Abstract
Background
The aim of present pilot study was to evaluate the changes in pulmonary function tests (PFTs) after locoregional post-mastectomy radiotherapy (PMRT) in breast cancer patients.
Materials and methods
Twenty consecutive patients with histopathologically confirmed breast carcinoma stages T1–T4, N1–N2, who were treated with modified radical mastectomy with neoadjuvant or adjuvant chemotherapy underwent PFTs, including forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), forced expiratory flow at 50%, and peak expiratory flow rate, maximum mid expiratory flow (MMEF25–75), maximal oxygen consumption (VO2max), and carbon monoxide diffusing capacity (DLCO) before, at 30 days, and at 90 days after locoregional PMRT. A two-tailed paired Student’s t-test was used to compare mean values among the variables between the groups
Results
A significant drop in FVC, FEV1, and DLCO was noticed at day 90 after the completion of locoregional PMRT with P-values 0.033, 0.042, and 0.031, respectively, while MMEF25–75 and VO2max were not significantly affected (P-values 0.075 and 0.062, respectively) favoring a restrictive lung injury pattern. However, no patient was found to be symptomatic.
Conclusion
A significant drop in reduction in PFTs occurred at day 90 after the completion of locoregional PMRT. PFTs shall be performed in all breast cancer patients receiving locoregional PMRT for early detection of radiation-induced lung toxicity as all patients in our cohort were found asymptomatic.
Introduction
During the post-mastectomy radiotherapy (PMRT) with tangential fields, usually a small portion of the underlying lung is included within the radiation portals, which may result in potential risk of radiation-induced lung toxicity (RILT).Citation1 RILT is typically manifested clinically either as acute radiation-induced pneumonitis (RIP), or late radiation-induced fibrosis (RIF).Citation2,Citation3 RIP usually occurs 4–12 weeks after the completion of PMRT and in most of the patients it is often indolent, although in few it can present as cough and shortness of breath. In most of cases, RIP is regressed spontaneously or with use of steroids; however, it can also evolve into RIF as consequential late toxicity.Citation4,Citation5 RIP and RIF are mainly related to 1) the volume of irradiated lung parenchyma, 2) mean lung dose, 3) fractionation schedule, and 4) concurrent chemotherapy.Citation6,Citation7 However, pulmonary functional reserve is not only limited by previously mentioned factors, but also affected by 1) involvement or not of supraclavicular (SC) region,Citation8 2) radiotherapy techniques,Citation9 3) smoking habits,Citation10 and 4) the use of concurrent tamoxifen.Citation11 Although RILT may not increase the risk of death, it has a negative impact on quality of life, and breast carcinoma patients with compromised pulmonary functional reserve may have relatively inferior long-term treatment outcomes.Citation3,Citation8 There is limited data available regarding the changes in pulmonary function tests (PFTs) in breast carcinoma patients receiving PMRT. Few studies have documented a significant decrement in PFTs in breast carcinoma patients who were treated with PMRT including SC region and were given adjuvant chemotherapy.Citation3,Citation9,Citation12
In the present study, we aimed to evaluate the acute changes in PFTs in breast carcinoma patients who received adjuvant chemotherapy and were treated with PMRT including SC regions.
Materials and methods
Eligibility
The study was approved by the Ethics Committee of King Saud University. All participants provided written informed consent to participate in this study. There were, 20 consecutive eligible women presenting with breast carcinoma enrolled in this study, and were treated with PMRT (chest wall, SC region ± axilla) after modified radical mastectomy (MRM). Inclusion criteria were 1) patients with histopathological confirmed breast carcinoma, 2) patients with T1–T4, N1–N2, and 3) patients who underwent MRM with neoadjuvant or adjuvant chemotherapy.
Exclusion criteria were 1) patients with N0 status and were not candidates for SC region PMRT, 2) patients with N3 status or in whom radiotherapy to internal mammary lymph node was given, 3) patients who did not receive neoadjuvant/adjuvant chemotherapy, 4) presence of distant metastasis, 5) history of bronchial asthma and chronic obstructive pulmonary disease, 6) history of smoking, and 7) any contraindications for spirometry (unstable angina, history of myocardial infarction, or comprised cardiac functions, aorta aneurysm, cerebral aneurysms, and syncope associated with forced exhalation).
Radiotherapy techniques
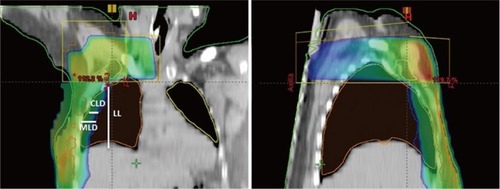
All patients underwent computed tomography (CT) simulation in supine position on breast board with head turned to contralateral side of region of interest (ROI), with both arms placed above the head. CT data were obtained with a high-speed 16-slice helical scanner at 5 mm slices through the ROI. A single isocenter was marked at the level of match line between the SC and breast below the medial end of clavicle. After the acquisition of CT data, clinical target volume (CTV) including chest wall, SC region, and level III axillary lymph node region was delineated. Organs at risk (OAR) including spinal cord, heart, and both lungs were also delineated on each CT slice. For all patients, three-dimensional conformal radiotherapy plans were created using Eclipse™ (Varian Medical Systems, Inc., Palo Alto, CA, USA) version 10.0. Opposed tangential fields were designed to encompass the contoured CTV chest wall. Superior border of the CTV chest wall field was defined by the inferior extent of the SC area, which corresponded to the single isocenter. The inferior edge was placed at 2 cm below the level of infra-mammary fold of contralateral breast. The angle of tangential fields was opted to avoid too much lung volume in tangential fields. Dynamic wedges were used to maintain homogeneity within ±10%. A single antero-posterior field was matched to the superior border of the CTV chest wall tangential fields to encompass the SC region (). A gantry angle of 5°–15° was applied to minimize the spinal cord dose. Wedges were used to create homogeneity in each plan. A dose of 50 Gy in 25 fractions (2 Gy/fraction/day) was prescribed to the chest wall, SC, and level III axillary nodes. A dose-volume histogram (DVH) was created to check the CTV dose coverage and the dose to each OAR. Treatment planning directed at good coverage of the CTV chest wall and SC and respected the International Commission on Reporting Units algorithms.
Figure 1 Treatment plans showing beam design and dose distributions for supraclavicular and tangential fields.
Abbreviations: LL, left lung; CLD, central lung distance; MLD, mean lung distance.
Pulmonary function tests
The study employed the ndd EasyOne Pro® Spirometry Lab system. This system was used to acquire baseline PFTs before the commencement of radiotherapy, consisting of forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), maximum mid expiratory flow (MMEF25–75), maximal oxygen consumption (VO2max), and carbon monoxide diffusing capacity (DLCO). These measurements were repeated after 30 and 90 days after completion of radiotherapy. FVC was considered as surrogate for lung volume, while FEV1 was considered a surrogate for the narrowing of large or medium-sized bronchi. MMEF25–75 was regarded as a surrogate for the narrowing of bronchioles; VO2max was defined as the oxygen uptake attained during maximal exercise intensity that could not be increased despite further increases in exercise workload, and DLCO was used as surrogate for the diffusing capacity through the alveolar-capillary barrier. Additionally, FEV1% pred (FEV1/FVC), forced expiratory flow at 50% when 50% of the FVC has been exhaled, and peak expiratory flow rate (PEFR) to assess expiratory muscle strength and large airway patency were noted down. Theoretically, FVC, FEV1, and DLCO are reduced in RIP, and all of aforementioned parameters are reduced in RIP and lung fibrosis.
DVH data
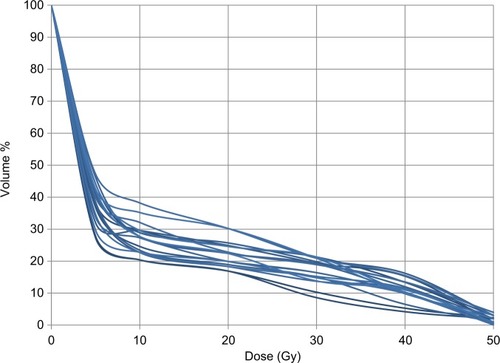
For the purpose of study, DVH to lungs and volume percentages of the ipsilateral lung absorbing 5, 10, 20, 30, 40, and 50 Gy (V5, V10, V20, V30, V40, and V50), respectively, were estimated. The continuous variables were dichotomized at respective median values.
Statistical analysis
Statistical Package for Social Science version 24.0 (IBM Corp., Armonk, NY, USA) was used for data analysis. Mean, minimum, maximum, and standard deviation were calculated for quantitative data description. A two-tailed paired Student’s t-test was subsequently used to compare mean values among the variables between the groups. A P-value of 0.05 was considered statistically significant.
Results
Median age of cohort was 44.95 years (range: 25–68). Three patients (15%) were known to have comorbidities (hypertension and diabetes). Majority of the cohort (17 patients; 85%) had left-side breast cancer. Majority of the cohort (85%) also had advanced primary (T3 and T4 stages) and N2 status (65%). Other histopathological features, receptor status, chemotherapy, and radiotherapy doses in the study population are shown in . Neoadjuvant and adjuvant chemotherapy regimen in the cohort was three cycles of 5-fluorouracil 600 mg/m2, epirubicin 60 mg/m2, and cyclophosphamide 600 mg/m2 every 3 weeks followed by three cycles of docetaxel 75 mg/m2. In all, 5/20 (25%) patients were given trastuzumab during and after radiation therapy.
Table 1 Patients’ baseline characteristics
Pulmonary function tests
Comparative analysis of different baseline PTFs parameters and those obtained at days 30 and 90 after the completion of radiotherapy are presented in . A significant decrease of FVC, FEV1, and DLCO was noticed at day 90 after the completion of radiotherapy with P-values 0.033, 0.042, and 0.031, respectively (–), while MMEF25–75 and VO2max were not significantly affected ( and ). All patients were found asymptomatic during the study period.
Figure 2 FVC measurements before and after treatment for breast cancer patients treated with local radiotherapy.
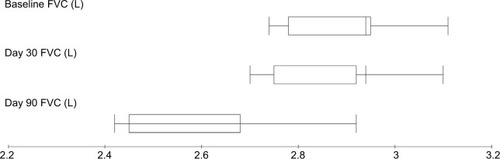
Figure 3 FEV1 measurements before and after treatment for breast cancer patients treated with local radiotherapy.
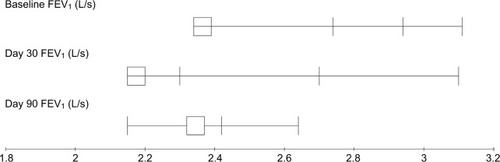
Figure 4 DLCO measurements before and after treatment for breast cancer patients treated with local radiotherapy.
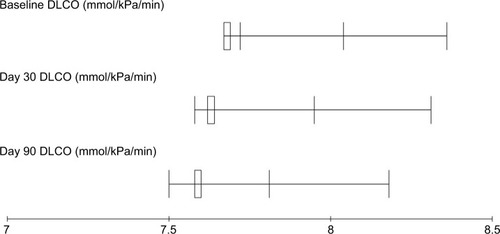
Figure 5 MMEF25–75 measurements before and after treatment for breast cancer patients treated with local radiotherapy.
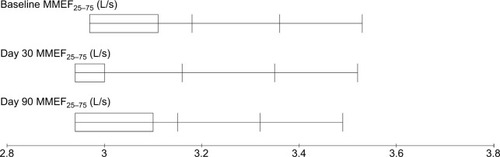
Figure 6 VO2max measurements before and after treatments for breast cancer patients treated with local radiotherapy.
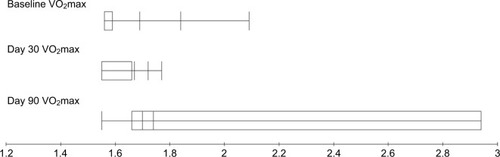
Table 2 Pulmonary function tests performed before, at day 30, and at day 90 after radiotherapy
DVH data
Fourteen patients (70%) had V20 lung dose constraint <30%; however, in 6 (30%) V20 >30% of accepted due to the nature of the patient’s contours. The mean ipsilateral lung V5, V10, V20, V30, V40, and V50 were 39.5%, 27.8%, 22.5%, 18.7%, 15.0%, and 1.8%, respectively (). Significant association between high V20 with decrease in FVC, FEV1, and DLCO was observed (P = 0.01, P = 0.002, and P = 0.001, respectively). Further, neoadjuvant chemotherapy was associated with decrease in DLCO at day 90 (P = 0.001).
Discussion
In our cohort, a significant reduction of FVC, FEV1, and DLCO and an association with ipsilateral lung V20 were observed at day 90 after the completion of PMRT; however, no deterioration of MMEF25–75 and VO2max was observed. These results are in agreement with previously published data.Citation12,Citation13 An equal reduction of FVC and FEV1 with normal FEV1% pred suggests an acute exudative inflammatory process in the alveolar spaces, favoring a restrictive lung injury pattern (RIP or RIF).Citation14 Further, unaltered MMEF25–75 supports the restrictive radiation-induced lung injury in our cohort, as deterio rated MMEF25–75 is related to damage to bronchioles leading to obstructive lung disease.Citation15 Similarly, VO2max, which is a measure of physical fitness of an individual, was found unchanged in our cohort. Possible explanation for this could be the compensation of RILT by contralateral healthy lung.Citation16
Although we did not aim to investigate the ability of PFTs to predict the risk of RILT, but topic is still arguable, as some studies have reported that better baseline PFTs predicted lower risk of RILT, whereas some studies did not see any correlation between PFTs and RILT.Citation17,Citation18 Impact of PFTs to predict the risk of RILT can be explained by the few hypotheses: 1) theoretically, individuals with better baseline PFTs have relatively higher level of cellular oxygenation, thus more radiosensitivity of alveoli and more risk of RILT;Citation19 2) on the other hand, patients with poor baseline PFTs are more likely to present with symptoms prior to radiotherapy, and in individuals with severe baseline pulmonary symptoms, the RILT score is less likely to decrease by one grade;Citation19,Citation20 3) as most of PFT data are available from lung cancer trials comprising patients who are mostly with emphysematous lungs. Since emphysema represents physiological missing lung parenchyma, theoretically one would expect less risk of RILT risk; and 4) physicians’ bias reduction of total dose or putting more stringent lung constraints in patients with poor baseline PFTs;Citation21 however, further studies using the radiological data are warranted. As RILT with clinical symptoms is relatively rare (incidence <3%) and of mild nature especially with use of modern radiation therapy techniques, the treatment is oral steroids; therefore prevention (reducing lung volume and fraction size) is the key buttress.Citation22 Further, treatment for patients with decrease in PFTs in the absence of clinical symptoms needs further investigation.
Limitations of the present study were 1) a relatively small sample size, 2) short follow-up duration, and 3) lack of correlation analysis between PFTs and RILT.
Conclusion
In conclusion, PFTs shall be performed in all breast cancer patients receiving PMRT for early detection of RILT as most of the patients in our cohort were asymptomatic. Studies incorporating longer duration of follow-up and large sample size are warranted to address clinical significance of PFTs in breast cancer patients receiving PMRT.
Disclosure
The authors report that no conflicts of interest in this work.
References
- KrengliMSaccoMLoiGPulmonary changes after radiotherapy for conservative treatment of breast cancer: a prospective studyInt J Radiat Oncol Biol Phys20087051460146717931797
- FragkandreaIKoulouliasVMavridisPRadiation induced pneumonitis following whole breast radiotherapy treatment in early breast cancer patients treated with breast conserving surgery: a single institution studyHippokratia201317323322824470733
- LindPAMarksLBJamiesonTAPredictors for pneumonitis during locoregional radiotherapy in high-risk patients with breast carcinoma treated with high-dose chemotherapy and stem-cell rescueCancer200294112821282912115368
- GongHYHuWGHuQYLiXPSongQBRadiation-induced pulmonary injury accelerated pulmonary metastasis in a mouse model of breast cancerOncol Lett20151063613361826788178
- TomaCLSerbescuAAlexeMCervisLIonitaDBogdanMAThe bronchoalveolar lavage pattern in radiation pneumonitis secondary to radiotherapy for breast cancerMaedica (Buchar)20105425025721977166
- KimseyFCMendenhallNPEwaldLMCoonsTSLayonAJIs radiation treatment volume a predictor for acute or late effect on pulmonary function? A prospective study of patients treated with breast-conserving surgery and postoperative irradiationCancer19947310254925558174052
- OoiGCKwongDLHoJCPulmonary sequelae of treatment for breast cancer: a prospective studyInt J Radiat Oncol Biol Phys200150241141911380228
- BudachWBolkeEKammersKGerberPANestle-KrämlingCMatuschekCAdjuvant radiation therapy of regional lymph nodes in breast cancer – a meta-analysis of randomized trials-an updateRadiat Oncol20151025826691175
- LindPAMarksLBHardenberghPHTechnical factors associated with radiation pneumonitis after local +/− regional radiation therapy for breast cancerInt J Radiat Oncol Biol Phys200252113714311777631
- BjermerLFranzenLLittbrandBNilssonKAngstromTHenrikssonREffects of smoking and irradiated volume on inflammatory response in the lung of irradiated breast cancer patients evaluated with bronchoalveolar lavageCancer Res199050202720302317792
- BentzenSMSkoczylasJZOvergaardMOvergaardJRadiotherapy-related lung fibrosis enhanced by tamoxifenJ Natl Cancer Inst199688139189228656444
- SpyropoulouDLeotsinidisMTsiamitaMSpiropoulosKKardamakisDPulmonary function testing in women with breast cancer treated with radiotherapy and chemotherapyIn Vivo200923586787119779125
- FleckensteinKGauter-FleckensteinBJacksonILRabbaniZAnscherMVujaskovicZUsing biological markers to predict risk of radiation injurySemin Radiat Oncol2007172899817395039
- SchytteTBentzenSMBrinkCHansenOChanges in pulmonary function after definitive radiotherapy for NSCLCRadiother Oncol2015117232826455451
- QuanjerPHWeinerDJPrettoJJBrazzaleDJBorosPWMeasurement of FEF25-75% and FEF75% does not contribute to clinical decision makingEur Respir J20144341051105824072211
- CaslaSLopez-TarruellaSJerezYSupervised physical exercise improves VO2max, quality of life, and health in early stage breast cancer patients: a randomized controlled trialBreast Cancer Res Treat2015153237138226293147
- RobnettTJMachtayMVinesEFMcKennaMGAlgazyKMMcKennaWGFactors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancerInt J Radiat Oncol Biol Phys2000481899410924976
- Dehing-OberijeCDe RuysscherDvan BaardwijkAYuSRaoBLambinPThe importance of patient characteristics for the prediction of radiation-induced lung toxicityRadiother Oncol200991342142619147245
- WangJCaoJYuanSPoor baseline pulmonary function may not increase the risk of radiation-induced lung toxicityInt J Radiat Oncol Biol Phys201385379880422836048
- YuanSTFreyKAGrossMDChanges in global function and regional ventilation and perfusion on SPECT during the course of radiotherapy in patients with non-small-cell lung cancerInt J Radiat Oncol Biol Phys201282e631e63822197235
- HernandoMLMarksLBBentelGCRadiation-induced pulmonary toxicity: a dose-volume histogram analysis in 201 patients with lung cancerInt J Radiat Oncol Biol Phys200151365065911597805
- ZhangXJSunJGSunJPrediction of radiation pneumonitis in lung cancer patients: a systematic reviewJ Cancer Res Clin Oncol2012138122103211622842662