests that we can predict the efficacy of bevacizumab by analyzing several blood cell count indexes.
Lung cancer ranks first both in the incidence and mortality rate among malignant tumors worldwide [Citation1]. Bevacizumab is a monoclonal antibody which exerts its anti-angiogenesis ability by targeting VEGF. At present, bevacizumab combined with platinum-based doublet chemotherapy has been widely administered as one of the standard first-line therapeutic options for patients with metastatic non-small-cell lung cancer (NSCLC) [Citation2]. However, there are still no validated clinical or biological biomarkers to predict the effects of and resistance to bevacizumab.
Inflammatory cells, including lymphocytes, neutrophils and monocytes, are a key component of the tumor microenvironment and substantially contribute to tumor proliferation, angiogenesis and metastasis [Citation3]. Several inflammatory indexes, such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic immune-inflammation index (SII) and lymphocyte-to-monocyte ratio (LMR), have been increasingly recognized as prognostic hematological biomarkers in a variety of malignancies, including colorectal cancer, ovarian cancer, prostate cancer, hepatocellular carcinoma and NSCLC [Citation4–9].
We performed this retrospective study with the purpose of assessing the prognostic value of NLR, PLR, SII and LMR in advanced NSCLC patients receiving bevacizumab and chemotherapy as the first-line treatment. If validated, these reproducible, cost effective and easily available peripheral immune-related biomarkers could be particularly useful in identifying NSCLC patients who may benefit from bevacizumab treatment.
Patients & methods
Patients
A total of 119 patients diagnosed with advanced NSCLC (stage IIIB/IV) at the First Affiliated Hospital of Bengbu Medical College between January 2017 and April 2021 were enrolled in our retrospective study. Eligible patients received at least one infusion of bevacizumab at a dose of 15 mg/kg together with paclitaxel (175 mg/m2) and carboplatin (area under the curve = 5) every 21 days. The therapy was continued until tumor progression, intolerable side effects, withdrawal or death. Patients who had used steroids or immunomodulatory drugs 1 month before the initiation of bevacizumab, or had coexisting active inflammatory diseases or immunodeficiencies, were excluded.
The patients’ clinical records were collected, including laboratory complete blood counts with differential count at the initiation of bevacizumab administration, sex, age, smoking history, EGFR mutation status, Eastern Co-operative Oncology Group performance status (ECOG PS) and the occurrence of bone, liver or brain metastases. All data were retrospectively collected by using the electronic patient medical records system to ensure consistent data collection. Disease progression was identified based on the Response Evaluation Criteria in Solid Tumors version 1.1. Progression-free survival (PFS) was calculated as the interval elapsed from the initiation of bevacizumab to first disease progression or death for any reason. Overall survival (OS) was characterized by the interval from commencing bevacizumab treatment to death or last follow-up. The follow-up of all patients ended on 15 November 2021.
Statistical analysis
Descriptive analysis was applied for all variables. Categorical parameters were described as frequencies and percentages, while continuous parameters were described as medians with ranges.
NLR was defined as the ratio of absolute neutrophil number to absolute lymphocyte number; PLR was derived from the quotient of the absolute platelet and lymphocyte count; SII was defined as the formula PLR × neutrophil count; and LMR was defined as the quotient of the absolute lymphocyte and monocyte count. The baseline markers were defined as the baseline counts immediately before the first cycle of bevacizumab.
The best cutoffs influencing the prognosis of baseline NLR/PLR/SII/LMR were defined using the X-tile v.3.6.1 software and the minimum p-value method as follows: 3.7 for NLR, 255.5 for PLR, 775.2 for SII and 3 for LMR, respectively () [Citation10]. Patients were categorized into a high baseline NLR/SII/LMR/PLR group and a low baseline NLR/SII/LMR/PLR group according to the cutoff values.
NLR: Neutrophil-to-lymphocyte ratio; LMR: Lymphocyte-to-monocyte ratio; PLR: Platelet-to-lymphocyte ratio; SII: Systemic immune-inflammation index.
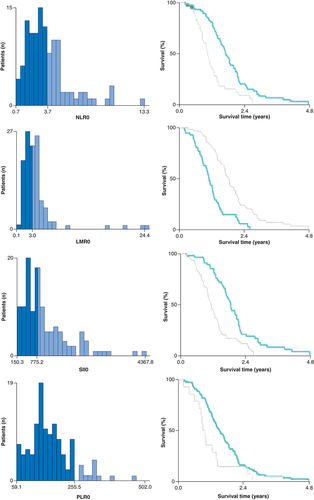
The relationship between the baseline NLR, SII, LMR and PLR levels and the patients’ clinical characteristics was calculated using the chi-square test. The distributions of PFS and OS were assessed using the Kaplan–Meier method, and the log-rank test was utilized for comparison. Univariate and multivariate Cox proportional hazards regression models were applied to evaluate the contribution of each potential prognostic factor to survival. Parameters entered into the multivariate analysis were chosen on the basis of the clinical relevance and statistical significance suggested by univariate Cox regression analysis (p < 0.05). A two-tailed p-value < 0.05 was regarded as statistically significant. All data were analyzed using the SPSS v. 24.0 software (IBM Corp., NY, USA).
Results
Characteristics of patients
A total of 119 patients with advanced NSCLC who received bevacizumab and chemotherapy as first-line treatment from January 2017 to April 2021 were included in the research. Baseline characteristics of all included patients are shown in . The median age was 61 years at diagnosis (range: 36–80 years). Sixty-seven patients (56.3%) were men and 73 (61.3%) were smokers or had a previous history of smoking. A majority of patients (72%) had an ECOG PS of 1 or 2. In relation to tumor characteristics, the most common site of metastasis was bone (43.7%), followed by brain (29.4%) and liver (15.1%). Regarding EGFR mutation status, 36.1% had a mutation, while 63.9% had the wild-type gene.
Table 1. Characteristics of patients.
Correlation between inflammatory indexes & clinical characteristics
Before the initiation of bevacizumab treatment, a total of 73 (61.3%) patients had low NLR and 105 (88.2%) low PLR, while 56 (47.1%) and 65(54.6%) patients had low SII and high LMR, respectively. We compared the baseline characteristics of patients grouped by inflammatory indexes cut-offs ( and ). Male patients trend to be more frequent in the high NLR (≥3.7), SII (≥755.2) and LMR (≥3) group. Patients with low NLR (<3.7) or low LMR (<3) exhibited more frequently an ECOG PS of 1–2. No significant difference was found among different groups with respect to any other characteristics.
Table 2. Patients' baseline characteristics classified by neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio (n = 119).
Table 3. Patients' baseline characteristics classified by systemic immune-inflammation index and lymphocyte-to-monocyte ratio (n = 119).
Correlation between inflammatory indexes & survival outcomes
The median PFS and OS for the entire cohort included in this study were 7.9 months (95% CI: 6.8–8.6) and 16.2 months (95% CI: 14.6–17.9), respectively. Kaplan–Meier survival analysis suggested that patients with high baseline NLR (≥3.7), PLR (≥255.5) and SII (≥775.2) and low LMR (<3) showed significantly worse PFS than those with low baseline NLR (<3.7), PLR (<255.5) and SII (<775.2) and high LMR (≥3), with median PFS of 5.9 versus 8.8 months (p = 0.002; A), 5.3 versus 8.5 months (p = 0.004; B), 5.8 versus 9.4 months (p < 0.001; C) and 6.1 versus 8.9 months (p = 0.003; D), respectively.
(A) PFS–NLR. (B) PFS–PLR. (C) PFS–SII. (D) PFS–LMR. (E) OS–NLR. (F) OS–PLR. (G) OS–SII. (H) OS–LMR.
LMR: Lymphocyte-to-monocyte ratio; NLR: Neutrophil-to-lymphocyte ratio; OS: Overall survival; PFS: Progression-free survival; PLR: Platelet-to-lymphocyte ratio; SII: Systemic immune-inflammation index.
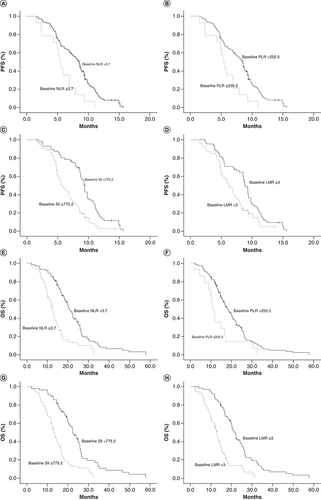
We also compared the relationship of OS with the inflammatory indexes. E–H shows that the OS in patients with baseline NLR ≥3.7, PLR ≥255.5, SII ≥775.2 and LMR <3 were significantly inferior to OS in those with baseline NLR <3.7, PLR <775.2, SII <775.2 and LMR ≥3, with median OS of 12.4 versus 20.4 months (p < 0.001; E), 10.6 versus 18.1 months (p = 0.018; F), 13.1 versus 22.3 months (p < 0.001; G) and 12.9 versus 21.4 months (p < 0.001; H), respectively.
Univariate & multivariate analysis for PFS & OS
Univariate and multivariate Cox regression were performed to analyze the prognostic importance of patients’ baseline characteristics and inflammatory indexes, and the results are summarized in & .
Table 4. Univariate and multivariable analysis of variables correlated to progression-free survival.
Table 5. Univariate and multivariable analysis of variables correlated to overall survival.
In univariate analysis of PFS, no significant associations with PFS were found for patients’ age, smoking history, ECOG PS score, EGFR status, or bone or brain metastasis. Patients with baseline NLR ≥3.7 (hazard ratio [HR]: 1.881; 95% CI: 1.251–2.82; p = 0.002), baseline PLR ≥255.5 (HR: 2.274; 95% CI: 1.279–4.041; p = 0.005), baseline SII ≥775.2 (HR: 2.407; 95% CI: 1.061–3.619; p < 0.001) and baseline LMR <3 (HR: 1.831; 95% CI: 1.220–2.749; p = 0.004) experienced shorter PFS. Furthermore, multivariate analysis demonstrated that only baseline SII ≥775.2 (HR: 2.338; 95% CI: 1.554–3.518; p < 0.001) and occurrence of liver metastasis (HR: 2.080; 95% CI: 1.232–3.509; p = 0.006) remained to be independently correlated with inferior PFS ().
In univariate analysis of OS, the presence of brain metastasis (HR: 1.746; 95% CI: 1.140–2.675; p = 0.010), liver metastasis (HR: 1.756; 95% CI: 1.062–2.904; p = 0.028), baseline NLR ≥3.7 (HR: 2.584; 95% CI: 1.709–3.908; p < 0.001), baseline PLR ≥255.5 (HR: 1.965; 95% CI: 1.111–3.474; p = 0.020), baseline SII ≥775.2 (HR: 2.799; 95% CI: 1.850–4.233; p < 0.001) and baseline LMR <4 (HR: 2.998; 95% CI: 1.969–4.565; p < 0.001) were indicated to be unfavorable prognostic factors. EGFR mutation (HR: 0.414; 95% CI: 0.265–0.647; p < 0.001) was indicated to be a favorable prognostic factor. The prognostic value of age, smoking status, ECOG PS and bone metastasis were evaluated and showed no significant correlation with OS. Furthermore, multivariate Cox regression analysis showed that baseline LMR ≥3 (HR: 2.619; 95% CI: 1.631–4.205; p < 0.001), baseline SII <775.2 (HR: 1.846; 95% CI: 1.165–2.923; p = 0.009), together with mutant EGFR (HR: 0.385; 95% CI 0.243–0.609; p < 0.001) and no brain metastasis (HR: 1.582; 95% CI 1.027–2.436; p = 0.037) remained strongly associated with superior OS ().
Discussion
The present study suggested that in advanced NSCLC patients receiving bevacizumab plus chemotherapy as first-line treatment, the baseline systemic inflammatory indexes exerted an influence on survival. In our cohort we observed that NLR ≥3.7, PLR ≥255.5, SII ≥775.2 and LMR <3 at baseline were significantly related to diminished PFS and OS. In multivariate analysis, high baseline SII was suggested to be an independent prognostic marker of PFS and OS, and low baseline LMR was found to contribute significantly to the prediction of OS. However, baseline NLR and baseline PLR lost their predictive values in terms of PFS and OS. Furthermore, the multivariate analysis also suggested the association of superior efficacy of bevacizumab in patients with EGFR gene mutation.
The underlying mechanism associated with the inflammatory indexes NLR, SII and LMR and prognosis could be partly explained by the association of cancer-related inflammation with tumor promotion and progression. Inflammation is regarded as the seventh hallmark of tumors and plays a major role in the proliferation, invasion and metastasis of multiple malignancies. Fewer lymphocytes and more neutrophils and monocytes are associated with progression or poor prognosis in various tumors [Citation3,Citation11,Citation12]. Mounting evidence suggests that VEGF is not only produced by tumor cells, but is also released to the tumor microenvironment by platelets and inflammatory cells such as neutrophils and monocytes during the tumor-associated inflammatory response and hypoxia [Citation13]. VEGF is involved in fostering immunosuppression in the tumor by downregulating the proliferation and differentiation of antigen-presenting cells as well as effector T cells while activating immunosuppressive cells including myeloid-derived suppressor cells, tumor-associated macrophages (TAMs) and regulatory T cells [Citation14,Citation15]. The peritumoral infiltration of these suppressive immune cells not only releases multiple angiostimulatory growth factors, but also facilitates and stimulates alternative angiogenesis pathways which contribute to the resistance to bevacizumab [Citation16]. In addition, the resistance to bevacizumab will aggravate the anaerobic state in the tumor microenvironment and enhance recruitment of neutrophils and macrophages, which can establish a positive feedback that further impairs the immune response and promotes tumor growth [Citation17–19].
Neutrophils, derived from the peripheral blood, are recruited at the site of inflammation and the tumor, and their number is always increased in tumors compared with healthy tissues [Citation20]. Neutrophilia is able to abolish the function of immune cells, increase peritumoral aggregation of macrophages, increase C-reactive protein (CRP) and reduce albumin synthesis. In addition, neutrophils contribute to tumor angiogenesis by delivering angiogenic factors including VEGF, protease, IL-1, IL-6 and IL-8, and eventually resulting in tumor progression [Citation21]. Early studies confirmed that lymphocytes, especially tumor-infiltrating lymphocytes, had a major impact on the antitumor inflammatory response by inducing cytotoxic cell apoptosis and suppressing the proliferation and dissemination of tumor cells; thus increased numbers of tumor-infiltrating lymphocytes are correlated with better prognosis [Citation22]. Lymphocytopenia is frequently found in malignancies, indicating an immune resistance status [Citation23]. This might be due to the higher susceptibility of T lymphocytes to apoptosis, caused by a chronic activation state in solid tumor tissues [Citation24]. Peripheral monocytes accumulate into the tumor tissue and are differentiated into TAMs under the action of different tumor-derived chemokines, such as VEGFA and CCL-2; TAMs are able to divide into tumor-supportive M2-polarized macrophages, which promote angiogenesis by secreting different proangiogenic factors, including VEGFA, MMP-9, bFGF and ADM [Citation25,Citation26].
Decreased lymphocytes and increased neutrophils, platelets and monocytes, in combination with higher NLR, PLR and SII and lower LMR, are confirmed to be helpful for estimating the status of tumor immunity during bevacizumab treatment, and have a predictive role in various malignancies [Citation27–30]. Our research is consistent with previous research suggesting that high NLR, PLR and SII and low LMR are independent factors predicting inferior survival in several malignancies, including lung, colorectal, ovarian and gastric cancer [Citation7,Citation31–33]. Especially in lung cancer, these inflammatory indexes have shown an association with poor survival outcomes in small-cell lung cancer and early and advanced NSCLC patients, and were able to predict the resistance to chemotherapy and target therapy as well as immunotherapy. In a comprehensive retrospective study, Mandaliya et al. illustrated that in patients with stage IV NSCLC, high baseline NLR and PLR and low baseline LMR, the advanced lung cancer inflammation index had a significant association with poorer survival [Citation32]. In another retrospective study, NLR was demonstrated to be a useful prognostic index for first-line chemotherapy in stage IIIB or IV NSCLC patients [Citation34]. Furthermore, Chan et al. showed that in a subpopulation of EGFR-mutated NSCLC patients, baseline NLR was recognized as an independent prognostic factor across early- and late-stage disease [Citation35]. In addition, Cao et al. suggested an association of high pretreatment NLR with worse outcome in NSCLC patients treated with nivolumab [Citation36]. Botta et al. found that NLR could be a useful prognostic marker for NSCLC patients treated with bevacizumab [Citation4]. Li et al. also confirmed that decreased NLR, PLR and SII and elevated LMR could indicate a favorable response to bevacizumab in NSCLC patients [Citation37].
Nevertheless, our present study had several limitations. First, the peripheral blood cell parameters such as neutrophils, platelets and lymphocytes are nonspecific parameters and may be influenced by some confounding factors including infections, the use of steroid drugs or cancer-related complications. We excluded patients with active infections or autoimmune disease from this study, but we could not confirm whether latent infections existed. Second, the low infiltration of CD4+ T lymphocytes and CD8+ T lymphocytes in the tumor microenvironment has been found to predict poorer survival in various malignancies. Compared with CD4+ helper T cells, the infiltration of CD8+ T cells in tumor was relatively higher [Citation38]. Nevertheless, we could not further analyze the specific T-lymphocyte subgroups due to the retrospective nature of the study. Thirdly, the possibility of selection bias and confounders could not be completely avoided because of the inherent characteristics of retrospective studies.
Conclusion
In conclusion, our study demonstrated that high baseline NLR, PLR and SII and low baseline LMR were significantly associated with inferior PFS and OS, and that pretreatment LMR and SII appeared to be independent prognostic indexes for advanced NSCLC patients treated with first-line bevacizumab plus chemotherapy. An increasing body of evidence has proved the important role of systemic inflammatory factors in tumor aggressiveness. Investigation of a further larger prospective dataset is warranted to validate our findings.
This study was designed to investigate the role of several inflammatory indexes in predicting the efficacy of bevacizumab in patients with advanced non-small-cell lung cancer (NSCLC).
In this retrospective study we aimed to evaluated the relationship between neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic immune-inflammation index (SII) and lymphocyte-to-monocyte ratio (LMR) and the prognosis of 119 patients with advanced NSCLC treated with bevacizumab combined with platinum-based doublet chemotherapy. Statistical analysis was evaluated using the Kaplan–Meier method and Cox regression.
The study suggested that median progression-free survival (PFS) and overall survival (OS) of patients with high baseline NLR (≥3.7), PLR (≥255.5) and SII (≥775.2) and low baseline LMR (<3) were significantly shorter than those of patients with low baseline NLR (<3.7), PLR (<255.5) and SII (<775.2) and high LMR (≥3).
Multivariable analysis indicated that high baseline SII was independently related to inferior PFS and OS, and that baseline LMR was an independent predictor for OS.
Author contributions
Study conception and design: J Yang and M Bi. Data collection and analysis: J Yang and M Deng. Methodology: J Yang, Y Wang and M Bi, M Deng. Writing – original draft: J Yang, Y Wang, M Deng, X Qiao and S Zhang. Writing – review and editing: J Yang, M Bi, Y Wang, M Deng, X Qiao and S Zhang.
Ethical conduct of research
This research was approved by the Institutional Review Board of the First Affiliated Hospital of Bengbu Medical College. Formal informed consent was not required because of the retrospective feature of our study, and all patient information were kept confidential. The study was carried out in accordance with the Declaration of Helsinki.
Acknowledgments
The authors thank all patients and investigators who participated in the study.
Financial & competing interests disclosure
This work received funding from the Natural Science Research Project of Anhui Educational Committee (no. KJ2019A0372) and Nature Science Research Project of Bengbu Medical College (no. 2021byzd172). The authors have no other relevantaffiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- BadeBC , DelaCruz CS. Lung cancer 2020: epidemiology, etiology, and prevention. Clin. Chest Med.41(1), 1–24 (2020).
- HannaN , JohnsonD , TeminSet al.Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol.35(30), 3484–3515 (2017).
- DiakosCI , CharlesKA , McMillanDC , ClarkeSJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol.15(11), e493–e503 (2014).
- BottaC , BarbieriV , CilibertoDet al.Systemic inflammatory status at baseline predicts bevacizumab benefit in advanced non-small cell lung cancer patients. Cancer Biol. Ther.14(6), 469–475 (2013).
- GuthrieGJ , CharlesKA , RoxburghCS , HorganPG , McMillanDC , ClarkeSJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit. Rev. Oncol. Hematol.88(1), 218–230 (2013).
- RossiS , BassoM , StrippoliAet al.Are markers of systemic inflammation good prognostic indicators in colorectal cancer?Clin. Colorectal Cancer16(4), 264–274 (2017).
- FarolfiA , ScarpiE , GrecoFet al.Inflammatory indexes as predictive factors for platinum sensitivity and as prognostic factors in recurrent epithelial ovarian cancer patients: a MITO24 retrospective study. Sci. Rep.10(1), 18190 (2020).
- TempletonAJ , PezaroC , OmlinAet al.Simple prognostic score for metastatic castration-resistant prostate cancer with incorporation of neutrophil-to-lymphocyte ratio. Cancer120(21), 3346–3352 (2014).
- YangZ , ZhangJ , LuYet al.Aspartate aminotransferase–lymphocyte ratio index and systemic immune-inflammation index predict overall survival in HBV-related hepatocellular carcinoma patients after transcatheter arterial chemoembolization. Oncotarget6(40), 43090–43098 (2015).
- CampRL , Dolled-FilhartM , RimmDL.X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 10(21), 7252–7259 (2004).
- GretenFR , GrivennikovSI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity51(1), 27–41 (2019).
- KumarR , GeunaE , MichalareaVet al.The neutrophil-lymphocyte ratio and its utilisation for the management of cancer patients in early clinical trials. Br. J. Cancer112(7), 1157–1165 (2015).
- KoehneP , WillamC , StraussE , SchindlerR , EckardtKU , BuhrerC. Lack of hypoxic stimulation of VEGF secretion from neutrophils and platelets. Am. J. Physiol. Heart Circ. Physiol.279(2), H817–H824 (2000).
- RahmaOE , HodiFS. The intersection between tumor angiogenesis and immune suppression. Clin. Cancer Res.25(18), 5449–5457 (2019).
- RamjiawanRR , GriffioenAW , DudaDG. Anti-angiogenesis for cancer revisited: is there a role for combinations with immunotherapy?Angiogenesis20(2), 185–204 (2017).
- HuijbersEJ , van BeijnumJR , ThijssenVL , SabrkhanyS , Nowak-SliwinskaP , GriffioenAW. Role of the tumor stroma in resistance to anti-angiogenic therapy. Drug Resist. Updat.25, 26–37 (2016).
- BrandauS , MosesK , LangS. The kinship of neutrophils and granulocytic myeloid-derived suppressor cells in cancer: cousins, siblings or twins?Semin. Cancer Biol.23(3), 171–182 (2013).
- LuT , RamakrishnanR , AltiokSet al.Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J. Clin. Invest.121(10), 4015–4029 (2011).
- Bottsford-MillerJN , ColemanRL , SoodAK. Resistance and escape from antiangiogenesis therapy: clinical implications and future strategies. J. Clin. Oncol.30(32), 4026–4034 (2012).
- MentzelT , BrownLF , DvorakHFet al.The association between tumour progression and vascularity in myxofibrosarcoma and myxoid/round cell liposarcoma. Virchows Arch.438(1), 13–22 (2001).
- NozawaH , ChiuC , HanahanD. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc. Natl Acad. Sci. USA103(33), 12493–12498 (2006).
- FarhoodB , NajafiM , MortezaeeK. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: a review. J. Cell. Physiol.234(6), 8509–8521 (2019).
- StoneRL , NickAM , McNeishIAet al.Paraneoplastic thrombocytosis in ovarian cancer. N. Engl. J. Med.366(7), 610–618 (2012).
- BremnesRM , BusundLT , KilvaerTLet al.The role of tumor-infiltrating lymphocytes in development, progression, and prognosis of non-small cell lung cancer. J. Thorac. Oncol.11(6), 789–800 (2016).
- MovahediK , LaouiD , GysemansCet al.Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res.70(14), 5728–5739 (2010).
- QianBZ , PollardJW. Macrophage diversity enhances tumor progression and metastasis. Cell141(1), 39–51 (2010).
- MiyagawaY , YanaiA , YanagawaTet al.Baseline neutrophil-to-lymphocyte ratio and C-reactive protein predict efficacy of treatment with bevacizumab plus paclitaxel for locally advanced or metastatic breast cancer. Oncotarget11(1), 86–98 (2020).
- PassardiA , ScarpiE , CavannaLet al.Inflammatory indexes as predictors of prognosis and bevacizumab efficacy in patients with metastatic colorectal cancer. Oncotarget7(22), 33210–33219 (2016).
- TrinhH , DzulSP , HyderJet al.Prognostic value of changes in neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and lymphocyte-to-monocyte ratio (LMR) for patients with cervical cancer undergoing definitive chemoradiotherapy (dCRT). Clin. Chim. Acta510, 711–716 (2020).
- SalatiM , FilippiR , VivaldiCet al.The prognostic nutritional index predicts survival and response to first-line chemotherapy in advanced biliary cancer. Liver Int.40(3), 704–711 (2020).
- ZhangJ , ZhangHY , LiJ , ShaoXY , ZhangCX. The elevated NLR, PLR and PLT may predict the prognosis of patients with colorectal cancer: a systematic review and meta-analysis. Oncotarget8(40), 68837–68846 (2017).
- MandaliyaH , JonesM , OldmeadowC , NordmanII. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl. Lung Cancer Res.8(6), 886–894 (2019).
- MiyamotoR , InagawaS , SanoN , TadanoS , AdachiS , YamamotoM. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur. J. Surg. Oncol.44(5), 607–612 (2018).
- LiuZL , ZengTT , ZhouXJet al.Neutrophil–lymphocyte ratio as a prognostic marker for chemotherapy in advanced lung cancer. Int. J. Biol. Markers31(4), e395–e401 (2016).
- ChanSWS , SmithE , AggarwalRet al.Systemic inflammatory markers of survival in epidermal growth factor-mutated non-small-cell lung cancer: single-institution analysis, systematic review, and meta-analysis. Clin. Lung Cancer22(5), 390–407 (2021).
- CaoD , XuH , XuX , GuoT , GeW. A reliable and feasible way to predict the benefits of nivolumab in patients with non-small cell lung cancer: a pooled analysis of 14 retrospective studies. Oncoimmunology7(11), e1507262 (2018).
- LiB , WangS , LiCet al.The kinetic changes of systemic inflammatory factors during bevacizumab treatment and its prognostic role in advanced non-small cell lung cancer patients. J. Cancer10(21), 5082–5089 (2019).
- ZikosTA , DonnenbergAD , LandreneauRJ , LuketichJD , DonnenbergVS. Lung T-cell subset composition at the time of surgical resection is a prognostic indicator in non-small cell lung cancer. Cancer Immunol. Immunother.60(6), 819–827 (2011).
