Abstract
A 62-year-old woman presented with a chronic fever and fatigue. Biological investigations showed leukocytosis and elevation of acute phase markers. Liver blood tests showed deterioration with both cholestatic and hepatocellular patterns (there were, respectively, elevations in serum alkaline phosphatase levels as well as in serum alanine and aspartate aminotransferases). Viral serologies were negative. Mycobacterial infection and endocarditis were excluded. Results from blood cultures were negative. Autoantibody tests including ANCA (anti-neutrophil cytoplasmic antibody), anti-nuclear, anti-smooth muscle and anti-mitochondria were all negative. A liver biopsy revealed epithelioid granulomatous necrotizing vasculitis. Subsequently, immunological testing was repeated revealing MPO-ANCA (myeloperoxidase-ANCA). A diagnosis of ANCA-associated vasculitis with liver involvement was then made. The patient was started on steroids and her clinical state gradually improved.
Plain language summary
A 62-year-old woman presented to the internal medicine department of our university hospital with chronic unresolved fever and an increase in liver function tests. Liver biopsy was performed. Microscopic examination revealed epithelioid granulomatous necrotizing vasculitis. Immunological testing revealed a perinuclear anti-neutrophil cytoplasmic antibody (ANCA) staining pattern with the presence of MPO-ANCA (Myeloperoxidase-ANCA). A diagnosis of ANCA-associated vasculitis with liver involvement was then made. The patient was started on steroids and her clinical state gradually improved.
Liver involvement in anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis is an extremely rare event.
Clinicians should consider ANCA-associated vasculitis as a potential etiology of liver dysfunction.
Negative results from ANCA tests do not exclude the diagnosis of ANCA-associated vasculitis.
Liver biopsy may play a crucial role in resolving diagnostic dilemma and revealing ANCA-associated vasculitis.
Keywords: :
Anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV) are necrotizing vasculitis predominantly affecting small vessels, with few or no immune deposits [Citation1]. The major clinicopathologic variants of AAV include microscopic polyangiitis, granulomatosis with polyangiitis (Wegener's), eosinophilic granulomatosis with polyangiitis (Churg-Strauss) and single-organ AAV [Citation1]. The different forms of AAV are differentiated by clinical characteristics, eosinophils and their corresponding anti-neutrophil cytoplasmic antibody (ANCA) patterns with specificity for proteinase-3 (PR3) or myeloperoxidase (MPO) [Citation2]. The causes of AAV are not well elucidated, and their underlying pathophysiological processes are still unclear [Citation2].
In AAV, kidneys and respiratory tract are commonly and severely affected. However, any organ may be affected in a vasculitic or granulomatous pattern of disease [Citation2]. Liver involvement in AAV is possible but remains uncommon and rarely described [Citation2,Citation3].
In the present paper, we report a unique and noteworthy case of hepatic involvement in AAV. We also conducted a literature review to contextualize our findings, aiming to underscore the importance of recognizing liver involvement in AAV.
Case presentation
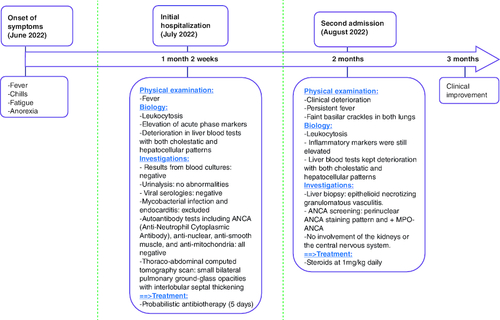
A 62-year-old woman presented to the internal medicine department of our university hospital with a 2-month history of fever, chills, fatigue and anorexia. Her past medical history was unremarkable. She did not smoke or consume alcohol.
Two weeks prior to the current admission, the patient had been referred to a local care clinic. During that hospitalization, the workup showed mild anemia (hemoglobin of 10.5 g/dl), with leukocytosis (white blood count (WBC) was 13450/mm3, with neutrophils: 11100/mm3, eosinophils: 400/mm3, lymphocytes: 1530/mm3, basophils: 0/mm and monocytes: 380/mm3). There was no thrombocytopenia. CRP (C-reactive protein) was 165 mg/l and the erythrocyte sedimentation rate was 106 mm/h. Liver function tests were as follows: Alanine aminotransferase (ALT) was 45 U/l, aspartate aminotransferase (AST) 57 U/l, alkaline phosphatase (ALP) 230 U/l and gamma-glutamyltransferase (GGT) 170 U/l. Urinalysis revealed no abnormalities. Results from blood cultures were negative. Thoraco-abdominal CT (computed tomography) scan revealed small bilateral pulmonary ground-glass opacities with interlobular septal thickening. Mycobacterial infection was excluded. A PCR (Polymerase Chain Reaction) test for SARS-CoV-2 was negative. Active viral infections caused by CMV (cytomegalovirus), EBV (Epstein-Barr virus), HBV (hepatitis B virus) and HCV (hepatitis C virus) were all excluded. Serologic tests for HIV (human immunodeficiency virus) and Brucella were negative. Autoantibody tests including ANCA, anti-nuclear, anti-smooth muscle and anti-mitochondria were all negative. Besides, transthoracic echocardiography ruled out endocarditis.
The patient was commenced on a probabilistic antibiotherapy (oral Imipinem and Amikacin). After a 5-day course of antibiotics, the patient's clinical condition gradually deteriorated. She became more tired and had night sweats in addition to persistent fever. She was therefore referred to our institution.
On admission, the patient's temperature was 39.9°C. Her appearance was distressed and tired but she was vitally stable. Pulmonary examination was notable for faint basilar crackles in both lungs. Otherwise, physical examination revealed no further abnormalities.
In comparison to initial laboratory data, WBC increased to 18,100/mm3. Inflammatory markers were still elevated. Liver blood tests kept deteriorating with both cholestatic and hepatocellular patterns: The total bilirubin level was 70 mg/dl, ALT 133.7 U/l, AST 153.2 U/l, ALP 378 U/l and GGT 376 U/l ().
Table 1. Evolution of blood analysis values.
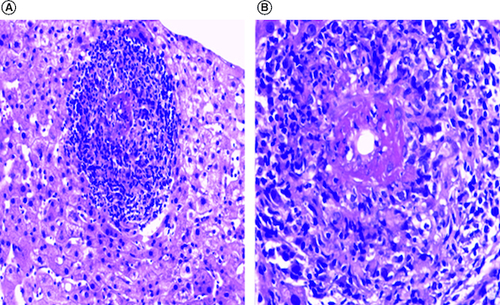
Owing to unresolved fever and sustained increase in liver function tests, a liver biopsy was then performed. Microscopic examination revealed epithelioid necrotizing granulomatous vasculitis within some portal tracts. Vasculitis lesions were surrounded by acute inflammation and scattered eosinophils. Steatosis and hemosiderosis were absent. Bile ducts were unremarkable. There was no lobular inflammation ().
Figure 1. Histological findings.
Microscopic features of liver biopsy demonstrated granulomatous necrotizing vasculitis (H&E staining X200 (A) and X400 (B)).
In that setting, immunological testing was repeated. By contrast to initial investigations, ANCA screening revealed a perinuclear ANCA staining pattern and demonstrated the presence of MPO-ANCA. These results were confirmed by indirect immunofluorescence assay and ELISA (enzyme-linked immunosorbent assay).
Further investigations showed no involvement of the kidneys or the central nervous system. A diagnosis of AAV with liver involvement was then made. The patient started on steroids at 1 mg/kg daily. Her clinical state gradually improved with apyrexia obtained on the second day. Complete normalization of inflammatory markers and liver function tests occurred respectively within 15 and 45 days.
Currently, the patient is doing well without any sign of relapse and she is monitored in the outpatient unit ().
Discussion
This case presents a challenging diagnosis of AAV. What was surprising and what made the case worth reporting was liver involvement in AAV in addition to initial negativity of ANCA. Besides, this case highlights the importance of liver biopsy in the diagnosis of AAV.
Our patient presented with chronic unresolved fever, unknown etiology of elevated inflammatory response and abnormal liver chemistries.
In routine practice, acute or progressing liver dysfunction is generally related to active viral hepatitis, drug-induced liver alteration or autoimmune hepatitis [Citation3,Citation4]. Active viral hepatitis was ruled out by negative serological screening. Drug-induced liver damage was highly unlikely as the liver blood tests deterioration was evident prior to antibiotics administration and persisted after discontinuation of the drug. Negativity of anti-nuclear, anti-smooth-muscle and anti-mitochondria antibodies along with the absence of characteristic microscopic features of auto-immune hepatitis stand against this diagnosis.
Because of low prevalence and nonspecific presentation, the diagnosis of vasculitis is often missed by clinicians early on in disease progression [Citation5]. According to the EULAR (European League Against Rheumatism), histopathological evidence of vasculitis in any organ remains the gold standard for the diagnosis of AAV [Citation6]. However, hepatic involvement in AAV is an exceedingly rare event [Citation7]. Goritsas et al. reported a case of severe anicteric febrile hepatitis coinciding with the diagnosis of granulomatosis with polyangiitis (GPA) which was revealed by lung biopsy. Liver biopsy, in that case, demonstrated mild nonspecific lobular hepatitis [Citation3]. Moreover, in a review including 109 patients with AAV, liver enzyme abnormalities were detected, during active disease, in 54 of cases and were normalized after initiating therapy. A liver biopsy was performed in eight patients. Eosinophilic infiltrates and granuloma-like lesions were observed only in one patient. The other biopsies showed no signs of liver vasculitis [Citation2].
Hepatic involvement in AAV has been associated with a fatal prognosis [Citation7]. However, in our case complete remission and good outcome were observed.
In clinical practice, the testing of ANCA plays an important role in the diagnostic workup of AAV [Citation8]. However, in our case screening for ANCA was initially negative which led to a diagnostic delay of AAV.
Similar to our case, Digby et al. described a case of GPA with negative repeated ANCA tests. Authors in that report emphasized that discrepancies in ANCA results might be related to high intermethod variation among ELISA assays, variation in antigen preparation, and a lack of international reference materials. They argued that the diagnosis of AAV must be clinical and not a laboratory one [Citation9]. In this regard, clinicians must be aware that negative results from ANCA tests do not exclude the diagnosis, especially when the clinical picture is sufficiently consistent with AAV.
Conclusion
Hepatic involvement in ANCA-associated vasculitis (AAV) is an extremely rare event. Physicians should consider AAV as a potential etiology of liver dysfunction. Besides they should be aware of the potential pitfalls of ANCA testing in the diagnosis of the disease. When the clinical picture is highly suggestive of AAV, negative ANCA screening should raise cautiousness and prompt clinicians to repeat immunological tests.
Open access
This work is licensed under the Creative Commons Attribution-NonCommercial 4.0 License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc/4.0/
Author contributions
Drafting the manuscript: B Lahbacha, R Mankai and A Bani. Patient management and data collection: W Garbouj, R Mankai and A Khsiba. Conception and design: A Chaabane, W Garbouj and R Amri. Analysis and interpretation of data: B Lahbacha, A Bani. Critical revision of the manuscript for important intellectual content: A Chaabane, A Khsiba, S Nechi and E Chelbi. Final approval of the manuscript, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: R Amri, S Nechi and E Chelbi.
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained verbal and written informed consent from the patient/patients for the inclusion of their medical and treatment history within this case report.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- Jennette JC, Falk RJ, Bacon PA et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthr. Rheum. 65(1), 1–11 (2013).
- Willeke P, Schlüter B, Limani A, Becker H, Schotte H. Liver involvement in ANCA-associated vasculitis. Clin. Rheumatol. 35(2), 387–394 (2016).
- Goritsas C, Paissios NP, Trigidou R, Delladetsima J. Hepatic involvement in Wegener's granulomatosis: a case report. J. Med. Case. Reports. 4(1), 9 (2010).
- Masiak A, Drobińska A, Zdrojewski Z. Hepatic involvement in granulomatosis with polyangiitis – diagnostic difficulties. Reumatologia 55(6), 318–322 (2017).
- Ahn E, Luk A, Chetty R, Butany J. Vasculitides of the gastrointestinal tract. Semin. Diagn. Pathol. 26(2), 77–88 (2009).
- Yates M, Watts RA, Bajema IM et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann. Rheum. Dis. 75(9), 1583–1594 (2016).
- Holl-Ulrich K, Klass M. Wegener's granulomatosis with granulomatous liver involvement. Clin. Exp. Rheumatol. 28(57 Suppl. 1), 88–89 (2010).
- Guchelaar NAD, Waling MM, Adhin AA, van Daele PLA, Schreurs MWJ, Rombach SM. The value of anti-neutrophil cytoplasmic antibodies (ANCA) testing for the diagnosis of ANCA-associated vasculitis, a systematic review and meta-analysis. Autoimmun. Rev. 20(1), 102716 (2021).
- Digby JW, Tinwell B, Sheldon J, Wang J. The missing antibody: the pitfalls of ANCA testing. Am. J. Med. 130(3), e93–96 (2017).