Abstract
Aim: The efficacy of antifibrinolytics in subarachnoid hemorrhage remains unclear due to conflicting evidence from studies. Materials & methods: Online databases were queried to include randomized controlled trials and propensity matched observational studies. We used Review Manager for the statistical analysis, presenting results as odds ratios with 95% CI. Results: The 12 shortlisted studies included 3359 patients, of which 1550 (46%) were in the intervention (tranexamic acid) group and 1809 (54%) in the control group. Antifibrinolytic therapy significantly reduced the risk of rebleeding (OR: 0.55; 95% CI: 0.40–0.75; p = 0.0002) with no significant decrease in poor clinical outcome (OR: 1.02; 95% CI: 0.86–1.20; p = 0.85) and all-cause mortality (OR: 0.92; CI: 0.72–1.17; p = 0.50). Conclusion: In patients with subarachnoid hemorrhage, antifibrinolytics reduce the risk of rebleeding without significantly affecting mortality or clinical outcomes.
Subarachnoid hemorrhage (SAH) accounts for approximately ten percent of all hemorrhagic strokes, with a global incidence of aneurysmal SAH of 7.9 per 100,000 person years [Citation1]. Aneurysmal SAH, the most common cause of SAH, is secondary to the rupture of a saccular aneurysm of one of the cerebral vessels. It occurs most frequently in younger individuals with half the patients less than 50 years of age and is responsible for significant morbidity and mortality [Citation2]. About one to two third of patients die after hemorrhage [Citation3], and about one in ten survivors remain unable to lead an independent life [Citation4].
Rebleeding, a common complication of SAH, is associated with increased mortality, brain death, poor outcomes and complications such as hydrocephalus and respiratory failure [Citation5]. Endovascular or neurosurgical obliteration of the aneurysm can prevent rebleeding. However, the risk (4–20%) is maximal within the first 24 h [Citation6], a window during which many patients are beyond the reach of centers that can provide timely intervention [Citation7]. Antifibrinolytic agents, such as aminocaproic acid and tranexamic acid (TXA), inhibit fibrinolysis of blood clots, and can potentially reduce the risk of rebleeding after a SAH. However, the use of antifibrinolytics in SAH has been limited due to concerns that it may increase the risk of cerebral ischemia – a common complication of SAH that arises 3–14 days after the event. It was thus proposed that short-term (i.e., continued no longer than 3 days) treatment with antifibrinolytics; before the cerebral ischemia window, may improve outcomes without increasing cerebral ischemia [Citation6].
The most recent review by Cochrane in 2013 [Citation2] included only a single study evaluating short-term antifibrinolytic treatment in patients with aneurysmal SAH. It showed promising yet inconclusive results on short-term TXA treatment and hypothesized that new studies exploring the use of early or ultra-early short-term TXA therapy might yield positive results [Citation2]. Since then, further studies evaluating short-term treatment have been published. These newer studies have a sample size larger than all the previous trials combined; have higher methodological rigor and are conducted in an era where the management of SAH is significantly different from the past practices. In the present meta-analysis, our aim was to incorporate the new studies to evaluate the potential benefit of antifibrinolytic therapy in terms of the effects on rebleeding risk, poor clinical outcome, delayed cerebral ischemia and all-cause mortality. By grouping studies based on the duration of TXA use, we specifically sought to determine if short-term versus long-term treatment shows evidence of clinical benefit in SAH. We also decided to group studies on the basis of antivasospasm treatments, seeking to know whether cerebral ischemia prevention offers any additional benefit.
Materials & methods
This systematic review and meta-analysis is compliant with the Preferred Reporting Items for Systematic review and Meta-Analyses (PRISMA) guidelines and has been registered with The International Prospective Register of Systematic Reviews (PROSPERO ID: CRD42022301879).
Data sources & search strategy
We searched a total of four electronic databases, namely PubMed/MEDLINE, Cochrane CENTRAL, Science Direct and Google Scholar from their inception to April 2023, and no filters were used for language or study design. We used a combination of the keywords and MeSH terms to generate our search string for PUBMED: (‘subarachnoid hemorrhage’ [MeSH terms] or ‘aneurysmal subarachnoid hemorrhage’ [all fields]) and (‘antifibrinolytic agents’ [MeSH terms] or ‘tranexamic acid’ [MeSH terms] or ‘aminocaproic acid’ [all fields] or ‘antifibrinolytics’ [all fields]). Additionally, the bibliographies of the included studies and previous meta-analyses were manually screened to identify all relevant articles. The details of the complete search strategy are given in Supplementary File 1.
Study selection
Published randomized controlled trials with a follow-up duration of at least 3 months for clinical outcome assessment or observational studies with adjusted or propensity matched data were selected for this meta-analysis. The included population consisted of adult patients (>18 years) presenting within the first 72 h of aneurysmal SAH. Patients were divided into intervention and control groups. The intervention group received antifibrinolytics (oral or intravenous), such as TXA and Epsilon aminocaproic acid, while the control group consisted of patients receiving placebo/standard of care. Only those studies that reported at least one of the four outcomes (i.e., mortality, poor clinical outcome, rebleeding and delayed cerebral ischemia) were included in our article. Studies that did not meet our inclusion criteria were excluded and only those observational studies were considered which had propensity matched data or provided adjusted odds ratios (OR).
Data extraction
All the articles retrieved from the literature search were exported to Mendeley software where duplicates were screened for and removed. The remaining articles were carefully assessed by two independent reviewers (Hareem Farooq and Muhammad Ebaad Ur Rehman), and articles were assessed using their titles and abstracts first, and all relevant articles were selected. Full articles were then reviewed and those that met the inclusion criteria were finalized for our study. A third investigator (Muhammad Aemaz Ur Rehman) was consulted to resolve any discrepancies that arose during the entire process. The authors then extracted data related to study design, the number of participants in the intervention and control group, details of the intervention (including drug name, dose, duration and route), ischemia prevention strategies, follow-up time and outcomes. Duration of therapy was divided into two categories, short-term (continued less than 72 h after SAH occurrence) and long-term (continued more than 72 h after SAH). We also grouped studies according to the presence or absence of ischemia prevention (defined as the use of calcium channel antagonists and/or hypervolemic therapy), hence four major subgroups of studies were identified: short-term therapy and medical prevention of ischemia; short-term therapy and no medical prevention of ischemia; long-term therapy and medical prevention of ischemia; long-term therapy and no medical prevention of ischemia.
Outcomes of interest
The four outcomes extracted from all the studies included: all-cause mortality, rebleeding, poor outcome and cerebral ischemia. Our primary outcome was poor clinical outcome assessed at a follow-up of 3–6 months and defined as death, severe disability, a vegetative state corresponding to a score of 4, 5 or 6 on the modified Rankin scale) or a score of 1, 2 or 3 on the Glasgow outcome scale. Among secondary outcomes, our study pooled data for the following: rebleeding, all-cause mortality and delayed cerebral ischemia (or simply, cerebral ischemia). All-cause mortality encompassed death due to any cause at a minimum of a 3-week follow-up. Due to the variability in the definition of cerebral ischemia among studies, we defined the term cerebral ischemia broadly in our study to mean the presence of any form of ischemia or infarction in the brain, after the SAH, seen on radiographic imaging or determined through clinical features.
Quality assessment of included studies
The Cochrane risk-of-bias tool [Citation8] was used for the quality assessment of randomized controlled trials while the Newcastle Ottawa Quality Assessment scale [Citation9] was used for the observational study. The risk-of-bias tool assessed all studies in the following domains: randomization, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, detection bias, attrition bias, bias in reporting and other biases. All domains were categorized as ‘high risk of bias’, ‘low risk of bias’ and ‘unclear risk of bias’. The Newcastle Ottawa Scale scored the observational study on the following three parameters: selection, comparability and outcome which were further divided into subcategories. The scale rates studies with a score of 7–9 as high quality, while those with a score of 4–6 are considered high risk and 0–3 as very high risk of having bias.
Statistical analysis
We conducted our statistical analysis using Review Manager (RevMan) (Computer program) version 5.4. The Cochrane Collaboration. Copenhagen: The Nordic Cochrane Center, 2020. The results from all studies were presented as ORs with 95% CI, were pooled using a Mantel–Haenszel random-effects model and forest plots were made for a graphical representation of the pooling. To assess for subgroup differences, the χ2 test was used. Heterogeneity across studies was evaluated using Higgins I2 and a value less than 50% for I2 was considered acceptable. Begg’s test and a visual inspection of the funnel plot were conducted to evaluate for publication bias. A p-value of less than 0.05 was considered significant in all cases.
Results
Literature search
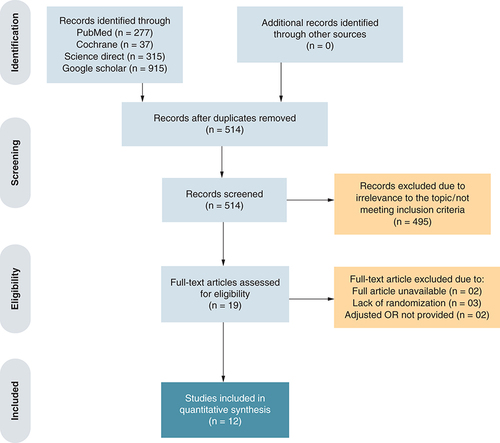
An initial electronic search of four databases yielded 1467 potential studies. Twelve studies were finally selected for analysis, including 11 randomized controlled trials [Citation10–20] and one observational study [Citation21]. The PRISMA flowchart () summarizes the results of our literature search.
Study characteristics & quality assessment
These studies assigned randomly a total of 3359 patients with SAH: 1550 patients (46%) in the antifibrinolytic treatment arm and 1809 patients (54%) in the control arm. TXA administered either orally or intravenously, was used as an antifibrinolytic in all 12 studies. The follow-up duration for the assessment of clinical outcome ranged from 3 to 6 months. Nine studies belonged to the long-term TXA therapy cohort whereas three studies were placed in the short-term TXA therapy cohort. The baseline study and major intervention characteristics are outlined in .
Table 1. Baseline study and major intervention characteristics of the included studies.
All studies were of a considerably high methodological quality as shown in Supplementary File 2. Funnel plots were made for outcomes that had ten or more studies. Symmetrical funnel plots (Supplementary Figures 1 & 2) suggest that there was no small study or publication bias.
Effect of intervention on outcomes
The outcomes included in the analysis were rebleeding risk, poor clinical outcome, delayed cerebral ischemia and all-cause mortality. Four major subgroups discussed previously were identified and then analyzed for each outcome.
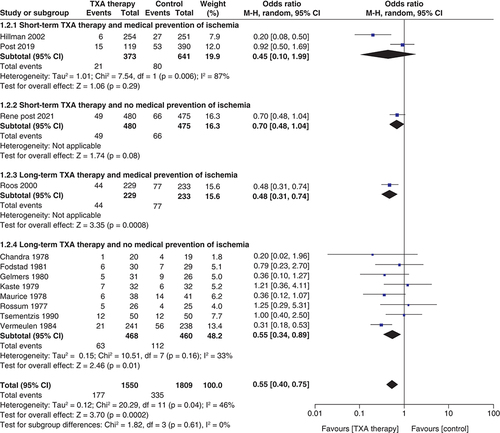
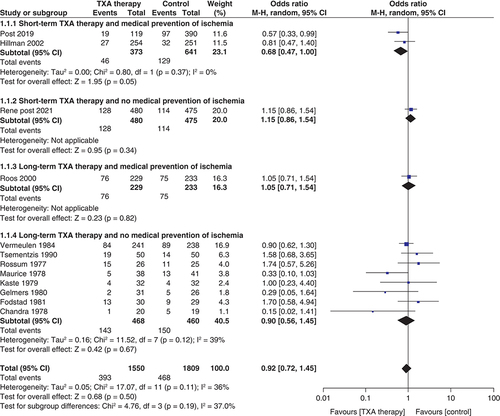
Risk of rebleeding ()
A total of 12 studies reported the effect of TXA therapy on the risk of rebleeding after SAH (n = 3359; TXA = 1550; control = 1809). The pooled result showed that TXA use significantly reduced the risk of rebleeding (OR: 0.55; 95% CI: 0.40–0.75; p = 0.0002]. The test for subgroup differences showed no statistically significant subgroup effect (p = 0.61), suggesting that the duration of antifibrinolytic administration and medical ischemia prevention does not change the effect of TXA therapy on rebleeding risk when compared with control.
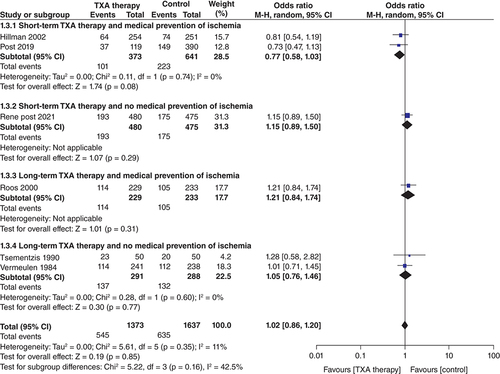
Poor clinical outcome ()
Six studies presented data on poor clinical outcomes at the end of follow-up (n = 3010; TXA = 1373; control = 1637). There was no significant decrease in poor clinical outcomes with TXA therapy as compared with control (OR: 1.02; 95% CI: 0.86–1.20; p = 0.85). This lack of effect was consistent among all four subgroups as shown in
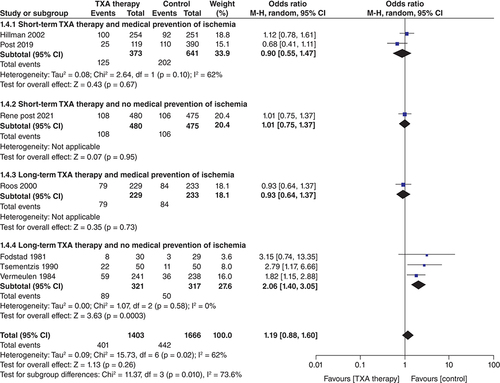
Delayed cerebral ischemia ()
In the seven studies that reported data on delayed cerebral ischemia (n = 3069; TXA = 1403; control = 1666), TXA treatment did not significantly increase the risk of cerebral ischemia (OR: 1.19; CI: 0.88–1.60; p = 0.26). Considerable heterogeneity (I2 = 62%) was found in the analysis of this outcome, but the result of the subgroup analysis explains this heterogeneity. The subgroup ‘Long-term TXA therapy and no medical prevention of ischemia’ showed a significantly increased risk of delayed ischemia (OR: 2.06; CI: 1.40–3.05; p = 0.0003) and the test for subgroup differences was statistically significant (pinteraction <0.1) ( 1.4.4). There was no increase in cerebral ischemia in the other subgroups; short-term TXA and medical prevention of ischemia (OR: 0.90; CI: 0.55–1.47; p = 0.67) ( 1.4.1), short-term TXA and no medical prevention of ischemia (OR: 1.01; CI: 0.75–1.37; p = 0.95) ( 1.4.2) and long term TXA and medical prevention of ischemia (OR: 0.93; CI: 0.64–1.37; p = 0.73).
All-cause mortality ()
Twelve studies reported data on all-cause mortality (n = 3359; TXA = 1550; control = 1809). TXA therapy had no significant effect on all-cause mortality (OR: 0.92; CI: 0.72–1.17; p = 0.50) and similar results were seen in the subgroup analysis. The overall heterogeneity was acceptable (<50%).
Discussion
This meta-analysis shows that neither short-term (started at the time of SAH diagnosis and continued <72 h) nor long-term (started at the time of SAH diagnosis and continued >72 h) antifibrinolytic therapies improve clinical outcome in patients with aneurysmal SAH, irrespective of whether or not the anticipated vasospasm (or cerebral ischemia) is medically prevented. Although there is a decrease in the rebleeding risk with long-term TXA therapy, the evidence for an overall efficacy in terms of a better clinical outcome and mortality benefit is lacking. Our findings conform with the previous studies but provide stronger evidence by including a larger pool of data from more recent trials and add significant value by answering some pertinent questions raised in the two previous meta-analyses. Gaberel et al. and Baharoglu et al. [Citation2,Citation22] suggested that short-term treatment, especially with medical prevention of vasospasm, may decrease rebleeding risk without an increase in the risk of cerebral ischemia, hence leading to improved clinical outcomes. Although our results showed that the risk of delayed cerebral ischemia was not increased with short-term TXA therapy, there was no significant decrease in the rebleeding risk nor an improvement in clinical outcomes.
Rebleeding is the most feared complication after SAH as it carries a high mortality [Citation23]. The role of TXA in clot stabilization and its antifibrinolytic properties initially nurtured the idea of using it in SAH, and the results of various clinical trials supported it as well [Citation24]. In 2003, Roos suggested that although there is a clear benefit in terms of rebleeding risk, there is an associated increase in delayed cerebral ischemia, resulting in an overall inefficacy when it comes to mortality and clinical outcome [Citation24]. Consequently, TXA therapy largely fell out of favor at most neurosurgical centers. In the earlier half of the last decade, it was proposed that short-term TXA therapy (given before the window of cerebral ischemia), especially along with antivasospasm measures like calcium channel blockers and hypervolemic therapy, etc., does not increase the risk of delayed cerebral ischemia in addition to significantly reducing rebleeding. In order to evaluate the credibility of this hypothesis, a large cohort study and the ULTRA trial were commenced, and have recently been completed [Citation19,Citation21]. The importance of newer evidence, especially the ULTRA trial, is paramount because most previous trials were carried out in an era when the practice of early aneurysm obliteration was almost non existent. According to our results, short-term TXA treatment did not significantly decrease the rebleeding risk and did not improve any other outcomes. Long-term treatment, on the other hand, significantly reduced the rebleeding risk, but this also came at the cost of increased cerebral ischemia. A recent review that investigated the role of fibrinolysis following SAH concluded that fibrinolysis does not have a proven role in SAH, and decreased platelet function is potentially the major factor in increasing rebleeding after SAH. Thus, while TXA may have some role in clot stabilization (especially when used long-term), there is little to no evidence to support its use at present. Recent studies have also suggested exploring alternative agents such as desmopressin to prevent rebleeding [Citation23].
Recent guidelines suggest that early surgical or endovascular management of the aneurysm (within 24 h) is the only definitive treatment [Citation25] to prevent rebleeding. The success of these procedures is dependent on the condition and resources of individual hospitals [Citation26]. Multidisciplinary teams are usually needed to decide the preferred technique (microsurgical clipping or endovascular coiling) depending on the patient and aneurysm characteristics [Citation25]. Multiple other factors serve as an obstacle to this management including a delay in referral to the neurosurgical units, lack of logistic support or unsuitable clinical condition of the patients at the time of admission [Citation27]. Therefore, additional measures to improve clinical outcomes (by preventing rebleeding and vasospasm) are now a part of standard care. Early hypertension management to keep systolic blood pressure less than 160 mmHg is the usual norm but it is vital to maintain the balance between hypertension and decreased cerebral perfusion to avoid ischemia [Citation25]. Nimodipine administration has consistently shown positive results in terms of clinical outcome and is therefore used in all patients as soon as they are stable [Citation28]. Antifibrinolytic therapies were also previously given as part of these conservative measures, but given the lack of improvement in clinical outcomes, most guidelines do not suggest any benefit from their routine use [Citation2]. The findings of this meta-analysis corroborate these current guidelines.
Although no increased risk of cerebral ischemia was observed with short-term TXA therapy compared with long-term use, a significant clinical improvement in this subgroup was still lacking. Therefore, other factors that might be responsible for poor outcome or mortality must be explored. A Spanish study highlighted that poor outcome in SAH was more likely to be seen in older (>65 years) age, female gender and patients who were hyperglycemic at the time of admission [Citation29]. Comorbidities such as hypertension and diabetes mellitus lead to poorer outcomes [Citation30] while smoking has been surprisingly shown to positively impact the outcome despite it being a risk factor for SAH [Citation31]. Moreover, other serious complications especially hydrocephalus (both acute and chronic), respiratory and cardiac abnormalities and electrolyte disturbances are also associated with poor prognosis after SAH [Citation32]. The abovementioned factors might play a role in the observed lack of improved clinical outcome despite a reduction in rebleeding. Furthermore, standardization of SAH trials and formulation of some core outcomes (specific to SAH) to be reported in each trial are needed to ensure an adequate comparison between trials and gain further progress in SAH management [Citation33].
Strengths & limitations
Compared with the previous meta-analyses, the power of this study is enhanced due to the inclusion of the most recent studies with a larger data pool. In order to reduce bias and yield reliable results, only the randomized controlled trials with a clearly reported follow-up period for clinical outcome and observational studies with propensity matched data were included. We believe that this significantly reduced the heterogeneity for all the outcomes except cerebral ischemia which might be accounted for by the variability in its definition and interpretation in different studies. This study adds significant value to the current literature by testing the proposed hypothesis regarding the possible benefit of short-term TXA therapy in SAH, especially with ischemia prevention. However, the authors would also like to acknowledge some limitations. The standard of care and treatment guidelines for aneurysmal SAH have significantly evolved over the last two decades (e.g., hypervolemia which was previously used for medical prevention of ischemia, has largely been replaced by euvolemia and calcium channel blockers now). This change in guidelines also explains the use of hypervolemia in older versus euvolemia in newer studies. Although we included the most recent available evidence in our meta-analysis, it must be noted that many trials addressing this topic are older, so all the results may not be completely applicable in modern practice. Clinical heterogeneity also exists owing to the variation in outcomes definitions, disease severity at baseline, the dose of TXA, and the start and duration of TXA after SAH diagnosis. At last, our literature search spanning four databases and bibliographies of all articles was not exhaustive, so it is possible that we did not find all relevant publications.
Conclusion
According to current evidence, both short-term and long-term antifibrinolytics cannot be recommended for presumed or proven aneurysmal SAH. Although the risk of rebleeding may be significantly reduced with long-term treatment, however, for reasons that are poorly understood, the evidence for an overall benefit in terms of better clinical outcome and mortality rates is lacking. TXA may have some role in clot stabilization, but fibrinolysis (which TXA primarily affects) has never been definitely proven after SAH, reinforcing that it may be time to explore other avenues besides TXA. It may be important to mention that most literature on this topic is old, so newer trials that are more clinically applicable in this era are needed to enhance the strength of evidence.
In patients with subarachnoid hemorrhage (SAH), rebleeding is the most important complication.
Antifibrinolytics reduce the risk of rebleeding in patients with SAH.
Antifibrinolytic use does not decrease mortality or poor clinical outcome after SAH.
Long-term antifibrinolytic use without antivasospasm measures increases rates of cerebral ischemia.
Author contributions
K Fatima's and M Aemaz Ur Rehman contributed equally to this article. M Aemaz Ur Rehman, K Fatima, A Asmar, H Farooq and N Ahmad contributed to the study design and conception, data extraction from the included studies, analysis, and manuscript writeup. A Danial, M Ebaad Ur Rehman, AA Khan, S Tahir, U Ahmed, A Khawaja and S Zubair contributed to the study design, literature search, data extraction, and manuscript writeup. All authors read and approved the final version of the manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations.
Supplemental Text 1
Download MS Word (214.9 KB)Supplemental Text 1
Download MS Word (14.9 KB)Supplemental Text 1
Download MS Word (68 KB)Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at:www.tandfonline.com/doi/full/10.2217/epi-2016-0184
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- EtminanN , ChangH-S , HackenbergKet al.Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta-analysis. JAMA Neurol.76(5), 588–597 (2019).
- BaharogluMI , GermansMR , RinkelGJEet al.Antifibrinolytic therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst. Rev.2013(8), CD001245 (2013).
- NieuwkampDJ , SetzLE , AlgraA , LinnFHH , de RooijNK , RinkelGJE. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol.8(7), 635–642 (2009).
- SchatloB , FungC , StienenMNet al.Incidence and outcome of aneurysmal subarachnoid hemorrhage: the Swiss study on subarachnoid hemorrhage (Swiss SOS). Stroke52(1), 344–347 (2021).
- LordAS , FernandezL , SchmidtJMet al.Effect of rebleeding on the course and incidence of vasospasm after subarachnoid hemorrhage. Neurology78(1), 31–37 (2012).
- StarkeRM , KimGH , FernandezAet al.Impact of a protocol for acute antifibrinolytic therapy on aneurysm rebleeding after subarachnoid hemorrhage. Stroke39(9), 2617–2621 (2008).
- SuarezJI. Diagnosis and management of subarachnoid hemorrhage. Contin. Minneap. Minn.21(5), 1263–1287 (2015).
- HigginsJPT , AltmanDG , GotzschePCet al.The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Br. Med. J.343(2), d5928–d5928 (2011).
- WellsG , SheaB , O'ConnellDet al.The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses (2013). http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- ChandraB. Treatment of subarachnoid hemorrhage from ruptured intracranial aneurysm with tranexamic acid: a double-blind clinical trial. Ann. Neurol.3(6), 502–504 (1978).
- GelmersHJ. Prevention of recurrence of spontaneous subarachnoid haemorrhage by tranexamic acid. Acta Neurochir. (Wien)52(1–2), 45–50 (1980).
- HillmanJ , FridrikssonS , NilssonO , YuZ , SavelandH , JakobssonK-E. Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: a prospective randomized study. J. Neurosurg.97(4), 771–778 (2002).
- VermeulenM , LindsayKW , MurrayGDet al.Antifibrinolytic treatment in subarachnoid hemorrhage. N. Engl. J. Med.311(7), 432–437 (1984).
- van RossumJ , WintzenAR , EndtzLJ , SchoenJH , de JongeH. Effect of tranexamic acid on rebleeding after subarachnoid hemorrhage: a double-blind controlled clinical trial. Ann. Neurol.2(3), 238–242 (1977).
- Maurice-WilliamsRS. Prolonged antifibrinolysis: an effective non-surgical treatment for ruptured intracranial aneurysms?Br. Med. J.1(6118), 945–947 (1978).
- RoosY. Antifibrinolytic treatment in subarachnoid hemorrhage: a randomized placebo-controlled trial. STAR Study Group. Neurology54(1), 77–82 (2000).
- KasteM , RamsayM. Tranexamic acid in subarachnoid hemorrhage. A double-blind study. Stroke10(5), 519–522 (1979).
- TsementzisSA , HitchcockER , MeyerCH. Benefits and risks of antifibrinolytic therapy in the management of ruptured intracranial aneurysms. A double-blind placebo-controlled study. Acta Neurochir. (Wien)102(1–2), 1–10 (1990).
- PostR , GermansMR , TjerkstraMAet al.Ultra-early tranexamic acid after subarachnoid haemorrhage (ULTRA): a randomised controlled trial. Lancet397(10269), 112–118 (2021).
- FodstadH , ForssellA , LiliequistB , SchannongM. Antifibrinolysis with tranexamic acid in aneurysmal subarachnoid hemorrhage: a consecutive controlled clinical trial. Neurosurgery8(2), 158–165 (1981).
- PostR , GermansMR , BoogaartsHDet al.Short-term tranexamic acid treatment reduces in-hospital mortality in aneurysmal sub-arachnoid hemorrhage: a multicenter comparison study. PloS ONE14(2), e0211868 (2019).
- GaberelT , MagheruC , EmeryE , DerlonJ-M. Antifibrinolytic therapy in the management of aneurismal subarachnoid hemorrhage revisited. A meta-analysis. Acta Neurochir. (Wien)154(1), 1–9 (2012).
- HvasCL , HvasAM. Hemostasis and fibrinolysis following aneurysmal subarachnoid hemorrhage: a systematic review on additional knowledge from dynamic assays and potential treatment targets. Semin. Thromb. Hemost.48(03), 356–381 (2022).
- RoosYBWEM , RinkelGJE , VermeulenM , AlgraA , van GijnJ. Antifibrinolytic therapy for aneurysmal subarachnoid hemorrhage. Cochrane Database Syst. Rev. (2), CD001245 (2003).
- ConnollyES , RabinsteinAA , CarhuapomaJRet al.Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke43(6), 1711–1737 (2012).
- ZipfelGJ. Editorial: ultra-early surgery for aneurysmal subarachnoid hemorrhage. J. Neurosurg.122(2), 381–382 (2015).
- RoosYB , BeenenLF , GroenRJet al.Timing of surgery in patients with aneurysmal subarachnoid haemorrhage: rebleeding is still the major cause of poor outcome in neurosurgical units that aim at early surgery. J. Neurol. Neurosurg. Psychiatry63, 490–493 (1997).
- AllenGS , AhnHS , PreziosiTJet al.Cerebral arterial spasm--a controlled trial of nimodipine in patients with subarachnoid hemorrhage. N. Engl. J. Med.308(11), 619–624 (1983).
- RodríguezDR , MatamorosCS , CúeLF , HernándezJLM , SánchezYP , NellarJP. Factors associated with poor outcome for aneurysmal subarachnoid haemorrhage in a series of 334 patients. Neurol. Barc. Spain32(1), 15–21 (2017).
- AvdagicSS , BrkicH , AvdagicH , SmajicJ , HodzicS. Impact of comorbidity on early outcome of patients with subarachnoid hemorrhage caused by cerebral aneurysm rupture. Med. Arch.69(5), 280–283 (2015).
- SlettebøH , KaricT , SortebergA. Impact of smoking on course and outcome of aneurysmal subarachnoid hemorrhage. Acta Neurochir. (Wien)162(12), 3117–3128 (2020).
- DanièreF , GascouG , Menjotde Champfleur Net al.Complications and follow up of subarachnoid hemorrhages. Diagn. Interv. Imaging96(7–8), 677–686 (2015).
- AndersenCR , PresseauJ , SaigleV , EtminanN , VergouwenMDI , EnglishSW. Core outcomes for subarachnoid haemorrhage. Lancet Neurol.18(12), 1075–1076 (2019).