Abstract
Transient receptor potential (TRP) channels contribute to the regulation of intracellular calcium, which can promote cancer hallmarks in cases of dysregulation of gene transcription and calcium-dependent pro-proliferative or anti-apoptotic mechanisms. Several studies have begun to elucidate the roles of TRPV1, TRPV6, TRPM8, and TRPC1 in cancer progression; however, no study has examined the expression profiles of human TRP channels in breast cancer on a large scale. This study focused on the expression and functionality of TRPV1, a nonselective cation channel that was found to be expressed in different carcinoma tissues. Next-generation sequencing analyses revealed the expression of TRPV1 in several native breast cancer tissues, which was subsequently validated via reverse transcriptase-polymerase chain reaction. Activation of TRPV1 by its ligand capsaicin was associated with the growth inhibition of some cancer cell types; however, the signaling components involved are complex. In this study, stimulation by the TRPV1 agonist, capsaicin, of SUM149PT cells, a model system for the most aggressive breast cancer subtype, triple-negative breast cancer, led to intracellular calcium signals that were diminished by the specific TRPV1 antagonist, capsazepin. Activation of TRPV1 by capsaicin caused significant inhibition of cancer cell growth and induced apoptosis and necrosis. In conclusion, the current study revealed the expression profiles of human TRP channels in 60 different breast cancer tissues and cell lines and furthermore validated the antitumor activity of TRPV1 against SUM149PT breast cancer cells, indicating that activation of TRPV1 could be used as a therapeutic target, even in the most aggressive breast cancer types.
Introduction
Breast cancer is one of the most common cancers affecting women worldwide.Citation1 A series of high-throughput methods have changed cancer research and therapy.Citation2–Citation4 Expression profiling on a genomic scale led to the classification of the intrinsic subtypes of breast cancer, thus allowing for the prediction of disease progression and patient stratification.Citation3 Treatment options are highly diverse; whereas human epidermal growth factor receptor 2 (HER2)-positive breast cancers can be targeted by specific drugs, the triple-negative breast cancer phenotype is defined by chromosomal instability and low expression levels of estrogen receptor (ER) alpha, progesterone receptor (PR), and HER2, rendering treatment rather difficult.Citation5 Triple-negative breast cancer is characterized by limited treatment options and frequent local recurrence.Citation6
Transient receptor potential (TRP) channels, which are membranous ion channels that conduct calcium and sodium ions, have been shown to influence cancer cell growth.Citation7,Citation8 These channels can be activated by many stimuli, including temperature or pH changes in the extracellular space.Citation9 TRP channels play an important role in the development of several diseases, and one of the most investigated and described TRP channel is TRPV1.Citation10 TRPV1 agonist, capsaicin, is capable of inducing apoptosisCitation11,Citation12 and inhibiting cancer cell growth by cell cycle arrest in many different types of cancer, for example, osteosarcoma, colon, and pancreatic cancer cells, while normal cells remained unharmed.Citation13–Citation17 A less-pungent capsaicin-like analog, RPF151, showed antitumor activity in a murine breast cancer model in vivo,Citation18 demonstrating the possibility of managing the induction of pain.
In this study, we aimed, on the one hand, to elucidate the expression profiles of TRP channels in a large amount of breast cancer tissues via RNA-Seq. On the other hand, we analyzed the expression of TRPV1 in detail to contribute to the understanding of the influences of TRPV1 activation in novel breast cancer therapy.
Materials and methods
Tissue preparation and reverse transcriptase (RT)-polymerase chain reaction (PCR)
Breast cancer tissues were obtained from Dr Bonatz, Augusta-Krankenanstalt Bochum, and Dr Wölk, Herz-Jesu Klinik Dernbach. Each patient provided written consent and gave permission for the storage of their samples for the use in research and development purposes. Identities were entirely concealed in accordance with the Declaration of Helsinki. The tumor tissue samples were maintained in RNAlater. RNA was isolated from the tumor samples using a homogenizer (Precellys®; Bertin Corp., Rockville, MD, USA) with 1.4 ceramic beads (Precellys®) and with an RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocols. cDNa synthesis was performed using the iScript™ cDNA Synthesis Kit according to the manufacturer’s protocol (Bio-Rad, Hercules, CA, USA). RT-PCR was conducted to validate the expression of the detected olfactory receptors. PCR was performed using GoTaq qPCR Master Mix. The PCR included the following steps: 3 min, 95°C, 40 cycles of 1 min at 60°C (TRPV1) and 1 min at 72°C. A Mastercycler® ep realplex (Eppendorf, Hamburg, Germany) was used.
Primers are as follows:
TRPV1 – forward: CAGGGAAGACCTGTCTGCTG;
reverse: ACAAGCTCCTTCAGGCTGTC.
Chemicals
Capsaicin was purchased from Sigma-Aldrich (St Louis, MO, USA). The inhibitor capsazepin and the TRP channel inhibitor ruthenium red were purchased from Abcam (Cambridge, MA, USA). Ringer’s solution contained 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, and 10 mM (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), at a pH of 7.4. The substances were prediluted in dimethyl sulfoxide (DMSO) prior to usage. The final concentration of DMSO solvent was maximally 0.1% for each experiment.
Next-generation sequencing
For standard mRNAseq (Illumina, San Diego, CA, USA), we used the TruSeq™ RNA Sample Prep Kit v2 protocol (Illumina). HiSeq 2000 (2 × 102 bp reads) was used for RNA-Seq. In total, 11 breast cancer tissue samples were analyzed in cooperation with the Cologne Center for Genomics (CCG). Raw data were generated for breast cancer samples in fastq format. Hg19 of the human genome and transcriptome was used to align the reads. The alignment was arranged by the Bowtie program included in TopHat (version 1.2.0).Citation19 The output files were sorted and indexed by the SAMtools program in BAM format.Citation20 The number of the aligned mRNA-Seq reads was calculated using the Cufflinks program,Citation21 which uses the RefSeq hg19 reference transcriptome in Gene Transfer Format (GTF; obtained from the USCS Genome Bioinformatic database, University of California, Santa Cruz, CA, USA). The Cufflinks program was used for the quantification of the relative abundance of transcripts for each gene, using the unit FPKM (fragments per kilobase of exon per million fragments mapped).Citation21 For visualization of the data sets and the analysis of the read distribution, the Integrative Genomic Viewer was used (www.broadinstitute.org/igv).
Cell culture
SUM149PT cells were purchased from Asterand Bioscience (Detroit, MI, USA). The cells were cultivated in Roswell Park Memorial Institute (RPMI) medium containing 10% fetal bovine serum, glutamine (5%) and 100 units/mL penicillin/streptomycin (Gibco®; Thermo Fisher Scientific, Waltham, MA, USA). Cultivation was performed in an incubator with a humidified atmosphere at 37°C in 10% CO2. SUM149PT cells were passaged every 3–4 days at a confluence of 85% using TrypLE™ (Thermo Fisher Scientific).
Calcium imaging
SUM149PT cells were incubated at 37°C for 30 min with Ringer’s solution containing 3 mM FURA-2-AM (fura-2-acetoxymethyl ester; Molecular Probes, Eugene, OR, USA). For calcium imaging experiments, the growth medium was replaced by extracellular medium. Fluorimetric imaging was performed according to the standard protocol by Spehr et al.Citation22 SUM149PT cells were exposed to capsaicin two to three times for 30–60 s, depending on the experimental approach. The concentrations ranged from 3–10 µM to 500 µM. The inhibitors ruthenium red and capsazepin were co-applied at concentrations of 5 and 10 µM. ATP (10 µM) served as a control and was applied after every measurement.
Scratch assays
Scratch assays were essentially performed as described in the study by Busse et al.Citation23 For stimulation, Dulbecco’s Modified Eagle’s Medium containing capsaicin was used at concentrations of 150 and 200 µM. The software TScratch was used to calculate the overgrowing gap relative to the initial scratch area.Citation24
Proliferation assay
SUM149PT cells were seeded at a density of 5 × 103 cells in 96-well plates. After 24 h of cultivation, the cells were stimulated with RPMI + capsaicin (150 and 200 µM) and RPMI + 0.1% DMSO. The CyQuant® NF assay (Thermo Fisher Scientific) was used according to the manufacturer’s protocol. Excitation at 485/20 nm and fluorescence detection at 528/20 nm were measured to calculate fluorescence intensities.
Annexin V-fluorescein isothiocyanate (FITC) staining
The apoptosis assay was performed by staining the SUM149PT cells with FITC-labeled Annexin V and propidium iodide (PI), using the Annexin V-FITC Apoptosis Detection Kit (Abcam), according to the manufacturer’s instructions. In brief, SUM149PT cells were treated with capsaicin (10 and 150 µM) or control for 48 h. The cells were washed twice with phosphate-buffered saline (PBS) and were resuspended with the Annexin V binding buffer. Then, the cells were incubated with FITC-labeled Annexin V and PI for 15 min in the dark at room temperature. For detection, a fluorescence microscope (Axioskop; Zeiss, Oberkochen, Germany) was used.
Caspase assay
For the detection of apoptosis, the Caspase-3 Colorimetric Assay Kit by Abcam was used according to the manufacturer’s protocol. SUM149PT cells were seeded and incubated on 12 mm coverslips and placed in 24-well plates containing medium. The medium was replaced by medium containing capsaicin (150 µM) or DMSO for 14 h. Cells were fixed for 20 min with 4% paraformaldehyde (PFA) and were washed twice with 2 mL of PBS + Triton X-100 for 10 min. The cells were blocked for 60 min with blocking buffer (PBS, 10% fish gelatin, 0.5% goat serum) at room temperature. Primary antibody-detecting caspase-3 (diluted 1:300 in blocking solution) was incubated overnight at 4°C. Before incubation for 1 h with the secondary antibody (purchased from Cell Signaling) at 1:1000 dilution including 2 µL of 4′,6-diamidino-2-phenylindole, the cells were washed thrice with PBS + TritonX-100. The cells were fixed with ProLong® Gold Antifade. Analysis was performed with an LSM 510 Meta (Zeiss).
Immunocytochemistry
SUM149PT cells were grown on coverslips in 24-well cell culture plates. PFA at a concentration of 4% was used for fixation of the cells. After fixation, the cells were washed and permeabilized in PBS−/− + 0.1% Triton X-100. The blocking reagent contained phosphate buffered saline tween-20 + 1% fish gelatin and was incubated for 1 h at room temperature. The TRPV1 antibody was incubated overnight at 4°C. Subsequently, the cells were washed three times, and the secondary antibody coupled to Alexa Fluor 488 (Thermo Fisher Scientific) was added for 45 min at room temperature. To analyze the images, a Zeiss LSM 510 Meta confocal microscope (Zeiss) was used.
Statistics
Every result was tested for normality (Shapiro–Wilk test) and equal variance. Normally distributed data were analyzed using Student’s two-tailed unpaired t-test. Data that were not normally distributed were analyzed by the Mann–Whitney U test. Every result contained at least three independent experiments so that the mean standard error of the mean is shown. Statistical significance was indicated as follows: *p<0.05, **p<0.01, ***p<0.001.
Results
Expression of TRP channels in breast cancer
Several studies have demonstrated the influence of some TRP channels on breast cancer cell progression.Citation25–Citation27 Therefore, we aimed to identify the expression patterns of 16 human TRP channels in native breast cancer tissues and cell lines, and we analyzed the transcriptome of 11 different breast cancer tissues via RNA-Seq (). We investigated the tissue samples in cooperation with the CCG and generated at least five Mio reads for each sample, using paired-end sequencing analysis and reanalyzed the data sets from the NCBI archive. In this study, we could detect a broad range of TRP channels with high expression values (FPKM > 10) in different breast cancer tissues.
Figure 1 Heat map of TRP channel expression in 11 native human breast cancer tissues and four healthy breast tissues.
Abbreviations: CCG, Cologne Center for Genomics; FPKM, fragments per kilobase of exon per million fragments mapped; TRP, transient receptor potential.
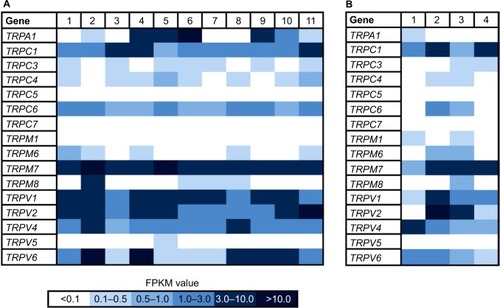
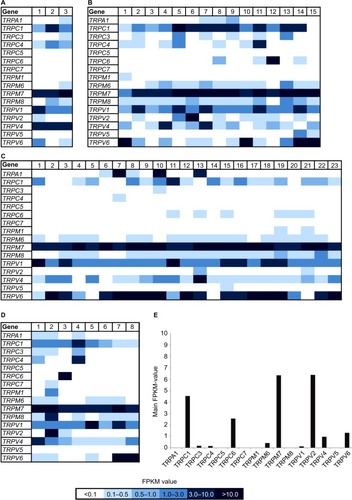
In addition, we reanalyzed the transcriptome data of 49 breast cancer cell lines originating from several different tumor types () with focus on the expression of TRP channels. In this study, we used the innovative classification of breast cancer subtypes, revealed by extensive genomic analyses of breast cancer tissues and based on the molecular profiles of the cells, for example, luminal A, luminal B, basal-like, triple-negative, or BRCA1 mutated.Citation28,Citation29 There were only two TRP channels expressed in every tissue sample, namely TRPM7 and TRPV1 (). Because TRPM7 is known to be expressed in a large number of different other cell types,Citation30 it could not be considered a suitable, specific target for cancer therapy. In comparison, TRPV1 is not expressed in such a broad range; furthermore, it is associated with breast tumor growth inhibition.Citation8,Citation31,Citation32 We showed the average expression level of TRP channels in breast cancer cell lines and the expression in healthy breast tissues, using data obtained from the Gtex database (www.gtexportal.org; ). TRPV1 was overexpressed in breast cancer tissues compared to normal breast tissues. Some TRP channels were expressed in neither tumor tissues nor healthy tissues, for example, TRPC5 and TRPC7. TRPA1 was differentially expressed; there was no expression in healthy breast tissues but particular expression in some luminal breast tumors. Another interesting TRP channel is TRPM8, which was not expressed in healthy breast tissues but was expressed in many breast cancer samples, regardless of the subgroup. An almost contrary effect could be observed for TRPV2, showing a high expression (FPKM > 6) in healthy breast tissues but low to almost no expression in the different breast cancer subtypes. Strikingly, the luminal breast cancer subtype showed the least expression, and in the majority of samples no expression existed at all.
Figure 2 Heat map of TRP channel expression in 49 breast cancer cell lines and healthy tissues.
Abbreviations: FPKM, fragments per kilobase of exon per million fragments mapped; TRP, transient receptor potential.
Expression of TRPV1 in breast cancer cells
With an average FPKM value of 4.9, TRPV1 showed the highest expression in triple-negative breast cancer cells (). Therefore, we focused on the investigation of TRPV1 and validated the expression of TRPV1 in breast cancer tissue samples via RT-PCR ().
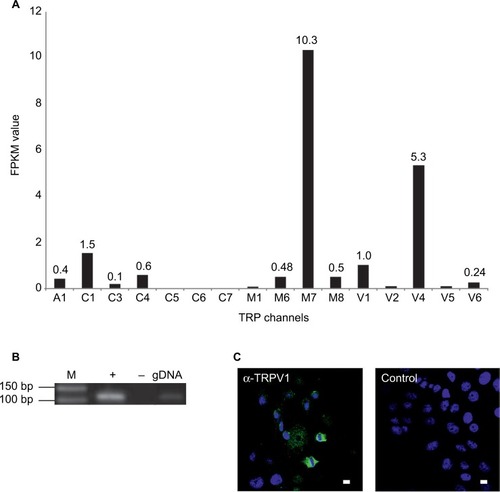
We analyzed the function of TRPV1 in SUM149PT cells, which were developed from an invasive, inflammatory triple-negative ductal carcinoma, one of the most aggressive breast cancer types. shows the self-generated RNA-Seq data of SUM149PT cells, as well as the expression of TRPV1 at the mRNA and protein levels via RT-PCR and immunocytochemistry, using specific TRPV1-detecting antibodies ().
Figure 3 Expression of TRPV1 in breast cancer tissues and cell lines.
Abbreviations: BC, breast cancer; M, marker; PCR, polymerase chain reaction; RT, reverse transcriptase; TNBC, triple-negative breast cancer.
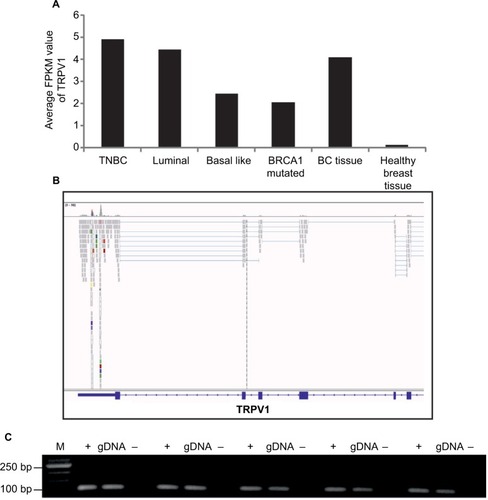
Figure 4 Expression profiles of TRP channels in the SUM149PT breast cancer cell line.
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; M, marker; NGS, next-generation sequencing; PCR, polymerase chain reaction; RT, reverse transcriptase; TRP, transient receptor potential.
Characterization of TRPV1 in SUM149PT cells
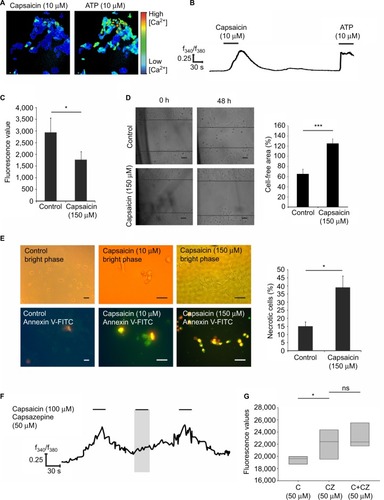
To analyze the function of TRPV1 in SUM149PT cells, we investigated the effect of ligand (capsaicin) stimulation. We conducted calcium imaging experiments and could elicit calcium signals at the concentration of 100 µM capsaicin (), which were abolished by coapplication of the TRPV1 antagonist capsazepin (). In total, 10% of the cells could be activated by capsaicin at the concentration of 10 µM, and ~20% of the cells showed calcium signals upon stimulation with 100 µM capsaicin in calcium imaging experiments. Next, we investigated the physiological effects of capsaicin by migration and proliferation assays. Stimulation of SUM149PT cells with capsaicin (150 µM) for 48 h induced a significant reduction in cell proliferation (). Furthermore, we performed scratch assays, which are used to analyze cell migration. When stimulated with capsaicin (150 µM) for 48 h, the cells showed a reduced migration into the gap and altered their morphology, indicating cell transition into apoptosis (). Therefore, we performed immunocytochemical staining for the detection of apoptosis and necrosis, using the Annexin V-FITC Kit, which allowed for differentiation among early apoptotic cells, necrotic cells, and viable cells (). Stimulation with capsaicin (10 and 150 µM) induced apoptotic and necrotic cell death (). Approximately 37% of the cells became necrotic upon stimulation with capsaicin (150 µM). Then, we examined whether the capsaicin-induced effect on cell proliferation was mediated by TRPV1 using the TRPV1-specific antagonist capsazepin (50 µM). The reduction in cell proliferation was diminished by the coapplication of capsaicin (50 µM) and capsazepin (50 µM) in comparison to capsaicin alone ().
Figure 5 Functional characterization of TRPV1 activation by capsaicin in SUM149PT cells.
Abbreviations: C, capsaicin; CZ, capsazepin; FITC, fluorescein isothiocyanate; PI, propidium iodide; F340/F380, ratio of the fluorescence intensities at 340 nm and 380 nm excitation; C, capsaicin; CZ, capsazepin; ATP, adenosine triphosphate; ns, not significant.
Discussion
In this study, we aimed to identify the expression profiles of TRP channels in different breast cancer subtypes and to investigate the effect of TRPV1 ligand on breast cancer progression. The targeting of different TRP channels in several cancer types has become increasingly important, because the roles of TRPs in tumorigenesis and cancer progression have been impressively proved by the detection of assigned oncogenic properties for most TRPs.Citation10 In this study, the comprehensive RNA-Seq analysis of 11 breast cancer tissues and 49 breast cancer cell lines revealed a considerable amount of data. To our knowledge, no studies have yet conducted a large-scale comparative study of the TRP expression profiles in breast cancer cell lines. In this study, TRPM7 showed the highest expression. TRPM7 is known to regulate many features of malignant cancers; for example, it mediates cell migration and invasion through the MAPK pathway in MDA-MB-435 breast cancer cells.Citation26,Citation33 Because of the broad expression of TRPM7 also in healthy cells, it is an unsuitable target for tumor therapy. Other TRP channels showed differential expression; TRPA1 is not expressed in the healthy tissue but in some breast tumor tissues, especially in the luminal subtype. Analyzing the association between TRPA1 expression and the progression of luminal breast cancer will be the aim of future studies. TRPM8 was also expressed in breast cancer tissues only, regardless of the molecular subtype. This finding was in accordance with studies describing TRPM8 as a potential cancer biomarker and a therapeutic target in pancreatic cancer.Citation34,Citation35 Furthermore, we showed the opposite expression pattern for TRPV2, which was highly expressed in healthy breast tissues and weakly expressed in breast cancer tissues. Of course, these data are not sufficient to draw conclusions concerning TRP channels as novel biomarkers for breast cancer subtypes, but they could serve as the basis for further investigations. TRPV1, a nonselective cation channel, was the second most highly expressed TRP channel in our data set and was detectable in all samples. TRPV1 is known to be expressed in several different neoplastic tissues, such as squamous cell carcinoma of the tongue, hepatocellular carcinoma, or transitional carcinoma of the human bladder,Citation31,Citation36,Citation37 and it has also been associated with growth and progression in breast cancer cells.Citation27,Citation38 In MCF-7 breast cancer cells, the mediation of cell death upon stimulation with the ligand capsaicin was demonstrated.Citation27 In preliminary investigations, Vercelli et alCitation39 demonstrated a reduction in cell proliferation upon application of the TRPV1 agonist capsaicin. Interestingly, even the inhibitor, capsazepin, showed the same effect.Citation39 Actually, the investigation of TRPV1 as a novel target in breast cancer is far from complete, not least because its functionality differs in healthy breast cells and cancerous cells. Therefore, we aimed to investigate the effect of TRPV1 in a triple-negative breast cancer cell line. TRPV1 showed the highest average expression in this study, followed by luminal and basal-like breast cancer subtypes. SUM149PT displayed a triple-negative breast cancer phenotype, defined by the absence of ER, PR, and HER2.Citation40 This cancer type accounts for 15%–20% of all breast cancer cases and shows poor prognosis with only a 40% 5-year survival rate due to the insensitivity to some of the most effective targeted therapies available for breast cancer treatment.Citation5,Citation40 We could validate the expression of TRPV1 in SUM149PT cells, as well as native breast cancer tissues, via RNA-Seq, RT-PCR, and immunocytochemical staining. The expression patterns of TRP channel genes in cancer suggested that TRP channel-mediated Ca2+ remodeling mechanisms might play a crucial role in tumor cell progression and survival.Citation41 In this study, we observed a clearly higher expression of TRPV1 in breast cancer tissues and cells than in healthy breast tissues. For TRPV1, high expression in hepatocellular carcinoma cells was associated with better prognosis.Citation37 In transitional cell carcinoma of the bladder, the expression of TRPV1 decreased progressively as the cancer stage increased.Citation31
Calcium imaging experiments revealed the functionality of TRPV1 in SUM149PT cells. In this study, the stimulation of the cells with its ligand capsaicin led to strong calcium increases in the cells, which could be diminished by the coapplication of the specific inhibitor capsazepin and the unspecific inhibitor ruthenium red. After we proved the existence and functionality of TRPV1, we conducted proliferation and migration analyses to investigate the long-term effects of capsaicin on SUM149PT cells. In a study by Sanchez et al,Citation43 the role of TRPV1 in prostate cancer cell proliferation was analyzed. In our experiments, a significant reduction in cell proliferation after capsaicin stimulation was observed, diminished by the coapplication of capsazepin. This finding was in accordance with the results of Vercelli et al,Citation43 who demonstrated a significant decrease in the cell growth rate of MCF-7 breast cancer cells upon capsaicin stimulation. Interestingly, Zheng et alCitation44 furthermore showed that capsaicin was capable of enhancing the antineoplastic effects of pirarubicin, which is an analog of the antibiotic doxorubicin.Citation45 In HCT116 cells, the inhibition of cell proliferation by TRPV1 signaling was demonstrated as well.
We showed the induction of apoptosis and necrosis upon capsaicin stimulation, in accordance with a study of TRPV1 in prostate cancer cells, in which its activation by capsaicin led to the induction of necrotic cell death.Citation46 This induction led to the suggestion that activation of TRPV1 by specific ligands could significantly enhance breast cancer cell death, even in the most aggressive breast cancer subtype, triple-negative breast cancer. The mechanisms involved leading to apoptosis remain elusive.
Conclusion
We conducted a meta-study of the expression patterns of all human TRP channels in different breast cancer subtypes and showed that TRPM7 and TRPV1 were the only channels expressed in every sample. Furthermore, TRPV1 showed the highest average expression in triple-negative breast cancer subtypes. Analyzing channel activation, we demonstrated anti-proliferative, anti-migratory and apoptosis/necrosis-inducing effects of capsaicin treatment due to the activation of TRPV1 in SUM149PT cells, which is a model system for triple-negative inflammatory breast cancer, the most aggressive breast cancer subtype.
Disclosure
The authors report no conflicts of interest in this work.
References
- DeSantisCELinCCMariottoABCancer treatment and survivorship statistics, 2014CA Cancer J Clin201464425227124890451
- DesmedtCVoetTSotiriouCCampbellPJNext-generation sequencing in breast cancer: first take home messagesCurr Opin Oncol201224659760423014189
- GarrawayLALanderESLessons from the cancer genomeCell20131531173723540688
- KarnTHigh-throughput gene expression and mutation profiling: current methods and future perspectivesBreast Care (Basel)20138640140624550747
- OPYRCHALMSALISBURYJLIANKOVIInhibition of Cdk2 kinase activity selectively targets the CD44+/CD24− Low stem-like sub-population and restores chemosensitivity of SUM149PT triple-negative breast cancer cellsInt J Oncol20144531193119924970653
- NavratilJFabianPPalacovaMTriple negative breast cancerKlin Onkol201528640541526673990
- BenemeiSPatacchiniRTrevisaniMGeppettiPTRP channelsCurr Opin Pharmacol201522182325725213
- BoddingMTRP proteins and cancerCell Signal200719361762417029734
- RamseyISDellingMClaphamDEAn introduction to TRP channelsAnnu Rev Physiol20066861964716460286
- ShapovalovGRitaineASkrymaRPrevarskayaNRole of TRP ion channels in cancer and tumorigenesisSemin Immunopathol201638335736926842901
- MistrettaFBuffiNMLughezzaniGBladder cancer and urothelial impairment: the role of TRPV1 as potential drug targetBiomed Res Int2014201498714924901005
- AmantiniCMoscaMNabissiMCapsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activationJ Neurochem2007102397799017442041
- PramanikKCBoreddySRSrivastavaSKRole of mitochondrial electron transport chain complexes in capsaicin mediated oxidative stress leading to apoptosis in pancreatic cancer cellsPLoS One201165e2015121647434
- KimYMHwangJTKwakDWLeeYKParkOJInvolvement of AMPK signaling cascade in capsaicin-induced apoptosis of HT-29 colon cancer cellsAnn N Y Acad Sci2007109549650317404062
- ClarkRLeeSAnticancer properties of capsaicin against human cancerAnticancer Res201636383784326976969
- BleyKBoormanGMohammadBMcKenzieDBabbarSA comprehensive review of the carcinogenic and anticarcinogenic potential of capsaicinToxicol Pathol201240684787322563012
- JinTWuHWangYPengHCapsaicin induces immunogenic cell death in human osteosarcoma cellsExp Ther Med201612276577027446273
- FerreiraAKTavaresMTPasqualotoKFMRPF151, a novel capsaicin-like analogue: in vitro studies and in vivo preclinical antitumor evaluation in a breast cancer modelTumour Biol20153697251726725894379
- LangmeadBTrapnellCPopMSalzbergSLUltrafast and memory-efficient alignment of short DNA sequences to the human genomeGenome Biol2009103R2519261174
- LiHHandsakerBWysokerAThe sequence alignment/map format and SAMtoolsBioinformatics200925162078207919505943
- TrapnellCRobertsAGoffLDifferential gene and transcript expression analysis of RNA-seq experiments with TopHat and CufflinksNat Protoc20127356257822383036
- SpehrMGisselmannGPoplawskiAIdentification of a testicular odorant receptor mediating human sperm chemotaxisScience200329956152054205812663925
- BusseDKudellaPGruningNA synthetic sandalwood odorant induces wound-healing processes in human keratinocytes via the olfactory receptor OR2AT4J Invest Dermatol2014134112823283224999593
- GebäckTSchulzMMKoumoutsakosPDetmarMTScratch: a novel and simple software tool for automated analysis of monolayer wound healing assaysBiotechniques200946426527419450233
- Ouadid-AhidouchHDhennin-DuthilleIGautierMSevestreHAhidouchATRP channels: diagnostic markers and therapeutic targets for breast cancer?Trends Mol Med201319211712423253476
- MengXCaiCWuJTRPM7 mediates breast cancer cell migration and invasion through the MAPK pathwayCancer Lett201333319610223353055
- WuTTPetersAATanPTRoberts-ThomsonSJMonteithGRConsequences of activating the calcium-permeable ion channel TRPV1 in breast cancer cells with regulated TRPV1 expressionCell Calcium2014562596724889371
- LiuZFChenCYaoXLClinicopathological characteristics and prognosis of different molecular types of breast cancerZhonghua Yi Xue Za Zhi201696221733173727356638
- BerseBLynchJAMolecular diagnostic testing in breast cancerSemin Oncol Nurs201531210812125951740
- ParaviciniTMChubanovVGudermannTTRPM7: a unique channel involved in magnesium homeostasisInt J Biochem Cell Biol20124481381138422634382
- LazzeriMVannucchiMGSpinelliMTransient receptor potential vanilloid type 1 (TRPV1) expression changes from normal urothelium to transitional cell carcinoma of human bladderEur Urol200548469169815992990
- BodeAMChoYZhengDThe transient receptor potential type vanilloid 1 suppresses skin carcinogenesisCancer Res200969390591319155296
- ZhouWGuoSXiongZLiuMOncogenic role and therapeutic target of transient receptor potential melastatin 7 channel in malignancyExpert Opin Ther Targets201418101177119625069584
- YeeNSRoles of TRPM8 ion channels in cancer: proliferation, survival, and invasionCancers (Basel)2015742134214626512697
- YeeNSTRPM8 ion channels as potential cancer biomarker and target in pancreatic cancerAdv Protein Chem Struct Biol201610412715527038374
- MarincsakRTothBICzifraGIncreased expression of TRPV1 in squamous cell carcinoma of the human tongueOral Dis200915532833519320840
- MiaoXLiuGXuXHigh expression of vanilloid receptor-1 is associated with better prognosis of patients with hepatocellular carcinomaCancer Genet Cytogenet20081861253218786439
- SharmaSKVijASSharmaMMechanisms and clinical uses of capsaicinEur J Pharmacol20137201–3556224211679
- VercelliCBarberoRCunibertiBOdoreRReGExpression and functionality of TRPV1 receptor in human MCF-7 and canine CF.41 cellsVet Comp Oncol201513213314223510405
- YangFZhangWShenYGuanXIdentification of dysregulated microRNAs in triple-negative breast cancer (review)Int J Oncol201546392793225571912
- ParkYRChunJNSoIData-driven analysis of TRP channels in cancer: linking variation in gene expression to clinical significanceCancer Genomics Proteomics2016131839026708603
- SanchezMGSanchezAMColladoBExpression of the transient receptor potential vanilloid 1 (TRPV1) in LNCaP and PC-3 prostate cancer cells and in human prostate tissueEur J Pharmacol20055151–3202715913603
- VercelliCBarberoRCunibertiBTransient receptor potential vanilloid 1 expression and functionality in mcf-7 cells: a preliminary investigationJ Breast Cancer201417433233825548580
- ZhengLChenJMaZCapsaicin enhances anti-proliferation efficacy of pirarubicin via activating TRPV1 and inhibiting PCNA nuclear translocation in 5637 cellsMol Med Rep201613188188726648574
- DhingraKFryeDNewmanRAPhase II clinical and pharmacological study of pirarubicin in combination with 5-fluorouracil and cyclophosphamide in metastatic breast cancerClin Cancer Res1995176916979816034
- ZiglioliFFrattiniAMaestroniUDinaleFCiufifedaMCortelliniPVanilloid-mediated apoptosis in prostate cancer cells through a TRPV-1 dependent and a TRPV-1-independent mechanismActa Biomed2009801132019705615
