Abstract
Background
Li–Fraumeni Syndrome (LFS) is a rare disease with autosomal dominant inheritance linked to germline mutations of tumor suppressor gene TP53. These patients are predisposed to malignancies such as sarcoma, breast cancer, leukemia, and other malignancies. Breast cancer, the most common malignancy in adult patients with LFS, has an early-onset presentation and is usually treated as per the guidelines for the general population due to the limited literature about breast cancer in LFS. We aimed to describe our institutional experience treating patients with breast cancer and LFS to contribute to literature about this entity.
Design
Retrospective single-institution case-series study. We searched for cases with LFS and breast cancer from 01/01/2000 to 12/31/2015 with treatment received at our institution.
Results
We identified 4 cases (2 African Americans, 1 Indian, and 1 Hispanic) in 4 different families, who were diagnosed with LFS after presenting with breast cancer. Three cases were triple-negative disease and 1 case was ER+, HER2 positive disease. They were treated with mastectomy and a third-generation breast chemotherapy regimen and/or trastuzumab-containing regimen. Radiation therapy was used in 2 patients. Breast cancer recurrence was seen in 1 patient, while three other malignancies were identified after breast cancer treatment (1 breast sarcoma, 1 leiomyosarcoma, and 1 myelodysplastic syndrome). A patient, who underwent surveillance with a positron emission tomography-computed tomography scan, was found to have a stage I leiomyosarcoma and was treated with surgical resection, but then developed metastatic disease requiring cytotoxic chemotherapy.
Conclusion
Breast cancer among patients with LFS needs a multidisciplinary treatment approach. Surgical management follows the guidelines for the general population. Risk–benefit assessment of chemotherapy and radiotherapy needs to be performed carefully in a case-by-case approach. Patients should undergo multimodality cancer surveillance, preferably in the context of a clinical trial.
Background
Breast cancer is the most common female malignancy worldwide. Only 5%–10% of breast cancer cases are associated with hereditary syndromes, with BRCA1/2 mutations being the most commonly found.Citation1 Li–Fraumeni syndrome (LFS) is a rare, highly penetrant, and autosomal dominant syndrome that occurs due to the germline mutation in the TP53 gene,Citation2 which encodes the tumor suppressor protein p53. TP53 is involved in cell cycle regulation, apoptosis, and genomic stability; hence, TP53 has been named the “guardian of the genome”. Missense mutations in the DNA-binding domain of the TP53 gene destroy the ability of the p53 protein to bind to its target DNA sequences, leading to not only loss of tumor suppressor function, but also gain of novel pro-oncogenic functions with different TP53 hotspot mutations exhibiting gain-of-function effects which may affect the age onset of certain cancers in LFS.Citation3 As a consequence of abnormal cell cycle regulation, about 50% of TP53 mutation carriers develop cancer before age 30 years, with a cumulative lifetime risk up to 70% in men and 100% in women.Citation4
The initial suggestion of this familial cancer syndrome was proposed by Frederick Li and Joseph Fraumeni when they reported 4 families that presented with soft-tissue sarcoma (STS), breast cancer, and other malignancies in children and young adults.Citation5 It was not until 1988 when the original definition of LFS was presented from 24 families with autosomal dominant transmission of early-onset tumors including STSs, breast carcinomas, brain tumors, leukemias, and adrenocortical carcinomas before age 45 years.Citation6 In 1990, TP53 mutations were identified in 5 families with LFS, and TP53 germline mutation is considered the hallmark of LFS;Citation7 however, genetic TP53 modifiers have also been implicated in LFS. Wu et al reported that MDM2 polymorphism (T>G variant of SNP 309) was associated with accelerated tumor formation in both carriers and noncarriers of germline TP53 mutations.Citation8 A recent study by Yao and Sherif revealed abrogation of DLL4 gene encoding a notch ligand, in LFS cells and tumor tissues and demonstrated that p53 binds to DLL4 promoter by association with CTCF. These findings suggest that DLL may play a role in cancer immune surveillance, and DLL4 dysregulation – such as in LFS – can contribute to widespread tumorigenesis.Citation9
LFS criteria have changed since the first proposal of classic criteria in 1988.Citation6 Birch et alCitation10 and EelesCitation11 defined the Li–Fraumeni-like (LFL) syndrome, to include patients who do not meet the classic LFS criteria, based on the occurrence of typical malignancies in both the proband and one or more relatives at a relatively early age. Moreover, Chompret criteria also included individuals at risk of carrying germline mutation in TP53 independently of family history ().Citation12
Table 1 LFS and LFL syndrome criteria
LFS criteria are used in clinical practice to identify individuals with high risk who would benefit from TP53 mutation testing. TP53 germline mutations can be identified in 70% of families who meet the classic LFS criteria and 40% of those families meeting the LFL criteria; furthermore, the frequency of de novo mutations in TP53 is ~7%–20%.Citation13 In addition, the combination of Chompret with classic criteria achieves the highest sensitivity (95%) to identify patients with TP53 mutations.Citation14
Early-onset breast cancer is one of the most common tumors in patients with LFS.Citation15 Literature is scarce about the optimal management of patients with LFS and breast cancer, such as choosing the best adjuvant chemotherapy regimen due to concern of increasing risk of other malignancies. Moreover, adjuvant radiation for patients with LFS has been questioned due to concern of increased risk of radiation-induced sarcomas. Therefore, current management is based on a case-by-case approach. We aimed to describe our own experience in the management of breast cancer patients with LFS at our institution to contribute to the oncology literature on the management of this entity.
Methods
After obtaining institutional review board approval, we carried out a retrospective case-series study. Using our institutional “Clinical Looking Glass” software, we identified patients who had been diagnosed with breast cancer and LFS from 01/01/2000 to 12/31/2015. We obtained baseline characteristics, pertinent history about breast cancer diagnosis, and management. Genetic counseling and testing records were also reviewed. Our study was approved by the Einstein East Campus Institutional Review Board. Patients provided informed consent to have their case details and imaging published as part of this study.
Results
During the study period, we identified 4 cases who met the inclusion criteria and who were treated at our institution. None of the patients were found to have BRCA 1/2 or any other deleterious mutations associated with hereditary breast cancer ().
Table 2 Characteristics of patients with LFS and breast cancer (n=4)
Case 1
A 27-year-old African American (AA) female presented with self-palpable right breast mass. No significant past medical or family history was noted. Right breast ultrasound and magnetic resonance imaging (MRI) showed multiple masses (largest diameter 2.1 cm). A positron emission tomography-computed tomography (PET-CT) scan showed heterogeneous uptake in at least 4 foci in the right breast, axillary, subpectoral, and interpectoral lymph nodes. Core biopsy of the right breast revealed poorly differentiated invasive ductal carcinoma, ER+ (99%), PR+ (85%), and HER2 3+ by immunohistochemistry. She underwent genetic counseling with BreastNext analysis and she was found to be heterogeneous for the p.R248W (c.742C>t) pathogenic mutation in the TP53 gene, consistent with the diagnosis LFS. She received neoadjuvant chemotherapy with docetaxel, carboplatin, trastuzumab, and pertuzumab for 6 cycles, with good clinical response evidenced by posttreatment MRI. She underwent a right modified radical mastectomy plus sentinel lymph node biopsy (SLNB) and left prophylactic mastectomy. Surgical pathology specimen revealed ypT1bN0 breast cancer. She completed 52-week trastuzumab therapy; however, she did not receive adjuvant radiation. She is currently on ovarian function suppression plus anastrozole and she is undergoing annual surveillance with whole-body MRI without clinicoradiologic evidence of cancer recurrence. Her 4-year-old son was recently diagnosed with a left temporal high-grade neuroepithelial tumor, which required surgical resection; his genetic testing revealed the same deleterious TP53 mutation as mother.
Case 2
A 21-year-old Indian female presented with a 4 cm self-palpable lump in the upper inner quadrant of the left breast. No significant family history or past medical history was noted. She underwent diagnostic mammogram and MRI of the left breast, which revealed a 5.2 cm lesion at 2 cm from the nipple, and biopsy of the same showed ER/PR/HER2 negative poorly differentiated infiltrating ductal carcinoma with admixed lymphoid component. A PET-CT scan revealed hypermetabolic left breast with necrotic central necrosis and mild uptake in ipsilateral axillary lymph node. She was evaluated for inherited mutations and was found to be heterozygous for pathogenic mutation in p.T155N (c.464C>A) variant in TP53 gene, consistent with a diagnosis of LFS. She was started on neoadjuvant chemotherapy with weekly paclitaxel and showed clinically progressive disease after the second dose; therefore, treatment was switched to dose-dense doxorubicin/cyclophosphamide for 6 cycles. She had excellent clinical response and underwent modified radical mastectomy with SLNB. She achieved complete pathologic response (ypT0N0) and received proton beam therapy to the left chest wall. She is currently disease-free and follows annual surveillance with breast MRI. Family members have not undergone genetic testing.
Case 3
A 16-year-old AA female presented with right shoulder osteosarcoma and received neoadjuvant therapy with methotrexate, doxorubicin, and cisplatin followed by surgical resection. At age 17, she experienced sarcoma recurrence as a solitary lung mass and underwent surgical resection followed by therapy with IGF-1R antibody for a year in a clinical trial setting. She declined genetic counseling at that time. At age 19, she presented with a self-palpable right breast lump. Diagnostic mammogram revealed a 3.1 cm solid lesion in the upper inner quadrant of the right breast. PET-CT scan revealed a large hypermetabolic right breast mass with no metastases and small nonavid lymph nodes in the right axilla. Core biopsy of the right breast mass revealed ER/PR/HER2 negative poorly differentiated ductal carcinoma. Due to prior anthracycline treatment, she received neoadjuvant carboplatin/paclitaxel for 6 cycles with clinical progression; therefore, she underwent right mastectomy with SLNB (ypT3N0) and a prophylactic contralateral mastectomy (). Given suspicion for LFS and its associated postradiation sarcoma risk, she did not receive adjuvant radiation, but was treated with adjuvant docetaxel/cyclophosphamide for 4 cycles. At age 20, she was found to have recurrent triple-negative breast cancer in the right axillary lymph nodes and lungs. The patient finally agreed to have genetic counseling, and BreastNext analysis found a heterozygous 5′UTR_3′UTR pathogenic mutation in the TP53 gene, consistent with the diagnosis of LFS. Multidisciplinary discussion recommended 5FU/doxorubicin/cyclophosphamide and dexrazoxane for cardioprevention, given to her prior to anthracycline exposure during sarcoma treatment. After 2 cycles, she developed severe pancytopenia, for which she underwent a bone marrow biopsy and aspirate showed 8.9% CD34+ and/or CD117+myeloblasts, partial loss of CD10 expression on 36.3% mature granulocytes, and ~25% erythroid precursor cells expressing CD71 and CD235. Cytogenetics revealed 5q deletion and monosomy 7, diagnosis consistent with myelodysplastic syndrome (MDS-RAEB). She was started on azacitidine. Unfortunately, she had progression with her lung metastases and succumbed to the disease 10 months after breast cancer recurrence. The patient had a paternal aunt with breast cancer diagnosed at age 50 years (unknown treatment) and maternal great grandmother with breast cancer diagnosed at an unknown age; however, her family members have not undergone genetic counseling.
Case 4
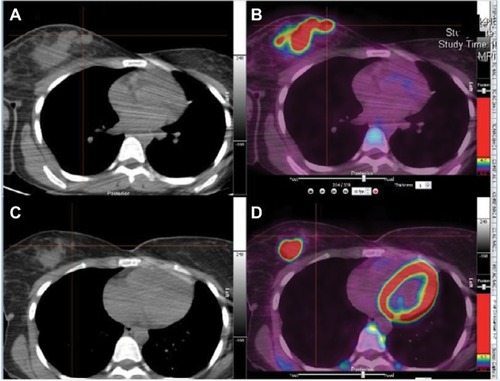
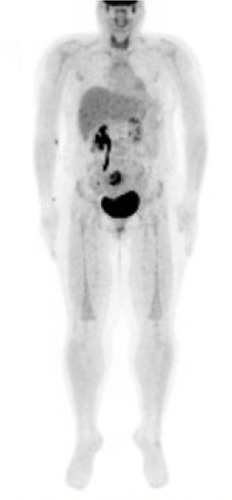
A 28-year-old Hispanic female presented with left breast malignant phyllodes tumor and was treated with surgical resection. At age 29 she noticed a right breast mass with right breast diagnostic mammogram and MRI revealing a 1.7 cm mass at the upper outer quadrant and prominent right axillary nodes. Breast mass core biopsy showed invasive mammary carcinoma, high nuclear grade with lymphoplasmacytic infiltrate, ER/PR/HER2 negative. She was referred for genetic counseling and was found to have a p.H193R TP53 pathogenic mutation compatible with LFS. She underwent right simple mastectomy with SLNB revealing a pT2N1mi ductal carcinoma. She then received adjuvant dose-dense doxorubicin/cyclophosphamide for 4 cycles and weekly paclitaxel for 12 cycles, followed by adjuvant radiotherapy to the chest wall. At age 33, a follow-up mammogram revealed a new left upper outer quadrant mass. Breast mass biopsy was consistent with highly cellular spindle cell neoplasm with moderate atypia. She underwent left mastectomy with SLNB and the surgical specimen was consistent with spindle cell sarcoma arising from the patient’s prior phyllodes tumor. At age 36, annual surveillance with PET-CT revealed avid uptake in the uterus (). Given the diagnosis of LFS and concern for malignancy, she underwent total abdominal hysterectomy plus bilateral oophorectomy which revealed a 5 cm high-grade leiomyosarcoma (pT1aNx). Eighteen months later, a surveillance computed tomography (CT) of the chest showed multiple subcentimeter nodules in both lungs; a biopsy revealed metastatic leiomyosarcoma and she was started on gemcitabine/docetaxel for 8 cycles. Most recent imaging revealed stable disease. Of note, her daughter was recently diagnosed with rhabdomyosarcoma at age 3 years.
Discussion and review of literature
Epidemiology of breast cancer in LFS
In adults, breast cancer is the most common cancer among female patients with LFS, which is present in up to 79% of affected TP53 mutation carriers, followed by STS (27% of mutation carriers). A study revealed that none of the 59 male patients with TP53 mutations were found to have breast cancer, suggesting a correlation of breast cancer risk only for females. Most patients are found to have HER2-amplified (55%) or ER+/PR+/HER2+ (37%) tumors,Citation16 and contralateral disease is present in ~31% patients with LFS. The annual hazard rate of breast cancer in LFS increases significantly after the second decade and peaks around age 40. A recent study revealed that the cumulative incidence of breast cancer iŝ85% by age 60, which is comparable to the cumulative incidence for BRCA 1/2 mutation carriers.Citation13 In our study, all our patients presented with early-onset breast cancer (before age 30 years), 3 patients had triple-negative disease, and 1 patient had ER+, PR+, HER2+ disease, and all of them revealed poorly differentiated features. These differences compared to other studies could be explained by our small sample size and diverse ethnical/racial population (2 AA, 1 Indian, and 1 Hispanic).
Breast cancer screening and work-up
National Comprehensive Cancer Network (USA) guidelines for breast cancer screening in LFS recommend clinical breast exam and breast screening with annual MRI ± mammogram until age 75 and consideration of risk-reducing mastectomy.Citation17 The work-up for patients with LFS should follow standard guidelines. Emphasis should be made that teenagers may benefit from sonogram and/or breast MRI due to dense breast tissue, while young adults would require mammogram and/or breast MRI. In our study, none of the patients was diagnosed by screening mammogram/MRI, since they were diagnosed with LFS after breast cancer presentation. Most patients required breast MRI and PET-CT due to dense tissue and/or locally advanced disease.
Surgical considerations in breast cancer and LFS
Several studies have demonstrated similar mortality rates when comparing total mastectomy to breast conserving surgery followed by radiotherapy in the management of early breast cancer.Citation18 Surgical local treatment in patients with LFS should follow the same guidelines as in the general population based on the clinicopathologic features of breast cancer and other medical comorbidities. However, in the absence of major contraindications, total mastectomy could be preferred over breast conserving surgery in order to avoid the need of adjuvant radiation, as radiation may predispose to secondary malignancies in patients with LFS. Axillary staging and management should follow standard guidelines. Due to the high risk for breast cancer in patients with LFS, discussion of risks and benefits of prophylactic mastectomy needs to be addressed in all cases. All of our patients were treated with mastectomy and 2 underwent prophylactic contralateral mastectomy. In our cohort, case 4 developed new contralateral breast sarcoma in the setting of a prior malignant phyllodes tumor and case 3 developed breast cancer recurrence in ipsi-lateral axillary lymph nodes despite bilateral mastectomies.
Adjuvant chemotherapy for breast cancer in LFS
Adjuvant chemotherapy with third-generation regimens, anthracycline–taxane based, has shown to reduce local and distant recurrence and decrease mortality bŷ30% in patients with early breast cancer.Citation19 Since TP53 is an important gene in the DNA damage response pathway, patients with LFS may be more susceptible to the carcinogenic effect of DNA-damaging drugs and ionizing radiation.Citation1 Moreover, literature suggests that patients with germline TP53 mutations have limited response to pre- or postoperative chemotherapy.Citation20,Citation21 Alkylating agents and anthracyclines induce p53-dependent apoptosis; therefore, their effect in patients with TP53 mutations might not be optimal, leading to treatment resistance.Citation22 Microtubule stabilizers, such as paclitaxel, act by inhibiting microspindle formation, independent of p53 involvement. Loss of cell cycle arrest due to p53 deficiency may allow cells to undergo S phase with more effective mitosis, thus making paclitaxel a potential better chemotherapy option for these patients.Citation22 In our study, case 2 developed rapid clinical progression after 2 cycles of paclitaxel, but achieved good clinical response after switching to doxorubicin/cyclophosphamide. Case 3 had clinical progression during neoadjuvant carboplatin/paclitaxel and also had recurrence despite adjuvant docetaxel/cyclophosphamide; she also developed MDS that may have developed due to prior anthracycline use. Her MDS was very aggressive, of high risk, and was poorly responsive to azacitidine. Chemotherapy regimen should be selected based on a case-by-case approach. We recommend that these patients should be treated with the regimen that offers the best improvement in breast cancer survival, such as anthracycline–taxane-based regimens, since the patients are at increased risk for carcinogenesis leading to breast cancer recurrence; also, they should be followed closely for the potential development of secondary malignancies associated with chemotherapy.
Adjuvant radiation for breast cancer in LFS
Radiotherapy is an important adjuvant therapy for early breast cancer, which reduces the 10-year recurrence rate (absolute reduction 15.7%) and the 15-year mortality rate (absolute reduction 3.8%).Citation23 Radiation-induced malignancies are rare, with an incidence ~2% and a latency period of 10–15 years after radiation treatment.Citation24,Citation25 Patients with LFS treated with radiation have increased risk of developing sarcomas or leukemias and a shorter latent period.Citation26 Limacher et al, in an anecdotal report, observed the development of metachronous cancers in the irradiated field of breast cancers in a patient with LFS.Citation27 Heymann et al reported that among 6 patients with LFS who received adjuvant radiotherapy, 11 events occurred (3 ipsilateral breast recurrences, 3 contralateral breast cancers, 2 radiation-induced cancers, and 3 new primaries).Citation24 p53-deficient cells are unable to arrest cell cycle or undergo apoptosis following radiation; moreover, these cells have increased resistance to DNA-damaging agents such as radiation.Citation28 Mice studies have shown that p53 plays a major role in tumor suppression by preserving genomic integrity through G1 arrest or apoptosis in response to radiation-induced damage to cells. A few mice models have reiterated that low-dose radiation in p53-deficient mice decreases the tumor latency with development of early-onset sarcomas and lymphomas.Citation29 Several case reports in LFS patients treated with radiation have shown rapid sarcoma development.Citation30,Citation31 In our cohort, in order to avoid surrounding tissue damage, case 2 received proton beam radiation and she remains disease-free; case 4 received ionizing radiation and developed contralateral breast sarcoma, and subsequent follow-up revealed metastatic uterine leiomyosarcoma. Given the risk of treatment-related complications, radiation as adjuvant therapy should be given careful consideration in LFS patients and offered in a case-by-case fashion.
Cancer surveillance in LFS
Patients with LFS and LFL syndrome are predisposed to a variety of cancers in their lifetime. Surveillance would be beneficial to detect early malignancies in asymptomatic patients. Masciari et al tested the use of F18-fluorodeoxyglucose-PET/CT as a potential screening tool. Among 15 asymptomatic LFS patients, they detected 2 papillary thyroid carcinomas and 1 esophageal carcinoma; all of the cancers were early stage and treated with curative intent.Citation32 However, the amount of radiation exposure in LFS patients using this screening strategy was concerning. The National Institutes of Health LFS study enrolled 100 patients who underwent blood tests, abdominal ultrasound, annual brain MRI, annual rapid whole-body MRI, annual breast MRI/mammogram, and colonoscopy every 3 years and identified 6 cancers (all identified by MRI), leading to a cancer detection rate of 6% and false-positive rate of 79%.Citation33 The multimodal Toronto protocol evaluated surveillance for adrenocortical carcinoma (abdominal ultrasound and blood/urine biomarkers), breast cancer (annual mammogram and MRI, breast self-exam, clinical breast exam), brain tumor (annual brain MRI), soft-tissue and bone sarcoma (annual rapid whole-body MRI, abdominal ultrasound), leukemia/lymphoma(complete blood count, erythrocyte sedimentation rate, lactate dehydrogenase), colorectal cancer (colonoscopy every 2 years), and melanoma (annual dermatologic exam) along with a general assessment (physical exam every 3–4 months). Among 39 families, 40 patients underwent surveillance and 49 declined surveillance (however, 19 patients then crossed to the surveillance group). With 90%–100% compliance rate to surveillance protocol, they identified 40 asymptomatic neoplasms in 19 of 59 patients who underwent surveillance and 61 symptomatic neoplasms among 43 of 49 patients in the nonsurveillance group. Eighty-four percent of individuals in the surveillance group were alive at 38 months, while only 49% in the nonsurveillance group were alive at 46 months. The 5-year overall survival rates were 88.8% and 59.6% for the surveillance and nonsurveillance groups, respectively.Citation34 Other studies are currently evaluating different screening strategies,Citation15 such as the LIFESCREEN French project (NCT01464086), the SIGNIFY trial in the UK (NCT01737255), US NIH (NCT01443468), Dana–Farber Cancer Institute study (NCT02950987), and the Australian surveillance study in multiorgan cancer prone syndromes (ACTRN12613000987763).
In our study, PET-CT surveillance for case 4 identified a stage I uterine leiomyosarcoma, which was treated with surgery but the patient developed metastatic disease later in the course. Case 1 recently started annual whole-body MRI surveillance without identification of any malignancy. Currently published recommendations for LFS cancer screening include National Comprehensive Cancer Network guidelines, National Institute for Health and Care Excellence guidelines, and eviQ cancer treatments online (). Available data suggest that an intensive screening strategy should be offered to patients with LFS. MRI-based screening seems to be an attractive option, given the absence of radiation exposure. Since no randomized trial has been reported, along with the psychologic stress of false-positive findings requiring invasive procedures, we suggest that cancer screening should be preferably performed in a clinical trial context.
Table 3 Cancer screening recommendations for patients with LFS
Conclusion
We report a cohort of 4 patients in different families, representing a heterogeneous racial/ethnical background, without significant prior family history suggesting TP53 mutations. Triple-negative disease was the most common histology, and all patients were treated with third-generation breast chemotherapy regimens and/or trastuzumab-containing regimens. Three patients are alive without breast cancer recurrence. Proton beam radiation was well tolerated in 1 patient, which was administered to limit exposure of radiation to the surrounding tissue. Risk–benefit assessment of chemotherapy and radiotherapy needs careful consideration due to the risk of treatment resistance and associated secondary malignancies. Cancer screening should be discussed in patients with LFS, and multimodality strategy in the context of a clinical trial should be preferred.
Author contributions
AGN helped in acquisition of relevant literature, data acquisition, drafting the manuscript, revising critical and intellectual content, tables creation, tables editing, figure creation and editing and final approval of the version to be published. SV contributed to acquisition of relevant literature, data acquisition, drafting the manuscript, final approval of the version to be published. JA carried out conception and design of the work, acquisition of relevant literature, data acquisition, manuscript writing, manuscript editing, revising critical and intellectual content, tables creation, tables editing, figure creation and editing, and final approval of the version to be published.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The research described was supported by NIH/National Center for Advancing Translational Science (NCATS) Ein-stein–Montefiore CTSA Grant Number UL1TR001073 and the Einstein Paul Calabresi Career Development Program (NIH 5K12CA132783-08)
References
- de BruinMAFordJMKurianAWA young woman with bilateral breast cancer: identifying a genetic cause and implications for managementJ Natl Compr Canc Netw201311551251723667202
- DamineniSRaoVRKumarSGermline mutations of TP53 gene in breast cancerTumour Biol20143599219922724929325
- XuJQianJHuYHeterogeneity of Li-Fraumeni syndrome links to unequal gain-of-function effects of p53 mutationsSci Rep20144422324573247
- ChompretABrugieresLRonsinMP53 germline mutations in childhood cancers and cancer risk for carrier individualsBr J Cancer200082121932193710864200
- LiFPFraumeniJFJrRhabdomyosarcoma in children: epidemiologic study and identification of a familial cancer syndromeJ Natl Cancer Inst1969436136513735396222
- LiFPFraumeniJFJrMulvihillJJA cancer family syndrome in twenty-four kindredsCancer Res19884818535853623409256
- MalkinDLiFPStrongLCGerm line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasmsScience19902504985123312381978757
- WuCCKraheRLozanoGJoint effects of germ-line TP53 mutation, MDM2 SNP309, and gender on cancer risk in family studies of Li-Fraumeni syndromeHum Genet2011129666367321305319
- YaoZSherifZAThe effect of epigenetic silencing and TP53 mutation on the expression of DLL4 in human cancer stem disorderOncotarget2016739629766298827542210
- BirchJMHartleyALTrickerKJPrevalence and diversity of constitutional mutations in the p53 gene among 21 Li-Fraumeni familiesCancer Res1994545129813048118819
- EelesRAGermline mutations in the TP53 geneCancer Surv1995251011248718514
- TinatJBougeardGBaert-DesurmontS2009 version of the Chompret criteria for Li Fraumeni syndromeJ Clin Oncol20092726e108e10919652052
- MaiPLBestAFPetersJARisks of first and subsequent cancers among TP53 mutation carriers in the National Cancer Institute Li-Fraumeni syndrome cohortCancer2016122233673368127496084
- GonzalezKDNoltnerKABuzinCHBeyond Li Fraumeni Syndrome: clinical characteristics of families with p53 germline mutationsJ Clin Oncol20092781250125619204208
- McBrideKABallingerMLKillickELi-Fraumeni syndrome: cancer risk assessment and clinical managementNat Rev Clin Oncol201411526027124642672
- BougeardGRenaux-PetelMFlamanJMRevisiting Li-Fraumeni syndrome from TP53 mutation carriersJ Clin Oncol201533212345235226014290
- NCCN guidelines (version 2.2017)2017 Available from: https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdfAccessed March 2, 2017
- JatoiIProschanMARandomized trials of breast-conserving therapy versus mastectomy for primary breast cancer: a pooled analysis of updated resultsAm J Clin Oncol200528328929415923803
- AnampaJMakowerDSparanoJAProgress in adjuvant chemotherapy for breast cancer: an overviewBMC Med20151319526278220
- KappelSJanschekEWolfBTP53 germline mutation may affect response to anticancer treatments: analysis of an intensively treated Li-Fraumeni familyBreast Cancer Res Treat2015151367167825981898
- LoweSWRuleyHEJacksTHousmanDEp53-dependent apoptosis modulates the cytotoxicity of anticancer agentsCell19937469579678402885
- Kandioler-EckersbergerDLudwigCRudasMTP53 mutation and p53 overexpression for prediction of response to neoadjuvant treatment in breast cancer patientsClin Cancer Res200061505610656431
- DarbySMcGalePCorreaCEffect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trialsLancet (London, England)2011378980417071716
- HeymannSDelalogeSRahalARadio-induced malignancies after breast cancer postoperative radiotherapy in patients with Li-Fraumeni syndromeRadiat Oncol2010510421059199
- RubinoCde VathaireFShamsaldinALabbeMLeMGRadiation dose, chemotherapy, hormonal treatment and risk of second cancer after breast cancer treatmentBr J Cancer200389584084612942115
- HisadaMGarberJEFungCYFraumeniJFJrLiFPMultiple primary cancers in families with Li-Fraumeni syndromeJ Natl Cancer Inst19989086066119554443
- LimacherJMFrebourgTNatarajan-AmeSBergeratJPTwo meta-chronous tumors in the radiotherapy fields of a patient with Li-Fraumeni syndromeInt J Cancer200196423824211474498
- LeeJMAbrahamsonJLKandelRDonehowerLABernsteinASusceptibility to radiation-carcinogenesis and accumulation of chromosomal breakage in p53 deficient miceOncogene1994912373137367970733
- KempCJWheldonTBalmainAp53-deficient mice are extremely susceptible to radiation-induced tumorigenesisNat Genet19948166697987394
- ChadazTHobbsSKSonHChest wall sarcoma: 18F-FDG PET/CT in a patient with Li-Fraumeni syndromeClin Nucl Med2013381081882024107814
- SalmonAAmikamDSodhaNRapid development of post-radiotherapy sarcoma and breast cancer in a patient with a novel germline ‘de-novo’ TP53 mutationClin Oncol (R Coll Radiol)200719749049317572079
- MasciariSVan den AbbeeleADDillerLRF18-fluorodeoxyglucose-positron emission tomography/computed tomography screening in Li-Fraumeni syndromeJAMA2008299111315131918349092
- MaiPLBaseline cancer screening findings from the NCI Li-Fraumeni syndrome study2016ASCO Annual meeting2016Chicago, IL, USA
- VillaniAShoreAWassermanJDBiochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: 11 year follow-up of a prospective observational studyLancet Oncol20161791295130527501770