Abstract
Implementation of the Oncotype DX assay has led to a change in the manner in which chemotherapy is utilized in patients with early stage, estrogen receptor (ER)-positive, node-negative breast cancer; ensuring that patients at highest risk of recurrence are prescribed systemic treatment, while at the same time sparing low-risk patients potential adverse events from therapy unlikely to influence their survival. This test generates a recurrence score between 0 and 100, which correlates with probability of distant disease recurrence. Patients with low-risk recurrence scores (0–17) are unlikely to derive significant survival benefit with adjuvant chemotherapy and hormonal agents derived from using adjuvant hormonal therapy only. Conversely, adjuvant chemotherapy has been shown to significantly improve survival in patients with high-risk recurrence scores (≥31). Trials are ongoing to determine how best to manage patients with recurrence scores in the intermediate range. This review outlines the introduction and impact of Oncotype DX testing on practice; ongoing clinical trials investigating its utility; and challenging clinical scenarios where the absolute recurrence score may require careful interpretation. We also performed a bibliometric analysis of publications on the topics of breast cancer and Oncotype DX as a surrogate marker of acceptability and incorporation of the assay into the management of patients with breast cancer.
Background
In 2004, the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 study showed that the vast majority (78%) of tamoxifen-treated patients with node-negative estrogen receptor (ER)-expressing tumors will not develop disease relapse within 15 years of diagnosis.Citation1 The NSABP B-20 study of the same year showed that the addition of cyclophosphamide, methotrexate, and fluorouracil (CMF) to tamoxifen improved 5-year recurrence-free survival to 89%.Citation1 However, the significant absolute benefit must be balanced with the risk of overtreatment of the significant proportion of women in whom relapse would not have occurred even without the addition of CMF. Traditional prognostic indicators and prognostic scoring systems (e.g., Nottingham Prognostic Index) that have been used to guide decision making with respect to application of adjuvant therapy in breast cancer include patient factors, such as age, and tumor factors, such as grade and stage.Citation2 Such tumor factors, particularly size, may reflect a time delay in presentation, as opposed to the biological activity of the disease.Citation3 It may be that the tendency of a tumor to metastasize is not simply related to chronology and increasing size, but may instead be derived from inherent biological factors,Citation3 and indeed tumor size and nodal status are considered independently as risk factors for recurrence.Citation4 It has been known for many years that gene expression can be used to more accurately provide prognostic information; and hierarchical clustering of gene expression patterns can discriminate between tumors of distinct molecular subtypes,Citation5 each of which is associated with different patterns of metastasis and response to treatment, independent of stage or grade.Citation6 Practically speaking, receptor status is used as a surrogate marker of tumor molecular subtype.Citation7 ER-negative tumors, in which human epidermal growth factor receptor 2 (HER2) is amplified, have special predilection for early metastasis, whereas ER-positive and HER2-negative tumors have a lesser tendency to metastasize, even at greater tumor size and higher burdens of nodal disease.Citation4 It has been known for many years that the low-grade ER-positive group of tumors also responds less often to neoadjuvant chemotherapy than HER2-overexpressing or triple-negative subtypes, again reflecting inherent differences in biology.Citation8–Citation10 Traditional prognostic indicators may, therefore, lack sufficient accuracy in identifying those patients who would benefit most from systemic treatment, and similarly may inappropriately assign patients to adjuvant therapy that would be cured by surgery alone.
Development of the Oncotype DX assay
The Oncotype DX genomic assay (Genomic Health, Inc., Redwood City, CA, USA) is a clinically validated assay that can be used to predict likelihood of recurrence of early stage breast cancer, and can be used therefore, in decision making with respect to systemic therapy. The Oncotype DX-automated algorithm assigns a score to the expression of 16 cancer-related genes multiplied by a factor specific to the group to which each individual gene is allocated, relative to the mean expression of five reference genes to generate an overall recurrence score (RS; ).Citation11,Citation12 The Oncotype DX assay is optimized for formalin-fixed paraffin-embedded tumor samples, and all testing is performed centrally in the clinical reference laboratory of Genomic Health, Inc.
Table 1 Genes included in Oncotype DX assay
The Oncotype DX assay was validated independently in two studies.Citation13,Citation14 The prospective NSABP-B14 study confirmed the RS as a continuous predictor of recurrence, independent of patient age and tumor size.Citation13 The NSABP B-20 study provided evidence that the RS can also be used to predict those patients with node-negative, ER-positive cancer in whom adjuvant chemotherapy will confer greatest disease-free and overall survival advantage compared to endocrine therapy alone.Citation15
These early validation studies categorized RS as low-(0–17), intermediate- (18–30), or high-risk (≥31). Paik et al determined Kaplan–Meier estimates of the rate of distant recurrence at 10 years as 6.8%, 14.3%, and 30.5% for patients with low-, intermediate-, and high-risk scores, respectively, and found that the RS was also shown to predict disease recurrence at 10 years when treated as a continuous variable,Citation13 more accurately than the adjuvant! online tool.Citation16 Habel et al also showed RS to be an independent predictor of breast cancer-related mortality in tamoxifen-treated patients with ER-positive, node-negative disease, with 10-year risk of death 2.8%, 10.7%, and 15.5% in low-, intermediate-, and high-risk patients, respectively.Citation14 Other genomic assays exist that purport to have higher predictive value for later onset disease recurrenceCitation17–Citation19 but their clinical utility in deciding in whom adjuvant chemotherapy will be beneficial above the use of extended endocrine therapy has not been fully elucidated. The beneficial impact of adjuvant systemic chemotherapy is in prevention of early disease recurrence.Citation20,Citation21
Clinical implementation
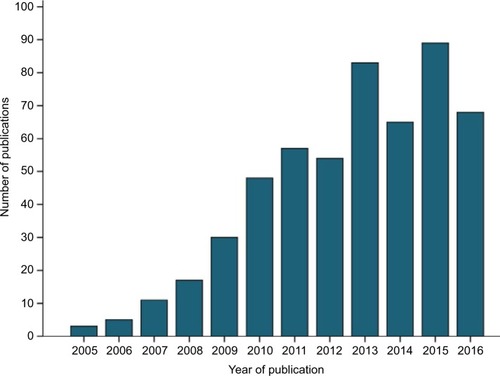
The increasing incorporation of Oncotype DX into clinical trials or routine clinical treatment of breast cancer can be illustrated by analysis of the number of publications based on these topics. Interrogation of the Web of Science Core Collection (31/10/2016) for publications based on topics “Oncotype DX” and “Breast cancer” using Boolean operators OR and AND and search terms “oncotype dx OR Oncotype DX OR oncotypedx OR oncotypeDX” AND “breast cancer OR breast carcinoma OR breast malignancy” generated 530 records (). The first manuscripts based on these topics were published in 2005, with dramatic increase in publications generated in the years following introduction of Oncotype DX to the market, and with incorporation of the assay into the clinical recommendations of American Society of Clinical Oncology in 2007 and St Gallen international expert consensus conference in 2009, and with increasing numbers in the years of publication of results of key trials such as NSABP B-14 and B-20 in 2010, transATAC in 2013, and TAILORx in 2015. The gradual increase in publications per year may also reflect the lag time in routine access to Oncotype DX in countries in which publicly funded health systems operate, for example, Ireland (2011), England, and Wales (2013).
The use of Oncotype DX RS in determining application of adjuvant chemotherapy has been incorporated into numerous national and international clinical recommendations and guidelines, including those of National Institute of Clinical Excellence,Citation22 National Comprehensive Cancer Network (NCCN),Citation23 and European Society for Medical OncologyCitation24 and has been successfully implemented in a number of countries, albeit with some regional variation in application, patient selection, and reliance of RS in decision making alongside traditional prognostic indicators.Citation25–Citation27
Incorporation of the Oncotype DX assay into routine clinical care has had a significant impact in decision making with respect to chemotherapy prescribing; with certain authors reporting a direct change in management plan in response to the RS in 31% to 44% patients, the change in most cases being from a decision to prescribe both chemotherapy and endocrine therapy to an endocrine-only regime.Citation28–Citation32 We have also identified a shift in prescribing patterns among Irish medical oncologists in the years since the development of Oncotype DX, even in intervals during which the assay could not be utilized,Citation26 reflecting a change in the paradigm of management of ER-positive lower-risk tumors.
Observations that the gene expression profile of ductal carcinoma in situ (DCIS) associated with invasive disease mirrored that of the invasive componentCitation33 prompted investigation into the use of the assay in DCIS as a predictor of invasion and recurrence. The Oncotype DX DCIS assay was developed by modifying the 21-gene assay, eliminating analysis of expression of genes related to proliferation.Citation34 Description of the use of this assay in DCIS is outside the scope of this article.
TAILORx: Redefining risk categories
While it is evident from these robust studies that patients with the lowest-risk RS clearly do not derive any additional disease-free or overall survival benefit from the addition of adjuvant chemotherapy to hormonal agents, and conversely, those individuals with highest scores derive substantial benefit from systemic treatment; it is unclear at what absolute score patients in the intermediate-risk category start to derive benefit. It is hoped that results of the TAILORx (Trial Assigning IndividuaLized Options for treatment [Rx]) will help to define the role of chemotherapy in patients with intermediate RS. However, to try to minimize the risk of undertreatment, the authors of this trial redefined the risk categories, with a reduced thresholds for low- (RS <11), intermediate- (11–25), and high-risk (>25) categories.Citation35 As part of this study, patients with scores ≤10 received hormonal agents only, patients with scores >25 received both adjuvant chemotherapy and hormonal therapy, whereas patients in the newly defined intermediate-risk category were randomized to receive either combination chemo- and hormonal therapy or hormonal monotherapy. While the majority of patients in the NSABP-B14 study had scores categorized as low-risk (51%), and lesser proportions as intermediate-risk (22%) and high-risk (27%),Citation13 using these new definitions, the vast majority of individuals in the TAILORx study (6907 [67%]) had scores now considered intermediate-risk, with only 16.9% and 15.9% of patients being considered at high- and low-risk, respectively.Citation20
Unsurprisingly, redefinition of the risk categories by TAI-LORx has created controversy in the management of patients with scores between 11 and 17. Wen et alCitation36 analyzed the outcome of a large cohort patients with RS <18, specifically analyzing the cohorts with RS 0–10 and 11–17 separately. In keeping with early results of TAILORx,Citation20 very low rates of disease recurrence at 5 years was recorded in patients with RS <11 (0.2%). In this study, only 4% of patients with RS 0–10 received chemotherapy, compared to 17% of patients with RS 11–17. The rate of distant relapse in patients with RS 0–10 was 0.2%, compared to 0.6% in patients with RS 11–17. Of patients who relapsed (n=6), only one had an RS <11. Only one patient with recurrent disease had received adjuvant chemotherapy, and had an RS of 12. The incidence of disease recurrence in that study was noted to be significantly higher in patients <40 years, suggesting that traditional prognostic factors should be considered in patient with intermediate RS.
The interpretation of the data from the intermediate-risk group in TAILORx is awaited, but will hopefully go some way to addressing the lack of clarity that exists surrounding the benefit of chemotherapy in this group. Currently, management of the patient with an intermediate RS poses a significant clinical challenge. In the absence of evidence-based practice regarding incorporation of Oncotype DX RS, we have observed that oncologists revert back to traditional prognostic indicators in decision making for such patients.Citation26 The absolute RS is, however, predictive of chemotherapy use when treated as a continuous variable, even in patients in the intermediate-risk group.Citation26,Citation37 At present, the clinical utility of this assay is not optimized, as most patients fall into this group, and only a small proportion of patients are ultimately spared chemotherapy on the basis of this result. This not only has clinical drawbacks, but also negates the cost-saving if the test does not alter management.
Utility of Oncotype DX in patients with positive nodal disease
Most data to date support the use of Oncotype DX profiling of tumors where nodal metastases have not occurred. Standard of care of patients with nodal metastases from ER-positive breast cancer is to offer adjuvant chemotherapy alongside hormonal modulators.Citation23,Citation24 However, compared to ER-negative, node-positive disease, where the survival benefit conferred by chemotherapy is unequivocal, several studies have suggested that patients with ER-positive, node-positive disease do not derive as great a survival benefit from systemic treatment.Citation38 The Southwest Oncology Group (SWOG)-8814 trial provided evidence that chemotherapy in advance of tamoxifen increased disease-free and overall survival in patients with postmenopausal, node-positive, ER-positive disease,Citation39 but identified a subset of patients within this cohort who did not benefit from systemic treatment.Citation40 The tumors of patients enrolled in this study, where available, were retrospectively assessed using the Oncotype DX assay, and RS were analyzed in the context of patient outcome. These data suggested that the Oncotype DX RS may be used to discriminate between patients with node-positive, ER-positive disease who will benefit from chemotherapy from those who will not, and the RS risk categories could stratify those patients at greatest risk of disease relapse and mortality at 10 years.Citation40 The TransATACCitation41 and ECOG2197Citation42 studies have provided robust evidence that the Oncotype DX RS significantly correlates with recurrence and with time to distant recurrence in both N0 and N+ subgroups. The NSABP-B28 study further showed that RS was an independent predictor of disease-free and overall survival, as well as of recurrence-free interval in postmenopausal women with ER-positive disease managed with chemo- and hormonal therapy.Citation43 The Phase III SWOG S1007/RxPONDER trial (NCT01272037) is still ongoing and aims to investigate the role of chemotherapy in patients with 1–3 positive lymph nodes and RS≤25.Citation44 Based on the analysis of data from the SWOG-8814 study, the NCCN guidelines have already been adjusted to include consideration of the assay in patients with 1–3 positive lymph nodes to guide treatment decisions.Citation23
Cost-effectiveness
Despite the high direct cost of the assay and heterogeneity of assay and health care costs between and within countries, many studies from different regions have shown the use of Oncotype DX in patients with node-negative disease to be cost-effective.Citation45–Citation47 There is also significant evidence to suggest that it might be cost-effective in patients with node-positive disease.Citation31,Citation48,Citation49 However, the cost-saving utility of the assay is derived from patients spared chemotherapy. Currently, although many similar assays exist, the Oncotype DX assay is the only such tool that is validated for use in predicting response to chemotherapy; such monopoly as well as patent protection means that the cost of the assay is unlikely to be reduced.Citation48 Furthermore, if the new risk category definitions as outlined in TAILORx are adopted, fewer patients will fall into the low-risk category. It is possible, therefore, that fewer patients will be spared chemotherapy than initial studies predicted, meaning that projections of costs saved might be overestimated.
Challenging clinical scenarios
In the case of synchronous bilateral breast cancer, the patient is at risk of relapse of either of the tumors. Where there is discordance in the molecular subtypes of the tumors, the use of Oncotype DX may not be appropriate. Where both tumors are ER-positive and HER2-negative; the utility of the Oncotype DX assay depends on the RS of each tumor. Some authors have found high rates of concurrence in RSs between bilateral tumors, particularly in older patients and in cases of tumors with high levels of progesterone receptor (PgR) expression.Citation50
Breast tumors in patients with germline mutations in BRCA1 and BRCA2 demonstrate different biological characteristics than patients with wild-type genotypes, with distinct somatic mutational signatures reflective of deficient homology-directed repair mechanisms.Citation51,Citation52 Deficient repair mechanisms may explain why tumors in BRCA1/2 gene mutation carriers may be more susceptible to the impact of chemotherapy; and application of chemotherapy in this cohort, even in early stage cancer, has been found to confer significant survival advantage.Citation53,Citation54 The majority of breast cancers in patients with BRCA1 gene mutations are triple negative, typically basal subtype. The spectrum of breast cancer phenotypes in BRCA2 gene mutation carriers is much more heterogeneous.Citation55,Citation56 Shah et al retrospectively investigated the utility of Oncotype DX in patients with BRCA1/BRCA2 gene mutations and ER-positive breast cancer.Citation57 Mutation carriers were more likely than age-matched controls to have RS in the intermediate- or high-risk range and overall had higher median RS. This trend has been observed by other authors.Citation58 Significantly, cases were also noted to have tumors of higher grade and lower PgR expression scores. Overall, mutation carriers were more likely, therefore, to receive chemotherapy, but proportions receiving chemotherapy within the risk categories were equivalent between carriers and noncarriers. Interestingly, within this study cohort, ER-positive cancers in BRCA1 mutation carriers tended to be of low risk more often than those in BRCA2 mutation carriers. Loss of heterozygosity analyses were not performed on all tumors. Furthermore, this subtype of breast cancer is not usually associated with germline BRCA1 mutations. The possibility that these tumors represent incidental sporadically occurring cancers cannot be dismissed; and in these patients, application of the Oncotype DX assay may be entirely appropriate, as the supposed favorable impact of chemotherapy conferred by BRCA deficiency may not be present if the tumor happened by chance. Further validation of this assay in this particularly unique subgroup of patients is required before implementation can be supported. Furthermore, its use in patients with germline mutations in other cancer predisposition genes (e.g., TP53 and STK11) has not yet been investigated.
Patient and clinician attitudes
Qualitative studies have revealed numerous factors influencing uptake of genomic profiling of tumors among oncologists. Most factors pertain to organizational/logistic and inter- and intrapersonal factors.Citation59 Furthermore, uncertainty regarding management of the intermediate-risk cohort represents a significant barrier to uptake.Citation60 Other factors that have been identified include concern with respect to patient understanding of the result and implications for treatment.Citation61 There is a wide variability in reported rates of patient misunderstanding about the risk of recurrence estimation between different centers (11%–83%).Citation62 Generally, attitudes of clinicians and patients are positive, and encourage incorporation of this technology into routine use. However, certain subsets of patients, particularly patients with high-Citation63 or intermediate-riskCitation64 RS, experience significant levels of distress and anxiety that cannot be discounted.
Conclusion
Oncotype DX has led to a shift in the paradigm of treatment of early-stage ER-positive breast cancer, with reduction in overall chemotherapy prescription in low-risk individuals. This shift is reflective of the move toward precision medicine in all realms of oncology in recent times. The application of this assay will spare a large proportion of patients with unnecessary over-treatment, while assuring that those patients with unfavorable biology will receive the whole gamut of available therapies to try to provide most survival benefit as possible. A number of challenges in its application still exist, for example, in patients with tumors that display biological intra-tumor heterogeneity, in patients with germline genetic mutations, or in patients with unfavorable traditional prognostic indicators and apparently conflicting RS categorization; and further longer term follow up is required for these particular patients. We await the results of the TAILORx and RxPONDER trials, which will help inform practice for intermediate-risk patients and patients with low nodal disease burden.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
Dr McVeigh was supported by the Health Research Board/Health Service Executive National Specialist Registrar Academic Fellowship Programme (Grant number NSAFP/14/1), and by Breast Cancer Research (chy9997).
References
- FisherBJeongJHBryantJNational Surgical Adjuvant Breast and Bowel Project randomised clinical trialsTreatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trialsLancet2004364943785886815351193
- GaleaMHBlameyRWElstonCEEllisIOThe Nottingham Prognostic Index in primary breast cancerBreast Cancer Res Treat19922232072191391987
- OakmanCBessiSZafaranaEGalardiFBiganzoliLDi LeoARecent advances in systemic therapy: new diagnostics and biological predictors of outcome in early breast cancerBreast Cancer Res200911220519435470
- FoulkesWDSize surprise? Tumour size, nodal status, and outcome after breast cancerCurr Oncol201219524124323144568
- SorlieTPerouCMTibshiraniRGene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implicationsProc Natl Acad Sci U S A20019819108691087411553815
- BlowsFMDriverKESchmidtMKSubtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studiesPLoS Med201075e100027920520800
- GoldhirschAWoodWCCoatesASGelberRDThürlimannBSennHJPanel membersStrategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011Ann Oncol20112281736174721709140
- McVeighTPAl-AzawiDKearneyDEAssessing the impact of neoadjuvant chemotherapy on the management of the breast and axilla in breast cancerClin Breast Cancer2014141202524157259
- BougheyJCMcCallLMBallmanKVTumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical TrialAnn Surg20142604608614 discussion 614–61625203877
- LippmanMEAllegraJCThompsonEBThe relation between estrogen receptors and response rate to cytotoxic chemotherapy in metastatic breast cancerN Engl J Med19782982212231228651963
- CroninMSangliCLiuMLAnalytical validation of the Oncotype DX genomic diagnostic test for recurrence prognosis and therapeutic response prediction in node-negative, estrogen receptor-positive breast cancerClin Chem20075361084109117463177
- SparanoJAPaikSDevelopment of the 21-gene assay and its application in clinical practice and clinical trialsJ Clin Oncol200826572172818258979
- PaikSShakSTangGA multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancerN Engl J Med2004351272817282615591335
- HabelLAShakSJacobsMKA population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patientsBreast Cancer Res200683R2516737553
- PaikSTangGShakSGene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancerJ Clin Oncol200624233726373416720680
- TangGShakSPaikSComparison of the prognostic and predictive utilities of the 21-gene Recurrence Score assay and adjuvant! for women with node-negative, ER-positive breast cancer: results from NSABP B-14 and NSABP B-20Breast Cancer Res Treat2011127113314221221771
- SgroiDCSestakICuzickJPrediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study populationLancet Oncol201314111067107624035531
- SestakICuzickJDowsettMPrediction of late distant recurrence after 5 years of endocrine treatment: a combined analysis of patients from the Austrian breast and colorectal cancer study group 8 and arimidex, tamoxifen alone or in combination randomized trials using the PAM50 risk of recurrence scoreJ Clin Oncol201533891692225332252
- SestakIDowsettMZabagloLFactors predicting late recurrence for estrogen receptor-positive breast cancerJ Natl Cancer Inst2013105191504151124029245
- SparanoJAGrayRJMakowerDFProspective validation of a 21-gene expression assay in breast cancerN Engl J Med2015373212005201426412349
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG)PetoRDaviesCGodwinJComparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trialsLancet2012379981443244422152853
- NICEGene expression profiling and expanded immunohistochemistry tests for guiding adjuvant chemotherapy decisions in early breast cancer management: MammaPrint, Oncotype DX, IHC4 and Mammostrat NICE Diagnostics guidance [DG10]2013 Available from: https://www.nice.org.uk/guidance/dg10Accessed March 1, 2017
- NCCN.orgNational Comprehensive Cancer Network (NCCN) Clinical Practic Guidelines in Oncology -Breast Cancer (Version 2.2016)2016 Available from: http://www.nccn.org/professionals/physicians_gls/pdf/breast.pdfAccessed March 1, 2017
- SenkusEKyriakidesSOhnoSESMO Guidelines CommitteePrimary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-upAnn Oncol201526Suppl 5v8v3026314782
- DinanMAMiXReedSDHirschBRLymanGHCurtisLHInitial Trends in the use of the 21-gene recurrence score assay for patients with breast cancer in the medicare population, 2005–2009JAMA Oncol20151215816626181015
- McVeighTPHughesLMMillerNThe impact of Oncotype DX testing on breast cancer management and chemotherapy prescribing patterns in a tertiary referral centreEur J Cancer201450162763277025240289
- JasemJAminiARabinovitchR21-Gene recurrence score assay as a predictor of adjuvant chemotherapy administration for early-stage breast cancer: an analysis of use, therapeutic implications, and disparity profileJ Clin Oncol201634171995200227001563
- HenryLRStojadinovicASwainSMPrindivilleSCordesRSoballePWThe influence of a gene expression profile on breast cancer decisionsJ Surg Oncol200999631932319204954
- LoSSMumbyPBNortonJProspective multicenter study of the impact of the 21-gene recurrence score assay on medical oncologist and patient adjuvant breast cancer treatment selectionJ Clin Oncol201028101671167620065191
- AsadJJacobsonAFEstabrookADoes oncotype DX recurrence score affect the management of patients with early-stage breast cancer?Am J Surg2008196452752918809056
- LoncasterJArmstrongAHowellSImpact of oncotype DX breast recurrence score testing on adjuvant chemotherapy use in early breast cancer: real world experience in greater Manchester, UKEur J Surg Oncol Epub201719
- RutterCEYaoXManciniBRInfluence of a 21-gene recurrence score assay on chemotherapy delivery in breast cancerClin Breast Cancer2016161596226483315
- BaehnerFSangliCMillwardCCherbavazDGoddardAShakSQuantitative gene expression analysis using Oncotype DX in ductal carcinoma in situ that is adjacent to invasive ductal carcinomaCancer Res200969Suppl 22066
- SolinLJGrayRBaehnerFLA multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breastJ Natl Cancer Inst20131051070171023641039
- SparanoJATAILORx: trial Assigning Individualized Options for Treatment (Rx)Clin Breast Cancer20067434735017092406
- WenHYKrystel-WhittemoreM2PatilSBreast carcinoma with an Oncotype Dx recurrence score <18: rate of distant metastases in a large series with clinical follow-upCancer2017123113113727526056
- AlbanellJSvedmanC2GligorovJPooled analysis of prospective European studies assessing the impact of using the 21-gene recurrence score assay on clinical decision making in women with oestrogen receptor-positive, human epidermal growth factor receptor 2-negative early-stage breast cancerEur J Cancer20166610411327544930
- BerryDACirrincioneCHendersonICEstrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancerJAMA2006295141658166716609087
- AlbainKSBarlowWERavdinPMBreast Cancer Intergroup of North AmericaAdjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: a phase 3, open-label, randomised controlled trialLancet200937497072055206320004966
- AlbainKSBarlowWEShakSBreast Cancer Intergroup of North AmericaPrognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trialLancet Oncol2010111556520005174
- DowsettMCuzickJWaleCPrediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC studyJ Clin Oncol201028111829183420212256
- GoldsteinLJGrayRBadveSPrognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic featuresJ Clin Oncol200826254063407118678838
- MamounasETangGPaikSPrognostic impact of the 21-gene recurrence score (RS) on disease-free and overall survival of node-positive, ER-positive breast cancer patients (pts) treated with adjuvant chemotherapy: results from NSABP B-28J Clin Oncol201230Suppl 27 Abstract 1
- SunZPratACheangMCGelberRDPerouCMChemotherapy benefit for ‘ER-positive’ breast cancer and contamination of nonluminal subtypes-Waiting for TAILORx and RxPONDERAnn Oncol2015261707425355719
- Bargallo-RochaJELara-MedinaFPérez-SánchezVCost-effectiveness of the 21-gene breast cancer assay in MexicoAdv Ther201532323925325740550
- SmythLWatsonG2WalshEMEconomic impact of 21-gene recurrence score testing on early-stage breast cancer in IrelandBreast Cancer Res Treat2015153357358226364296
- KipMMontebanHSteutenLLong-term cost-effectiveness of Oncotype DX® versus current clinical practice from a Dutch cost perspectiveJ Comp Eff Res20154543344525872415
- HallPSMcCabeCSteinRCCameronDEconomic evaluation of genomic test-directed chemotherapy for early-stage lymph node-positive breast cancerJ Natl Cancer Inst20121041566622138097
- BrufskyAMPredictive and prognostic value of the 21-gene recurrence score in hormone receptor-positive, node-positive breast cancerAm J Clin Oncol201437440441024853663
- KarstenMStempelMRadosaJPatilSKingTAOncotype DX in bilateral synchronous primary invasive breast cancerAnn Surg Oncol201623247147626340863
- AlexandrovLBNik-ZainalSWedgeDCSignatures of mutational processes in human cancerNature2013500746341542123945592
- Nik-ZainalSDaviesH1StaafJLandscape of somatic mutations in 560 breast cancer whole-genome sequencesNature20165347605475427135926
- ArunBBayraktarSLiuDDResponse to neoadjuvant systemic therapy for breast cancer in BRCA mutation carriers and noncarriers: a single-institution experienceJ Clin Oncol201129283739374621900106
- NarodSAMetcalfeKLynchHTShould all BRCA1 mutation carriers with stage I breast cancer receive chemotherapy?Breast Cancer Res Treat2013138127327923381743
- FoulkesWDStefanssonIMChappuisPOGermline BRCA1 mutations and a basal epithelial phenotype in breast cancerJ Natl Cancer Inst200395191482148514519755
- LakhaniSRVan De VijverMJJacquemierJThe pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2J Clin Oncol20022092310231811981002
- ShahPDPatilSDicklerMNOffitKHudisCARobsonMETwenty-one-gene recurrence score assay in BRCA-associated versus sporadic breast cancers: differences based on germline mutation statusCancer201612281178118426859126
- LewinRSulkesAShochatTOncotype-DX recurrence score distribution in breast cancer patients with BRCA1/2 mutationsBreast Cancer Res Treat2016157351151627225387
- WeldonCBTrosmanJRGradisharWJBensonAB3rdSchinkJCBarriers to the use of personalized medicine in breast cancerJ Oncol Pract201284e24e3123180995
- RobertsMCBrysonAWeinbergerMOncologists’ barriers and facilitators for Oncotype Dx use: qualitative studyInt J Technol Assess Health Care201632535536127958190
- BombardYRozmovitsLTrudeauMLeighlNBDealKMarshallDAThe value of personalizing medicine: medical oncologists’ views on gene expression profiling in breast cancer treatmentOncologist201520435135625746345
- LeggettLELorenzettiDLNoseworthyTTiwanaSMackeanGClementFExperiences and attitudes toward risk of recurrence testing in women with breast cancer: a systematic reviewBreast Cancer Res Treat2014144345746524596049
- TzengJPMayerDRichmanARWomen’s experiences with genomic testing for breast cancer recurrence riskCancer201011681992200020213682
- O’NeillSCBrewerNTLillieSEWomen’s interest in gene expression analysis for breast cancer recurrence riskJ Clin Oncol200725294628463417925559