Abstract
PD-1 blockade is now routine for nearly all patients with non-small lung cancer. Acquired resistance to PD-1 blockade – defined generally as an initial response followed later by progression [Citation1-3] is a common yet poorly understood concept. A key clinical challenge to insight has been a lack of standard guidance for clinical management of a case of suspected acquired resistance. The infrequency of performing tumor biopsies and the uncertainty of actionability from tissue sampling likely also contribute to limited insight into the biology of acquired resistance [Citation4]. To address this knowledge gap and to highlight the value of tumor and liquid biopsy, we present a representative case of suspected acquired resistance to PD-1 blockade and propose a multi-modal guide for approaching this clinical scenario.
Acquired resistance to immunotherapy should not be assumed but should be thoroughly interrogated.
Liquid biopsies provide invaluable real-time insights into treatment response and should be utilized whenever possible.
Incongruity in imaging, clinical status, and sequencing should not be dismissed, and an alternative explanation for the discrepancy needs to be explored.
PD-1 blockade is now routine for nearly all patients with non-small lung cancer. However, the proper investigation of alternative explanations and the cause remains not intuitive for many clinicians. We present a case of a patient who benefitted from PD-1 blockade for more than a year, but later developed what was initially suspected to be radiological progression. A systematic approach to investigating with the utilization of clinical examination, radiology and comprehensive molecular profiling with tissue and liquid biopsy of the new pulmonary nodule revealed an alternate explanation, preventing the premature discontinuation of treatment and providing a framework for approaching this clinical scenario. In presenting this individual case, we aim to highlight the approach, considerations, and tools that can be used to address new radiologic changes suspected, but not certain, to be acquired resistance to PD-1 blockade.
Case presentation
A current smoker with a 50-pack-year smoking history presented to care with bilateral lung nodules and a suspicious left adrenal gland lesion. A diagnostic biopsy showed metastatic lung adenocarcinoma; scant tissue limited PD-L1 expression. Tissue underwent comprehensive molecular testing with MSK-IMPACT (Integrated Mutation Profile of Actionable Cancer Targets), the in-house FDA approved platform that can detect many mutations even at very low allele frequencies (<2%) [Citation5]. As a companion diagnostic, he was offered MSK-ACCESS (Analysis of Circulating cfDNA to Evaluate Somatic Status), an in-house liquid biopsy platform. MSK-ACCESS can detect over 120 mutations even at a variable allele frequency of 0.5% with more than 99% sensitivity when paired with tissue sequencing, as with our patient [Citation6]. MSK IMPACT tissue platform demonstrated no targetable mutations, but was notable for TP53 and ARID1A mutations. MSK-ACCESS liquid biopsy platform was congruous with the initial tissue biopsy, but additional mutations were discovered and were notable for detectable alterations in TP53, ARID1A, KEAP1, MAP2K and SMARCA4 (). The patient initially received carboplatin, pemetrexed, and pembrolizumab and immediately achieved an excellent clinical and a complete radiological response. After four cycles of combination therapy, both carboplatin and pemetrexed were ceased and pembrolizumab was continued as maintenance therapy every 3 weeks. The deep response persisted for more than 14 months while on pembrolizumab alone, most consistent with an ongoing response to PD-1 blockade therapy.
Figure 1. Timeline of the patient.
At baseline, no targetable mutations, but liquid biopsy detected TP53, ARID1A, KEAP1 and SMARCA4 mutations. After complete response, at 14 months, a solitary pulmonary nodule was detected on CT, and it was PET avid indicative of potentially acquired resistance to PD-1 therapy. Liquid biopsy detected clearance and was inconsistent with radiology. Further investigation with tissue biopsy at 16 months revealed a benign diagnosis and refuted the progression of the disease.
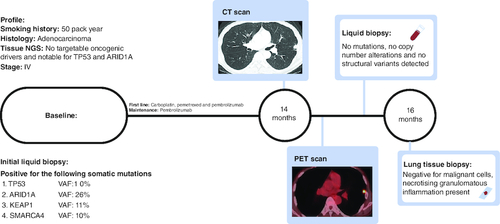
More recently, a routine interval CT scan showed a new 1.2 cm subpleural nodule in the lingula. The prior primary and metastatic lesions remained well controlled without progression. The patient clinically felt well. To inform the persistence and avidity of the new nodule, and to assess for additional suspicious sites of distant metastasis, we evaluated his disease status with a PET scan. This test redemonstrated the PET avid new nodule with an SUVmax of 6.2 and was suspicious for recurrent malignancy but identified no other distant sites of metastasis. We also surveyed for radiologically occult systemic recurrence using ctDNA testing, which returned no detectable somatic variants, with the clearance of previously detected somatic alterations. Given the radiologic suspicion of acquired resistance to PD-1 blockade, but the patient feeling clinically well and with no detectable ctDNA shedding in the blood, we next pursued a biopsy of the nodule to determine the diagnosis. Fortunately, pathology revealed no malignant cells and rather a necrotizing, granulomatous inflammation. No further intervention was pursued on the nodule, and the patient has remained well on pembrolizumab with no further evidence of progression.
Discussion
This case represents a common clinical scenario clinicians face in patients with non-small cell lung cancer and response to PD-1 blockade. Acquired resistance can occur in nearly 2/3rds of initial responders and, akin to this case, commonly occurs in a single site or oligo-pattern of progression [Citation7]. Most clinicians in this context would acknowledge progression, especially after PET avidity, and explore other options such as local therapy or change of systemic treatment. Our approach to this scenario is multimodal and includes a comprehensive diagnostic evaluation rather than empirically pursuing a change in systemic therapy or palliative radiation (). This multimodality approach utilizes comprehensive molecular profiling through tissue or liquid biopsies, traditional radiology, functional imaging and the patient's clinical status. The systematic approach helps prevent unnecessary complications associated with invasive investigations such as a tissue biopsy in a cohort of people already at high risk of complications such as pneumothorax [Citation8,Citation9]. This stepwise approach will guide a clinician through systemic interrogation to determine the cause for the acquired resistance and efficient deployment of resources yielding the maximum benefit to the patient and the healthcare setting.
Figure 2. Schema for investigation of acquired resistance to PD-1 blockade.
After CT diagnosis of the pulmonary nodule, proceed with functional imaging with a PET scan. If PET avid, proceed to liquid biopsy to confirm progression, identify therapeutic targets, and for consideration of management change. If the liquid biopsy confirms clearance, i.e., ongoing response, proceed to tissue biopsy to characterize the pulmonary nodule.
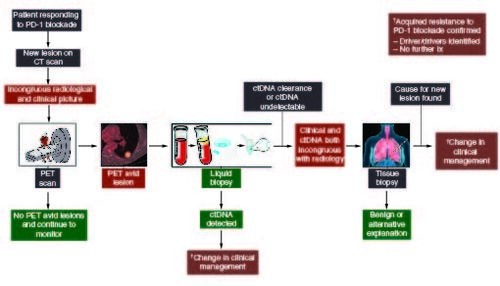
Specifically, faced with a new nodule concerning acquired resistance to PD-1 blockade, we evaluated the patient's clinical status with an exam and history, informing the persistence relative suspicion of the nodule with a subsequent PET scan. Then we assessed the systemic status of detectable cancer with ctDNA testing to confirm the resistance and potentially identify new drivers and therapeutic options, including clinical trials if a driver was identified.
In our patient, the clinical and the complete ctDNA clearance [Citation10] indicated ongoing response, which was incongruous with the radiology and necessitated proceeding to the final step in the scheme: a diagnostic biopsy. It is important to note that if the ctDNA was congruent with the clinical picture, it confirmed oligoprogression and could provide invaluable information regarding the underlying driver of progression and allude to targets and therapeutic options. In addition, it makes the next step, as suggested in , proceeding to an invasive biopsy redundant. Fortunately, a benign diagnosis was discovered, and no further intervention was required preventing premature discontinuation of treatment or unnecessary radiation. This case serves as a reminder that acquired resistance should not be assumed but instead approached thoughtfully and carefully examined prior to pursuing interventions that may not be necessary or prematurely stopping the current line of therapy. Additionally, this case highlights the value of pursuing a more comprehensive molecular characterization, either from a traditional tissue biopsy or liquid biopsy, in the context of suspected acquired resistance to PD-1 blockade. This may facilitate the discovery of the molecular underpinnings of resistance and inform the development of rational therapeutic strategies in the future.
Conclusion
A new lesion in a patient otherwise responding well to PD-1 blockade should not be presumed to be acquired resistance and should instead be investigated in a stepwise manner.
Author contributions
All authors listed on the title page (DL De Silva, J Luo) has contributed to the following aspects of the manuscript. Substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work. Drafting the work or revising it critically for important intellectual content. Final approval of the version to be published.
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The study governing genomic testing (MSK IRB#12–145) of ctDNA was evaluated and approved by the local ethic committee of our institution and conducted in accordance with the 1964 Declaration of Helsinki. The patient enrolled in this and gave their consent for participation. The report has been anonymized to remove patient identifiers. The patient has consented to MSK IRB #12–245, including anonymized publication of results.
Acknowledgments
The authors thank the staff at Thoracic Oncology Service, Division of Solid Tumor Oncology, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Competing interests disclosure
J Luo reports honoraria from Targeted Oncology, Physicians' Education Resource, VJ Oncology, CancerGRACE, and Community Cancer Education, Inc.; advisory board participation from AstraZeneca; research support to her institution from Erasca, Revolution Medicines, and Genentech; and personal fees from Erasca, Blueprint Medicines, and Daiichi Sankyo. The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- Brahmer JR, Govindan R, Anders RA et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of non-small cell lung cancer (NSCLC). J. Immunother. Cancer 6(1), 75 (2018).
- Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 168(4), 707–723 (2017).
- Kluger HM, Tawbi HA, Ascierto ML et al. Defining tumor resistance to PD-1 pathway blockade: recommendations from the first meeting of the SITC Immunotherapy Resistance Taskforce. J. Immunother. Cancer 8(1), 398 (2020).
- Schoenfeld AJ, Hellmann MD. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell 37(4), 443–455 (2020).
- Cheng DT, Mitchell TN, Zehir A et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J. Mol. Diagn. 17(3), 251–264 (2015).
- Rose Brannon A, Jayakumaran G, Diosdado M et al. Enhanced specificity of clinical high-sensitivity tumor mutation profiling in cell-free DNA via paired normal sequencing using MSK-ACCESS. Nat. Commun. 12(1), 3770 (2021).
- Shah S, Wood K, Labadie B et al. Clinical and molecular features of innate and acquired resistance to anti-PD-1/PD-L1 therapy in lung cancer. Oncotarget 9(4), 4375–4384 (2018).
- Huo YR, Chan MV, Habib AR, Lui I, Ridley L. Pneumothorax rates in CT-Guided lung biopsies: a comprehensive systematic review and meta-analysis of risk factors. Br. J. Radiol. 93(1108), 20190866 (2020).
- Boskovic T, Stanic J, Pena-Karan S et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J. Thorac. Dis. 6(Suppl. 1), S99–S107 (2014).
- Song Y, Hu C, Xie Z et al. Circulating tumor DNA clearance predicts prognosis across treatment regimen in a large real-world longitudinally monitored advanced non-small cell lung cancer cohort. Transl. Lung Cancer Res. 9(2), 269–279 (2020).
