Abstract
Mycobacterium ulcerans is the causative agent of Buruli ulcer – a necrotizing skin infection. As an environmental pathogen, it has developed stress response mechanisms for survival. Similar to endospore formation in M. marinum, it is likely that M. ulcerans employs sporulation mechanisms for its survival and transmission. In this review, we modeled possible transmission routes and patterns of M. ulcerans from the environment to its host. We provided insights into the evolution of M. ulcerans and its genomic profiles. We discuss reservoirs of M. ulcerans as an environmental pathogen and its environmental survival. We comprehensively discuss sporulation as a possible stress response mechanism and modelled endospore formation in M. ulcerans. At last, we highlighted sporulation associated markers, which upon expression trigger endospore formation.
Plain Language Summary
Buruli ulcer is an infectious disease characterized by extensive sores on the skin and soft body tissues. The disease is caused by a bacterium called Mycobacterium ulcerans and is mainly found in tropical countries. Over the years, several attempts to understand the means by which humans get into contact with this bug as well as how it thrives in its host remain futile. In this review, we describe a possible survival strategy, known as sporulation, that is adopted by the pathogen for dispersal and survival.
Mycobacterium ulcerans is the causative agent of Buruli ulcer (BU) and it is an environmental mycobacterium usually found in an aquatic and swampy environment. These environments expose the pathogen to harsh conditions such as extreme temperature, nutrient deprivation and harsh chemicals [Citation1]. As such, the pathogen may develop stress response mechanisms to survive these harsh conditions. The Formation of dormant spores is a major stress response mechanism for most environmental pathogens including some species of Bacillus, Clostridium and Mycobacterium. While this kind of spore forming mechanism allows the pathogen to survive under stressed conditions, it may also help with dispersal to new environments [Citation2]. Recently, sporulation has been a topic of interest among members of the mycobacteria genus such as M. marinum, Mycobacterium bovis and M. avium subsp. paratuberculosis [Citation3]. However, little has been shown in M. ulcerans, although the identification of its route for transmission is still unclear. Therefore, reviewing its spore-forming potential as a survival mechanism and possible means of disease transmission is essential to public health.
Buruli ulcer
BU is a neglected tropical disease characterized by progressive damage to the skin and soft tissues [Citation4]. The disease has been reported in countries found in America, Africa, Asia and Western Pacific and it affects people of all ages. However, children below 15 years are the most affected [Citation5], and lesions usually appear on the lower limbs [Citation6]. BU is the third most common mycobacterial infection after leprosy and tuberculosis; however, identification of a definite mode of transmission is a major public health concern. Unlike TB and leprosy, human-to-human spread of BU is rare. Hypothetically, M. ulcerans is acquired from the environment and individuals living in remote areas near aquatic environments are usually at higher risk of developing infections [Citation7]. From these environments M. ulcerans can gain entry into the human host through skin cuts or wounds [Citation8]. Also, BU transmission has been associated with insect bites with mosquitoes as possible reservoirs or vectors [Citation9]. The possibility of transmission through aerosols has been proposed [Citation10]. Aquatic insects could also serve as reservoirs for M. ulcerans [Citation11]. Water bugs and insects that belong to the Naucoridae and Belostimadae families are considered reservoirs and possible transmission vectors ( [Citation12,Citation13]).
(Figure designed by authors: 1[Citation5], 2[Citation7], 3[Citation13], 4[Citation38], 5[Citation72], 6[Citation11] and 7[Citation15]).
![Figure 1. Proposed transmission patterns of Mycobacterium ulcerans showing the role of the aquatic ecosystems and animals in the transmission of M. ulcerans from the environment to humans.(Figure designed by authors: 1[Citation5], 2[Citation7], 3[Citation13], 4[Citation38], 5[Citation72], 6[Citation11] and 7[Citation15]).](/cms/asset/e6ad7d64-04fe-42ba-8b6f-d4d04b7742b4/ifso_a_12364536_f0001.jpg)
BU usually starts as a painless nodule or papule that progresses into severe skin ulceration with undermined edges [Citation14]. Typically, these ulcers are associated with severe coagulative necrosis [Citation15]. Mycolactone, a lipid-like plasmid encoded toxin secreted by M. ulcerans is the major virulence factor that mediates pathogenesis. Unlike other Mycobacterial spp., M. ulcerans exists as an extracellular cluster of bacilli that usually concentrates at the center of the necrotic areas [Citation16]. M. ulcerans could exist in macrophages within inflammatory filtrates, suggesting that the pathogen can be intracellular and extracellular. The possibility of mycolactone inhibiting M. ulcerans uptake by macrophages is high [Citation16]. The determinants of the pathogenesis of M. ulcerans includes ability to behave as an intracellular pathogen and synthesize mycolactone [Citation17]. Mycolactone diffuses through surrounding tissues exerting multiple effects (cytotoxicity and immunosuppression) on cells; although its mechanism is less understood, several targets of this toxin have been identified. Purified vesicles extracted from M. ulcerans extracellular matrix were highly toxic, which shows that mycolactone is secreted and stored in the extracellular matrix [Citation16]. As a result of mycolactone secretion, host cells cytoskeleton and tissue structure are disrupted, leading to cell death. Mycolactone also targets the Wiskott–Aldrich syndrome proteins (WASP) and neural WASP, which controls actin dynamics in adherent cells [Citation18]. Ulceration and an eventual cell loss result from induced apoptosis caused by inhibition of protein translocation into the endoplasmic reticulum and cell detachment [Citation19].
Mycobacterium ulcerans
M. ulcerans has evolved diverse mechanisms including highly resistant cell envelope to dehydration [Citation20], and the synthesis of photoprotective compounds [Citation21], to survive under harsh environmental conditions. Also, a waxy cell envelope rich in mycolic acids and lipids, conferring the ability to resist decolorization with acidic organic solvents. M. ulcerans is a slow growing nontuberculous mycobacterium which mostly affects humans. It grows at 29–33°C under microaerophilic conditions (5% CO2) [Citation22]. Although M. ulcerans is an environmental mycobacterium, attempts to isolate pure cultures from their supposed habitats have been unsuccessful and difficult; however, it has been isolated from aquatic insects and biofilms [Citation23]. In the human host, M. ulcerans extracellularly produce macrolide toxin encoded by a plasmid (174 kb), increasing its virulence and enabling it to establish severe BU infections. M. ulcerans is thought to have evolved from M. marinum by acquiring this virulence conferring plasmid. M. marinum is an atypical mycobacterium that causes infections in fishes and humans. Human infections may occur following exposure to aquatic environments and animals (fish and aquatic bugs). However, M. marinum infections are minor and are characterized by intracellular bacteria and granulomatous lesions [Citation24].
Genome structure & evolution of M. ulcerans
M. ulcerans and M. marinum share 98% similar genome and genetic markers [Citation25]; however, the differences are as a result of gene insertions or deletions. Analysis of the 3′ end of the highly conserved 16S rRNA sequence of mycobacteria shows that M. ulcerans differs from M. marinum at just one nucleotide position or single nucleotide polymorphism [Citation26]. The M. ulcerans strain Agy99 isolated in Ghana, was the first genome sequenced [Citation26]. There were two replicons, 631,606 bp chromosome and the 174,155 bp virulence plasmid pMUM001 with the genome. The chromosome consists of about 4160 protein coding genes and 771 pseudogenes. The genome of M. ulcerans has a high G + C content (65% chromosomal and 62.5% plasmid genome) with 12 insertion elements, including the well characterized IS2404 and IS2606 insertion sequence [Citation27]. These IS2404 is present in high copy numbers in the M. ulcerans core and plasmid genome, however absent in M. marinum [Citation28]. The insertion sequences are distributed within the genome, and are sometimes inserted in the middle of some genes. Insertion of these sequences in the middle of genes interrupts coding sequences and inhibits their expression. The genes that are interrupted by insertion sequences become inactivated and are likely to form pseudogenes [Citation29]. In the M. ulcerans genome, there are about 771 pseudogenes, and this may be because M. ulcerans has a higher number of insertion sequences that are constantly inserted into functional genes [Citation29]. An example of gene interruption and pseudogenization is observed in the otsB1 and otsB2 genes present in both M. tuberculosis and M. ulcerans, which are essential for cell wall biogenesis. However, in the M. ulcerans genome, the otsB1 gene has been disrupted by an IS2404 and has lost its function [Citation30]. Genomic DNA deletion, loss of gene function and pseudogene formation are features of genome reduction or downsizing in the M. ulcerans [Citation31]. Some pathogens that undergo genomic reduction adapt easily to a foreign host and harsh environment [Citation30]. Consequently, genes that are not very essential for their survival in this environment or host become functionally silent and redundant. This suggests that M. ulcerans is capable of undergoing genetic alterations to adapt to a particular ecological niche [Citation30].
The M. ulcerans genome also contains two prophages that are structurally similar to other mycobacteriophages [Citation29]. These are the 18 kb phiMU01 and the 24 kb phiMU02 with an 18 and a 17 coding sequences (CDs). Comparative genomic analysis of the M. ulcerans genome has also shown the deletion or absence of some genes that encode immunogenic proteins. For example, the esxB-esxA gene clusters are highly immunogenic mycobacterial proteins essential for host’s immune response [Citation32]. The M. marinum genome contains two copies of these esxB-esxA cassettes, but several M. ulcerans strains have only one copy of this gene, and others have none. This evolutionary feature can enhance the immune evasion of the pathogen in its host. The hallmark of M. ulcerans evolution is the acquisition of a virulent plasmid pMUM001, which differentiates the pathogen from M. marinum. It harbors three essential genes: mlsA1, mlsA2 and mlsB, encoding polyketide synthases required for mycolactone synthesis [Citation33]. Six structural types of mycolactone have been characterized from the various mycolactone producing mycobacteriums (A/B, C, D, E, F and G2). Other mycolactone producing mycobacterium, including M. shinshuense, M. pseudoshottsii and M. ‘liflandii,’ (which may not be necessarily associated with BU) have been described with a common M. marinum progenitor [Citation34]. They have very similar phenotypic and genotypic characteristics to M. ulcerans and have been argued not to be classified as separate species [Citation34]. Overall, the main events in M. ulcerans evolution include DNA rearrangements, gene deletions, a proliferation of Insertion sequences and the acquisition of plasmid, which are characteristic of bacteria that have undergone a bottleneck evolution. These features, however, enable the pathogen to adapt to a specific environment or host, escape immune defence and contribute immensely to its pathogenicity [Citation30].
Reservoirs of M. ulcerans as an environmental pathogen
The genome or genetic diversity of M. ulcerans supports its adaptation and survival under different environments; hence, it could be described as an environment pathogen [Citation26]. M. ulcerans DNA has been isolated from several swampy environments which further establishes its environmental habitat. Molecular techniques including DNA sequencing and PCR amplification of the KR-B gene (mycolactone ketoreductase-B gene) and IS2404 sequence showed that M. ulcerans are present in water filtrates, detritus and plants in some endemic and nonendemic regions of Ghana and Ivory Coast [Citation35,Citation36]. The isolation of M. ulcerans from water bodies in BU endemic regions has made it important to consider water as a major reservoir for the pathogen. Also, aquatic plants provide suitable habitat for M. ulcerans [Citation37]. M. ulcerans can possibly be transmitted through aquatic vegetations such as Imperata sp. (satin tails) and Panicum sp. (panic grass) [Citation37]. The pathogen has been reported to form biofilms on the surfaces of these aquatic vegetations [Citation38]. Marsollier et al. further showed that aquatic plants stimulate the growth and biofilm formation of M. ulcerans by addition of a crude extract from green algae to BACTEC 7H12B culture medium [Citation39].
Aquatic environments indicating the presence of M. ulcerans had different plant communities, suggesting that the pathogen’s dispersal is unrestricted. The concept of M. ulcerans biofilm formation is unclear; however, Marsollier and his colleagues subsequently demonstrated that it adopts a biofilm like structure in vivo and in vitro with different biofilm layers or components different from other bacterial biofilms [Citation39]. These biofilms have a high amount of extracellular matrix, which contains vesicles shown to be reservoirs of mycolactone [Citation40]. Aquatic insects such as creeping water bugs and giant water bugs have also been implicated in BU disease transmission. The ability of some infected aquatic insects to transmit M. ulcerans to laboratory mice through bites has also been demonstrated [Citation11], however, the insect transmission hypothesis is mostly mechanical and not biological. Also, some aquatic insects (Naucoridae, Belostomatidae, Nepidae and Heteroptera) can serve as transient hosts of the pathogen before transmission to their respective host [Citation41]. Aquatic heteropterans could contaminate water and can further infect people away from their sources as they fly around. Animals including wildlife (possums, rodents, shrews, koalas), livestock (goats), pets (dogs) and recreational (horses) are possible reservoirs of M. ulcerans (zoonotic transmission) () [Citation42,Citation43]. In the endemic region of Australia, 43% of ringtail possums and 29% of brushtail possums were positive for M. ulcerans and 1% for possum faecal samples in nonendemic regions [Citation44]. These indicated terrestrial mammals as possible reservoirs of M. ulcerans. In Ghana, however, an attempt to isolate M. ulcerans from rodents and shrews yielded negative results [Citation45]. In Ghana and Ivory coast, M. ulcerans has been isolated from fish, amphibians (tadpoles and adult frogs) and fecal samples mice and grasscutter [Citation46,Citation47].
M. ulcerans survival in the environment
The optimum temperature for most Mycobacteria spp. is 30–32°C [Citation48]; M. ulcerans grows best at 29–33°C, partly accounting for its strict extracellular nature [Citation49], initiating infections on external body parts, its transmission and pathogenesis. Rapid intracellular replication of M. ulcerans has been observed in amphibian cell lines at 28°C [Citation49]. The survival of M. ulcerans in the environment is temperature specific [Citation49]. Also, availability of appropriate nutrients is essential for its growth and survival. M. ulcerans has a broader spectrum of growth requirements including carbohydrates, alcohols, amino acids, fatty and carboxylic acids that serve as carbon and energy sources [Citation50]. Phosphorus and nitrogen containing solutes are also essential for M. ulcerans nucleic acid synthesis. In the laboratory, M. ulcerans is routinely cultured on media supplemented with ‘Oleic Albumin Dextrose Catalase’ and glycerol. Oleic Albumin Dextrose Catalase contains oleic acid, bovine albumin, sodium chloride, dextrose and catalase. The long-chain fatty acids are essential for its metabolic activities, and glycerol serves as a carbon source. In hostile environments such as nutrient limitations and extreme temperature, bacteria engage adaptive survival mechanisms [Citation51]. For example, Vibrio cholerae exist in a viable, nonculturable state upon nutrient deprivation [Citation52]. Nutrient deprivation in Escherichia coli has no effect on cell viability and integrity, however it induces a temperature dependent reduction in cell-culturability [Citation51]. In S. aureus, multiple-nutrient (glucose, amino acid and phosphate) starvation results in a loss of viability, decrease in cell size and increased resistance to acid shock and oxidative stress [Citation53]. For Mycobacterium species, M. smegmatis has been shown to undergo a cellular differentiation to form small resting cell morphotype when exposed to mild nutrient starvation conditions (phosphate buffered saline with fatty acids that can be metabolized by mycobacteria) [Citation54]. Starved M. tuberculosis cells showed altered morphology and staining properties [Citation55]. It is unclear what happens when M. ulcerans is nutrient deprived, however its growth dynamics is similar to typical growth kinetics of lag to log and finally to death phase [Citation56]. Unlike normal bacteria cells, M. ulcerans takes a relatively longer period to grow in culture with a doubling time of about 3.5 days [Citation57]. Fast growing M. smegmatis shows visible colonies on Middlebrook 7H10 agar plates in less than 7 days, M. ulcerans will take about 4–8 weeks.
M. ulcerans stress response mechanisms
Mycobacteria species are exposed to environmental stresses including low pH, high temperature, nutrient depletion, oxidation and salinity [Citation58]. Since these conditions are not mostly favourable, M. ulcerans have developed stress response mechanisms by altering their metabolic activities [Citation59]. This is achieved by expressing specific genes and subsequent synthesis of proteins to survive this hostile environment. Also, expression of certain essential genes might be halted; for example, sigE, sigH, sigB control oxidative stress responses and are required for vegetative growth [Citation60]. This response mechanism initiates morphological changes which might include decrease in cell size or rate of cell division and resistance to stress factors or agents, thereby increasing virulence or pathogenicity [Citation61]. This might also make M. ulcerans to be dormant or exist in a latent state with the cells nonreplicating, however viable () [Citation62].
(A) Environmental stress factors that affect the survival of M. ulcerans and their sources. (B) Possible stress response mechanisms that are mounted by mycobacterium in response to environmental stress. (C) Stress response mechanisms or an alteration of cellular processes under stress conditions that may result in dormancy or the formation of distinct cell types such as endospores or cellular aggregates such as biofilms.
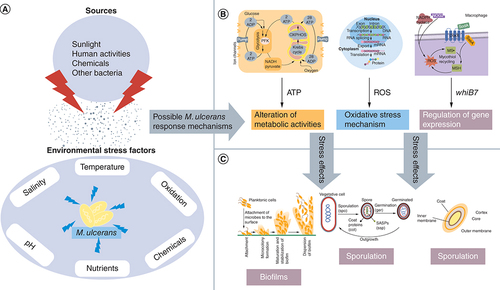
M. ulcerans also engage a ‘stringent response’ mechanism against nutritional stress [Citation62]. This involves the accumulation of nucleotide tetraphosphate (ppGpp) in the cell to downregulate the expression of ribosomal proteins and biosynthetic enzymes [Citation62]. These proteins also activate the expression of other genes that are involved in resistance to stressed conditions. This stringent response mechanism is also common in M. tuberculosis, M. smegmatis and M. leprae [Citation55]. Formation of specialized cells including spores and multicellular aggregates such as biofilms are potential response mechanisms [Citation63]. Species of mycobacteria can form biofilms [Citation40] and endospores [Citation63].
Sporulation as a stress response mechanism
Bacterial cells can differentiate into specialized cells called spores in response to starvation and stress [Citation64]. Endospores are the toughest of these cells, the longest surviving and remain dormant in the environment for many years [Citation65]; this persistence contributes to the geographic distributions of spores in ecosystems [Citation66]. Endospores can withstand high temperatures, toxic/acidic solvents, oxidizing agents (superoxide and hydrogen peroxides), irradiation (UV) and antibiotics. Endospore formation plays a critical role in bacterial resistance to antimicrobial agents. Spores are resistant to antibiotics and disinfectants that will otherwise readily kill vegetative forms of the same cell. Bacteria endospores may encounter these extreme conditions either in the external (natural) environment or experiments that mimic natural conditions in the laboratory [Citation67]. Bacterial endospores produced by species of the Bacillus and Clostridium are the most resistant cellular structures [Citation68]. Sporulation in B. subtilis has been used as a model system to improve the understanding of various basic processes in bacteria including mycobacteria [Citation69]. Spore-like particles with structural similarity to Bacillus spp. endospores have been identified in M. marinum [Citation63]. These particles were identical to well-known spores in terms of their physical, biochemical, morphological and cell biological properties, while maintaining the genetic materials which identify them as M. marinum [Citation63]. For instance, the presence of dipicolinic acid, a major biochemical feature of bacterial endospores [Citation70]. Transmission electron microscopy showed that the spore-like particles are morphologically similar to spores of Bacillus spp. [Citation63]. M. bovis bacillus Calmette–Guerin also forms particles similar to M. marinum spores under similar conditions; an indication that sporulation is not restricted to M. marinum. It is, however, likely a general characteristic or an adaptation strategy among mycobacteria [Citation63]. Since M. ulcerans evolved from M. marinum and 98% genetically similar [Citation25], they likely shared similar sporulation profiles.
Model of endospores formation in M. ulcerans
As M. ulcerans encounters unfavorable environmental conditions, it switches cellular processes in response [Citation69]. Such a process includes its ability to produce endospores, although the development of spores may not be immediate; however, it is initiated as its growth rate and metabolic pathway becomes altered. This alteration enables M. ulcerans to explore other alternative processes before committing to sporulation activities, which is similar to the prespore formation process in B. subtilis [Citation69]. These alternative responses might include activation of sliding motile structure as found in M. smegmatis [Citation71] to pursue new nutrient sources, to release antibiotics that can kill other competing microbes and produce hydrolytic enzymes that can degrade extracellular proteins. This cascade of activities could further trigger the sporulation process in M. ulcerans especially when nutrients such as dextrose, oleic acid, glycerol and in some cases, nitrogen are in short supply. Sporulation is initiated by the phosphorylation and activation of the spo0A, a master regulator transcription factor [Citation72], which induces asymmetric division and triggers subsequent transcription of other sporulation markers such as spoIIE and the Spo loci. The Spo loci and spoIIE encodes spore-developmental regulators and proteins that form a normal polar septum respectively [Citation69]. The cells would thereby differentiate into a smaller prespore, later developing into a spore and relatively larger mother cell that is also required for spore formation ().
The diagram illustrates only key morphological stages of the cycle; The cell differentiates into a smaller prespore, which goes through several stages, accompanied by differential gene regulation to form a mature spore that is highly resistant to stress.
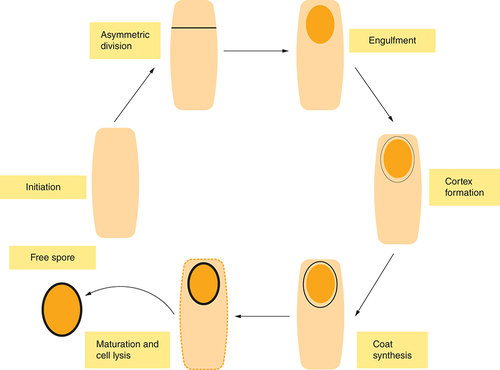
This would be complemented with different gene expression profiles influenced by sporulation-specific RNA polymerase, Sigma factors: δF in the prespore and δE in the mother cell. The prespore is engulfed by the mother cell about after cell division. A significant change in transcription should occur after completion of the engulfment stage, leading to the activation of δG in the prespore and δK in the mother cell [Citation73]. The change in gene regulation should be accompanied by specific morphogenesis, which leads to the development of the resilience observed in matured spores [Citation73].
Sporulation associated genes in mycobacteria
Comparative genomics showed that mycobacterial genome has putative gene orthologs similar to sporulation markers in B. subtilis and Streptomyces coelicolor [Citation63]. Some of these genes, for example sigF, sigJ, spo0A, spoVE when expressed are involved in the sporulation process [Citation69]. For example, while sigJ is a key regulator for desiccation tolerance, spoVE encodes for proteins that are involved in spore cortex and peptidoglycan synthesis. This expression is controlled by master regulons (transcriptional co-regulated operons), protein profiling of spore contents and RNA sequence profiling of sporulation gene expression [Citation74]. These genes are characterized based on the sporulation cycle (), rather than their biochemical functions [Citation74].
Table 1. Sporulation associated markers.
Despite the clinical, industrial and environmental importance of spores, sporulation mechanisms are mostly restricted to B. subtilis and B. anthracis. There has been relatively little information on sporulation in Clostridium spp. [Citation75]. Although comparative genome analyses have profiled certain genes associated with the regulation of sporulation process, there is paucity of data on the essential set of sporulation genes required for producing a complete and viable heat-resistant spore [Citation76]. Some of these essential genes may be triggered by expression of mutation in the parental cells [Citation76]. In addition, some sporulation genes may be essential only in certain species or mutants due to the occurrence of several alternative regulatory pathways [Citation76]. Therefore, defining essential genes that are required for sporulation is nontrivial, however needed to profile bacterial behavioural patterns especially in hostile environments.
Conclusion
This review provided succinct insights into the evolution and genomic profiles of M. ulcerans as the causative agent of BU. It highlighted possible transmission patterns, associated transmission factors and survival strategy of M. ulcerans as an environmental pathogen. Also, the mechanisms of spore formation in response to stress conditions as driven by genetic factors including sporulation markers, which might influence the strain pathogenesis and BU severity.
Future perspective
M. ulcerans is evolving with increasing threat to public health, especially in developing countries including Africa. Its involvement in BU is challenging as the transmission patterns and profiles are unclear. Evidence of its genome diversity pointed to its ability to develop stress response and survival mechanisms in hostile environments, an indication that in vivo it has the tendency to surmount the odds of the immune systems thereby increasing the severity of its associated infections. The expression of associated genetic factors that triggers virulence of the strains apart from ‘mycolactone’ would provide insights into emerging phenotypic traits such as sporulation and consequently influence disease dynamics. It is obvious that the paucity of data on the pathogenesis, reservoirs, evolution and genomic signatures of M. ulcerans provide progressive and notable reasons for further studies. Robust profiling techniques with sensitive assays, genomic algorithms and proteomic tools would provide clarity and balanced perspective into the immunology that can facilitate hypothesis driven research.
Background
M. ulcerans is an environmental pathogen that evolved from M. marinum and they share similar genetic features.
As informed and facilitated by their genetic similarities, it is likely they employ similar mechanisms for survival and disease transmission.
Buruli ulcer
The genomic profiles and diversity of M. ulcerans (the causative agent of Buruli ulcer) might confer survival advantage especially in hostile environment and against immune factors.
Sporulation is a phenotypic response mechanism of M. ulcerans to unfavorable conditions and expression of spore-forming markers might trigger the development of pathogenic and virulent traits.
Future perspective
The expression of associated genetic factors that triggers virulence of M. ulcerans aside mycolactone would provide insights into emerging phenotypic traits including sporulation and consequently influence disease dynamics.
Author contributions
A Isawumi and EA Ayerakwa conceived and prepared the first draft of the manuscript. A Isawumi and MK Abban revised the draft for important intellectual content. Mosi made substantial contributions to the draft, critically reviewed the manuscript and approved the final version for publication. All the authors approved the final draft of the manuscript for submission.
Acknowledgments
The authors appreciate Mosi Research Lab and AMR Research Group led by A Isawumi at the Department of Biochemistry, Cell and Molecular Biology and West African Centre for Cell Biology of Infectious Pathogens at the University of Ghana for support.
Financial & competing interests disclosure
EA Ayerakwa and MK Abban are supported by a WACCBIP-World Bank ACE PhD fellowship (ACE02-WACCBIP: Awandare) and a DELTAS Africa grant (DEL-15-007: Awandare). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Stanaszek-TomalE. Environmental factors causing the development of microorganisms on the surfaces of national cultural monuments made of mineral building materials – review. Coatings10(12), 1203 (2020).
- MüllerAL , DeRezende JR , HubertCRJet al.Endospores of thermophilic bacteria as tracers of microbial dispersal by ocean currents. ISME J.8(6), 1153–1165 (2014).
- SinghB , GhoshJ , IslamNM , DasguptaS , KirsebomLA. Growth, cell division and sporulation in mycobacteria. Antonie van Leeuwenhoek, International Journal of General and Molecular Microbiology.98(2), 165–177 (2010).
- WalshDS , PortaelsF , MeyersWM. Buruli ulcer (Mycobacterium ulcerans infection). Trans. R. Soc. Trop. Med. Hyg.102(10), 969–978 (2008).
- PhanzuDM , MahemaRL , SuykerbuykPet al.Mycobacterium ulcerans infection (Buruli ulcer) on the face: a comparative analysis of 13 clinically suspected cases from the Democratic Republic of Congo. Am. J. Trop. Med. Hyg.85(6), 1100–1105 (2011).
- AgbenorkuP , DonwiIK , KuadziP , SaundersonP. Buruli Ulcer: treatment challenges at three centres in Ghana. J. Trop. Med.2012, 371915 (2012).
- BoccarossaA , DegnonviH , BrouTYet al.A combined field study of Buruli ulcer disease in southeast Benin proposing preventive strategies based on epidemiological, geographic, behavioural and environmental analyses. PLOS Glob. Public Heal.2(1), e0000095 (2022).
- HammoudiN , CassagneC , MillionMet al.Investigation of skin microbiota reveals Mycobacterium ulcerans-Aspergillus sp. trans-kingdom communication. Sci. Rep.11(3777), 2045–2322 (2021).
- PortaelsF , ElsenP , Guimaraes-PeresA , FonteynePA , MeyersWM. Insects in the transmission of Mycobacterium ulcerans infection. Lancet353(9157), 986 (1999).
- VeitchMG , JohnsonPD , FloodPE , LeslieDE , StreetAC , HaymanJA. A large localized outbreak of Mycobacterium ulcerans infection on a temperate southern Australian island. Epidemiol. Infect.119(3), 313–318 (1997).
- MarsollierL , RobertR , AubryJet al.Aquatic insects as a vector for Mycobacterium ulcerans. Appl. Environ. Microbiol.68(9), 4623–4628 (2002).
- MarionE , ChautyA , YeramianE , BabonneauJ , KempfM , MarsollierL. A case of guilt by association: water bug bite incriminated in M. ulcerans infection. Int. J. Mycobacteriology3(2), 158–162 (2014).
- WuJ , TschakertP , KlutseE , FerringDet al.Buruli ulcer disease and its association with land cover in Southwestern Ghana. PLOS Negl. Trop. Dis.9(6), e0003840 (2015).
- OnwuchekwaEC , EkelemeUG , Onu-OsiO , OsezeleOP. Buruli ulcer (Acha-ere): pathogenesis and manifestation. J. Adv. Microbiol.15(3), 1–5 (2019).
- BolzM , RuggliN , RufMT , RicklinME , ZimmerG , PluschkeG. Experimental infection of the pig with Mycobacterium ulcerans: a novel model for studying the pathogenesis of Buruli ulcer disease. PLOS Negl. Trop. Dis.8(7), e2968 (2014).
- TorradoE , FragaAG , CastroAGet al.Evidence for an intra macrophage growth phase of Mycobacterium ulcerans. Infect. Immun.75(2), 977–987 (2007).
- GoodingTM , JohnsonPDR , SmithM , KempAS , Robins-BrowneRM. Cytokine profiles of patients infected with Mycobacterium ulcerans and unaffected household contacts. Infect. Immun.70(10), 5562–5567 (2002).
- SarfoFS , PhillipsR , Wansbrough-JonesM , SimmondsRE. Recent advances: role of mycolactone in the pathogenesis and monitoring of Mycobacterium ulcerans infection/Buruli ulcer disease. Cell Microbiol.18(1), 17–29 (2016).
- HallBS , HillK , McKennaMet al.The pathogenic mechanism of the Mycobacterium ulcerans virulence factor, mycolactone, depends on blockade of protein translocation into the ER. PLOS Pathog.10(4), 15–17 (2014).
- JacksonM. The mycobacterial cell envelope-lipids. Cold Spring Harb. Perspect. Med.4(10), a021105 (2014).
- RobledoJA , MurilloAM , RouzaudF. Physiological role and potential clinical interest of mycobacterial pigments. IUBMB Life63(2), 71–78 (2011).
- RealiniL , DeRidder K , PalominoJC , HirschelB , PortaelsF. Microaerophilic conditions promote growth of Mycobacterium genavense. J. Clin. Microbiol.36(9), 2565–2570 (1998).
- ChatterjeeM , BhattacharyaS , KarakK , DastidarSG. Effects of different methods of decontamination for successful cultivation of Mycobacterium tuberculosis. Indian J. Med. Res.138(4), 541–548 (2013).
- ElAmrani MH , AdouiM , PateyO , AsselineauA. Upper extremity Mycobacterium marinum infection. Orthop. Traumatol. Surg. Res.36(6), 706–711 (2010).
- StinearTP , JenkinGA , JohnsonPDR , DaviesJK. Comparative genetic analysis of Mycobacterium ulcerans and Mycobacterium marinum reveals evidence of recent divergence. J. Bacteriol.182(22), 6322–6330 (2000).
- QiW , KäserM , RöltgenK , Yeboah-ManuD , PluschkeG. Genomic diversity and evolution of Mycobacterium ulcerans revealed by next-generation sequencing. PLOS Pathog.5(9), e1000580 (2009).
- StinearT , RossBC , DaviesJKet al.Identification and characterization of IS2404 and IS2606: two distinct repeated sequences for detection of Mycobacterium ulcerans by PCR. J. Clin. Microbiol.37(4), 1018–1023 (1999).
- ChemlalK , HuysG , LavalFet al.Characterization of an unusual mycobacterium: a possible missing link between Mycobacterium marinum and Mycobacterium ulcerans. J. Clin. Microbiol.40(7), 2370–2380 (2002).
- DoigKD , HoltKE , FyfeJAMet al.On the origin of Mycobacterium ulcerans, the causative agent of Buruli ulcer. BMC Genom.40(7), 1471–2165 (2012).
- StinearTP , SeemannT , PidotSet al.Reductive evolution and niche adaptation inferred from the genome of Mycobacterium ulcerans, the causative agent of Buruli ulcer. Genome Res.17(2), 192–200 (2007).
- RondiniS , KäserM , StinearTet al.Ongoing genome reduction in Mycobacterium ulcerans. Emerg. Infect. Dis.13(7), 1008–1015 (2007).
- HuberCA , RufMT , PluschkeG , KäserM. Independent loss of immunogenic proteins in Mycobacterium ulcerans suggests immune evasion. Clin. Vaccine Immunol.15(4), 598–606 (2008).
- StinearTP , Mve-ObiangA , SmallPLCet al.Giant plasmid-encoded polyketide synthases produce the macrolide toxin of Mycobacterium ulcerans. Proc. Natl Acad. Sci. USA101(5), 1345–1349 (2004).
- PidotSJ , AsieduK , KäserM , FyfeJAM , StinearTP. Mycobacterium ulcerans and other mycolactone-producing mycobacteria should be considered a single species. PLOS Negl. Trop. Dis.4(7), e663 (2010).
- DassiC , MosiL , NarhCAet al.Distribution and risk of mycolactone-producing mycobacteria transmission within Buruli ulcer endemic communities in Côte d'Ivoire. Trop. Med. Infect. Dis.2(1), 3 (2017).
- WilliamsonHR , BenbowME , NguyenKDet al.Distribution of Mycobacterium ulcerans in Buruli ulcer endemic and non-endemic aquatic sites in Ghana. PLOS Negl. Trop. Dis.2(3), e205 (2008).
- McIntoshM , WilliamsonH , BenbowMEet al.Associations between Mycobacterium ulcerans and aquatic plant communities of West Africa: implications for Buruli ulcer disease. Ecohealth11(2), 184–196 (2014).
- MerrittRW , BenbowME , SmallPLC. Unraveling an emerging disease associated with disturbed aquatic environments: the case of Buruli ulcer. Front. Ecol. Environ.8(1), e2660 (2005).
- MarsollierL , StinearT , AubryJet al.Aquatic plants stimulate the growth of and biofilm formation by Mycobacterium ulcerans in axenic culture and harbor these bacteria in the environment. Appl. Environ. Microbiol.70(2), 1097–1103 (2004).
- MarsollierL , BrodinP , JacksonMet al.Impact of Mycobacterium ulcerans biofilm on transmissibility to ecological niches and Buruli ulcer pathogenesis. PLOS Pathog.3(5), 0582–0594 (2007).
- MosiL , WilliamsonH , WallaceJR , MerrittRW , SmallPLC. Persistent association of Mycobacterium ulcerans with West African predaceous insects of the family belostomatidae. Appl. Environ. Microbiol.74(22), 7036–7042 (2008).
- DjouakaR , ZeukengF , BigogaJDet al.Domestic animals infected with Mycobacterium ulcerans – implications for transmission to humans. PLOS Negl. Trop. Dis.12(7), e0006512 (2018).
- BolzM , RufMT. Buruli ulcer in animals and experimental infection models. In: Buruli Ulcer: Mycobacterium Ulcerans Disease.Springer Cham, NY, USA, 159–181 (2019).
- FyfeJAM , LavenderCJ , HandasydeKAet al.A major role for mammals in the ecology of Mycobacterium ulcerans. PLOS Negl. Trop. Dis.4(8), e791 (2010).
- VandelannooteK , DurnezL , AmissahDet al.Application of real-time PCR in Ghana, a Buruli ulcer-endemic country, confirms the presence of Mycobacterium ulcerans in the environment. FEMS Microbiol. Lett.304(2), 191–194 (2010).
- WillsonSJ , KaufmanMG , MerrittRW , WilliamsonHR , MalakauskasDM , BenbowME. Fish and amphibians as potential reservoirs of Mycobacterium ulcerans, the causative agent of Buruli ulcer disease. Infect. Ecol. Epidemiol.3(10.3402), 19946 (2013).
- NarhCA , MosiL , QuayeCet al.Source tracking Mycobacterium ulcerans infections in the Ashanti Region, Ghana. PLOS Negl. Trop. Dis.9(1), e0003437 (2015).
- JunghanssT , BoockAU , VogelM , SchuetteD , WeinlaederH , PluschkeG. Phase change material for thermotherapy of Buruli Ulcer: a prospective observational single centre proof-of-principle trial. PLOS Negl. Trop. Dis.3(2), 1–7 (2009).
- DrancourtM , JarlierV , RaoultD. The environmental pathogen Mycobacterium ulcerans grows in amphibian cells at low temperatures. Appl. Environ. Microbiol.68(12), 6403–6404 (2002).
- NiderweisM. Nutrient acquisition by mycobacteria. Microbiology154(3), 679–692 (2008).
- OrruñoM , ParadaC , KaberdinVR , AranaI. Survival of Escherichia coli under nutrient-deprived conditions: effect on cell envelope sub proteome. Escherichia coli - Recent Advances on Physiology, Pathogenesis and Biotechnological Applications.IntechOpen Limited, London, UK (2017). https://doi.org/10.5772/intechopen.68309
- XuHS , RobertsN , SingletonFL , AttwellRW , GrimesDJ , ColwellRR. Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb. Ecol.8(4), 313–323 (1982).
- WatsonSP , ClementsMO , FosterSJ. Characterization of the starvation-survival response of Staphylococcus aureus. J. Bacteriol.180(7), 1750–1758 (1998).
- WuML , GengenbacherM , DickT. Mild nutrient starvation triggers the development of a small-cell survival morphotype in mycobacteria. Front. Microbiol.7, 947 (2016).
- BettsJC , LukeyPT , RobbLC , McAdamRA , DuncanK. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol.43(3), 717–731 (2002).
- Peñuelas-UrquidesK , Villarreal-TreviñoL , Silva-RamírezB , Rivadeneyra-EspinozaL , Said-FernándezS , de LeónMB. Measuring of Mycobacterium tuberculosis growth: a correlation of the optical measurements with colony forming units. Brazilian J. Microbiol.44(1), 287–289 (2013).
- ZingueD , PandaA , DrancourtM. A protocol for culturing environmental strains of the Buruli ulcer agent, Mycobacterium ulcerans. Sci. Rep.8(1), 6778 (2018).
- RonEZ. Bacterial stress response. In: The Prokaryotes: Prokaryotic Physiology and Biochemistry.RosenbergE, DelongEF, LoryS, StackebrandtE, ThompsonF ( Eds). Springer, Heidelberg, Germany, 509–603 (2013).
- ŚwieciłoA , Zych-WezykI. Bacterial stress response as an adaptation to life in a soil environment. Polish J. Environ. Stud.22(6), 1577–1587 (2013).
- BobekJ , StrakovaE , ZikovaA , VohradskyJ. Changes in activity of metabolic and regulatory pathways during germination of S. coelicolor. BMC Genomics15(1), 1173 (2014).
- JaishankarJ , SrivastavaP. Molecular basis of stationary phase survival and applications. Front. Microbiol.8, 2000 (2017).
- GuptaS , ChatterjiD. Stress responses in mycobacteria. IUBMB Life57(3), 149–159 (2005).
- GhoshJ , LarssonP , SinghBet al.Sporulation in mycobacteria. Proc. Natl Acad. Sci. USA106(26), 10781–10786 (2009).
- ErringtonJ. Regulation of endospore formation in Bacillus subtilis. Nat. Rev. Microbiol.1(2), 117–126 (2003).
- CrammMA , ChakrabortyA , LiC , RuffSE , JørgensenBB , HubertCRJ. Freezing tolerance of thermophilic bacterial endospores in marine sediments. Front. Microbiol.10(945), (2019).
- MwakapejeER , NdimuligoSA , MosomtaiGet al.Ecological niche modeling as a tool for prediction of the potential geographic distribution of Bacillus anthracis spores in Tanzania. Int. J. Infect. Dis.79, 142–151 (2019).
- AbecasisAB , SerranoM , AlvesR , QuintaisL , Pereira-LealJB , HenriquesAO. A genomic signature and the identification of new sporulation genes. J. Bacteriol.195(9), 2101–2105 (2013).
- TanIS , RamamurthiKS. Spore formation in Bacillus subtilis. Environ. Microbiol. Rep.6(3), 212–225 (2014).
- PiggotPJ , HilbertDW. Sporulation of Bacillus subtilis. Curr. Opin. Microbiol.7(6), 579–586 (2004).
- PaidhungatM , SetlowB , DriksA , SetlowP. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol.182(19), 5505–5512 (2000).
- MartínezA , TorelloS , KolterR. Sliding motility in mycobacteria. J. Bacteriol.181(23), 7331–7338 (1999).
- Ramos-SilvaP , SerranoM , HenriquesAO. From root to tips: sporulation evolution and specialization in Bacillus subtilis and the intestinal Pathogen Clostridioides difficile. Mol. Biol. Evol.36(12), 2714–2736 (2019).
- HilbertDW , PiggotPJ. Compartmentalization of gene expression during Bacillus subtilis spore formation. Microbiol. Mol. Biol. Rev.68(2), 234–262 (2004).
- EichenbergerP , JensenST , ConlonEMet al.The σE regulon and the identification of additional sporulation genes in Bacillus subtilis. J. Mol. Biol.327(5), 945–972 (2003).
- EdwardsAN , McbrideSM. Initiation of sporulation in Clostridium difficile: a twist on the classic model. FEMS Microbiol. Lett.358(2), 110–118 (2014).
- GalperinMY , MekhedovSL , PuigboP , SmirnovS , WolfYI , RigdenDJ. Genomic determinants of sporulation in Bacilli and Clostridia: towards the minimal set of sporulation-specific genes. Environ. Microbiol.14(11), 2870–2890 (2012).
- SprusanskyO , ZhouL , JordanSet al.Identification of three new genes involved in morphogenesis and antibiotic production in Streptomyces coelicolor.. J. Bacteriol.185(20), 6147–6157 (2003).
- SalernoP , PerssonJ , BuccaGet al.Identification of new developmentally regulated genes involved in Streptomyces coelicolor sporulation.BMC Microbiol.13(281), (2013).
- DeMaioJ , ZhangY , KoCet al.Mycobacterium tuberculosis sigF is part of a gene cluster with similarities to the Bacillus subtilis sigF and sigB operons.. Tuber. Lung Dis.78(1), 3–12 (1997).
