Abstract
Breast cancer is one of the major causes of cancer-related deaths among women worldwide. Aberrant regulation of various growth factors, cytokines, and other proteins and their receptors in cancer cells drives the activation of various oncogenic signaling pathways that lead to cancer progression. Semaphorins are a class of proteins which are differentially expressed in various types of cancer including breast cancer. Earlier, these proteins were known to have a major function in the nerve cell adhesion, migration, and development of the central nervous system. However, their role in the regulation of several aspects of tumor progression has eventually emerged. There are over 30 genes encoding the semaphorins, which are divided into eight subclasses. It has been reported that some members of semaphorin classes are antiangiogenic and antimetastatic in nature, whereas others act as proangiogenic and prometastatic genes. Because of their differential expression and role in angiogenesis and metastasis, semaphorins emerged as one of the important prognostic factors for appraising breast cancer progression.
Introduction
Breast cancer accounts for one of the largest causes of morbidity and mortality among women globally.Citation1 Intricate signaling pathways regulated by an array of growth factors, cytokines and other proteins are known to be involved in the progression of the incipient neoplasm to higher grades of breast tumors.Citation2 Advanced stages of breast cancer are mostly untreatable due to its aggressive nature and lack of effective therapies for the heterogeneous disease. It takes several measures toward identifying the diagnostic and prognostic factors and methods for detection and prevention of cancer at early stages of the disease. Differentially expressed genes/proteins between cancer and adjacent normal/healthy breast tissues are routinely used for the diagnosis and prognosis of breast cancer. Proteomic and microarray studies have identified various genes that are differentially expressed among normal and different grades of breast cancer.Citation3,Citation4 Semaphorins are a class of one such proteins that are differentially expressed in normal vs. different grades of breast cancer patients.Citation5,Citation6 Semaphorins are a class of secreted, membrane-bound, or glycophosphatidylinositol-anchored glycoproteins and are characterized by the presence of a sema and plexin-semaphorin integrin (PSI) domains. Semaphorins were originally discovered as axon guidance cues for the developing nervous system.Citation7 However, the role of semaphorins in regulation of several hallmarks of cancer has been eventually recognized. Semaphorins exert their tumor modulatory function by binding to receptors, plexins, and neuropilins, or holoreceptor complexes associated with plexins/neuropilins such as integrins and receptor tyrosine kinases (RTKs) such as C-Met and ErbB2. By binding to these receptors, semaphorins regulate various downstream signaling molecules such as extracellular signal-regulated kinases1/2 (ERK1/2), Akt, phosphatase and tensin homolog (PTEN), and Rho-associated protein kinase, leading to cancer cell survival, angiogenesis, and metastasis.Citation8–Citation10 Mishra et al have shown that Sema3A inhibits the tumor growth and angiogenesis by inducing MelCAM expression.Citation11 Several reports suggest that semaphorins regulate angiogenesis and metastasis by competing with vascular endothelial growth factor (VEGF) family members for neuropilin binding.Citation12–Citation14 Semaphorins also induce the epithelial to mesenchymal transition (EMT) to increase the migratory and invasive potentials of breast cancer cells.Citation15 Differences in semaphorin expression can be used clinically to predict the breast cancer subtype, disease progression, and patient’s survival.Citation6,Citation16–Citation18 Based on the clinical relevance of semaphorin expression and its critical role in disease progression, semaphorins emerged as one of the intriguing therapeutic targets for breast cancer management. In this review, we summarize the clinical relevance of semaphorin expression and the pro- and antiangiogenic and metastatic effects of semaphorins in breast cancer progression.
General structure and classification of semaphorins
Twenty different types of semaphorins in humans, five in Drosophila, and two in DNA viruses have been identified. To simplify the understanding of the biology of these semaphorins, semaphorins have been categorized into eight subclasses on the basis of their structural elements and amino acid sequence homology. Out of the eight subclasses, class 1 and 2 semaphorins are present in invertebrates; classes 3, 4, 6, and 7 semaphorins are only expressed in vertebrates; and the eighth group (class V, where V stands for the virus) contains semaphorins that are encoded by viral genomes (). However, class 5 semaphorins are expressed in both invertebrates and vertebrates. In the current unified nomenclature of semaphorins, the abbreviation Sema is followed by a number indicating the subclass and a capital letter designating the individual member (eg, Sema3A).Citation19 All semaphorins possess a conserved sema domain, consisting of 500 amino acids at the N-terminal region ().Citation20 The sema domain constitutes distinctive structural and functional element of semaphorins and is responsible for various functions.Citation21 Interestingly, the sema domain is also present in some of the semaphorin receptors such as plexins and RTKs such as MET and RON ().Citation22,Citation23 Semaphorin, MET, and RON axes are known to play a major role in the development, tissue regeneration, and carcinogenesis.Citation24,Citation25 The structure of the sema domain is a seven-blade β-propeller fold that shows complete structural similarity to the extracellular domain of α-integrin.Citation20,Citation25 Nevertheless, studies on crystal structures suggest that the mode of dimerization and the regions of the domain involved in ligand–receptor interactions are considerably different among these families.Citation23 Next to the sema domain, semaphorins contain a cysteine-rich PSI domain, which is also referred as a MET-related sequence ().Citation26 Semaphorins also harbor other distinctive protein domains such as basic charged C-terminal domain, thrombospondin repeats, and immunoglobulin (Ig)-like domains. Class 3 semaphorins are characterized by a conserved, basic charged domain at the C-terminal region and these are secreted semaphorins ().Citation10 Class 4–7 semaphorins are cell membrane-anchored proteins that are characterized by their distinct structural elements. Thrombospondin repeats are present in case of class 5 semaphorins, whereas a glycophosphatidylinositol anchor is present in class 7 semaphorins (). Membrane-anchored semaphorins can be further processed into soluble forms through the proteolytic cleavage at a specific site as in the case of class 4 and 7 semaphorins by ADAMTS1 and furin-like proprotein convertase (FPPC; ).Citation27,Citation28
Figure 1 Graphic representation of semaphorins and their receptors.
Abbreviations: FPPCs, furin-like proprotein convertase; GAPs, GTP-ase activating proteins; GPI, glycophosphatidylinositol; IPT, Ig-like fold shared by plexin and transcription factors; PSI, plexin-semaphorin integrin; RTKs, receptor tyrosine kinases; VEGFR, vascular endothelial growth factor receptor.
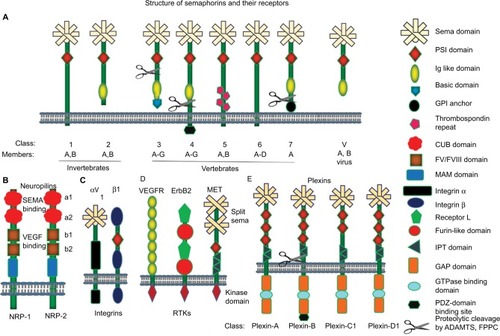
Semaphorins bind to plexins and neuropilins to exert their functions through the activation of downstream signaling molecules.Citation29,Citation30 Plexins are high-affinity receptors for semaphorins and expressed in both vertebrates and invertebrates. The genome of invertebrates contains two plexin genes, whereas the genome of vertebrates harbors nine plexin genes. Plexins are divided into four subfamilies, which are plexin-A (1–4), plexin-B (1–3), plexin-C1, and plexin-D1 (). Plexin contains a sema and PSI domains as similar to their ligands, semaphorins, along with three distinctive Ig-like fold shared by plexin and transcription factors domains. However, plexins lack the homology to any known proteins or functional motifs at the cytoplasmic tail.Citation31 The cytoplasmic tail possesses two stretches of amino acids that are rarely similar to GTPase activating proteins ().Citation32 Neuropilins act as obligate coreceptors for class 3 semaphorins and these are only expressed in vertebrates. Neuropilins are divided into two subclasses, NRP1 and NRP2. Neuropilins, single-pass transmembrane proteins, were initially identified as coreceptors for class 3 semaphorins and VEGF family proteins.Citation33,Citation34 Neuropilin comprises two complement-like (CUB) domains and two FV/FVIII coagulation factor-like domains, which are useful for the binding to semaphorins and VEGF family members, respectively. In addition, neuropilins also exhibit meprin-like MAM domain, which is an evolutionarily conserved domain likely to have an adhesive function ().Citation35 Additionally, semaphorins engage with holoreceptor complexes associated with plexins and neuropilins, such as αVβ1integrin, and RTKs such as Met, ErbB2, and VEGFR2 ().Citation28
Semaphorin signaling in breast cancer
Semaphorins regulate several pleiotropic changes that are associated with tumor progression by influencing the behavior of tumor cells.Citation36 Except for Sema3E, all other members of class 3 semaphorins bind to plexin-A (1–4) only in the presence of their coreceptors, NRP1 and NRP2. However, they can also directly bind to NRPs to perform their various functions. Sema3E is known to exhibit its tumor-promoting function by binding to plexin-D1.Citation9,Citation30,Citation37,Citation38 Sema4D, another semaphorin, binds to plexin-B1 and B2, whereas Sema4C engages only with plexin-B2.Citation39,Citation40 Moreover, Sema5A is known to bind to plexin-B3, while Sema7A is recognized to bind to plexin-C1.Citation30,Citation41
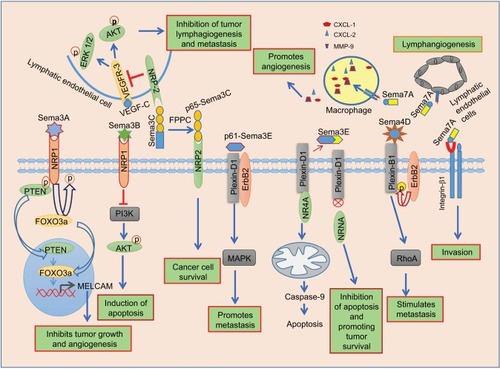
Sema3A stimulates the α2β1 integrin expression, and it ultimately leads to a reduction in the migratory and invasive potential of breast cancer cells.Citation42 However, recent studies have shown that Sema3A shows both promoting and inhibitory effects on breast cancer cell migration. Moreover, cell migration and spreading are influenced by the strength of cell–substratum interaction. Optimal concentration of substratum leads to increased migration and spreading of cells. Gehler et al have recently demonstrated that Sema3A increases the cancer cell migration and spreading even at a low concentration of the substratum (ECM coating with collagen and fibronectin) by inducing FAK phosphorylation at the focal adhesions in Rho-associated protein kinase-dependent manner, while it inhibits the migration at a high concentration of the substratum.Citation43 Mishra et al have shown that Sema3A suppresses the breast tumor growth and angiogenesis through PTEN/FOXO 3a axis-dependent MelCAM expression by binding to the receptor, NRP1 ().Citation11 Sema3A inhibits VEGF-induced activation of ERK1/2 without efficiently disturbing VEGF-induced phosphorylation of VEGFR2 in endothelial cells.Citation44 Acevedo et al have revealed that Sema3A suppresses VEGF-induced angiogenesis by interrupting VEGF-mediated FAK and Src signaling in endothelial cells.Citation45 Moreover, Sema3A shows a causative role in cancer cell metastasis to the bone by stimulating osteoblastic differentiation.Citation46 Another member of class 3 semaphorins, Sema3B, also acts as a tumor suppressor in breast cancer. Sema3B suppresses tumor proliferation and induces apoptosis in NRP1-positive breast cancer cells by inactivating PI3K/Akt signaling ().Citation47 Sema3B and VEGF165 act as antagonists to each other in the regulation of apoptosis in breast cancer cells. This might be due to the competitive binding of Sema3B and VEGF165 to the NRP1 receptor.Citation14 Shahi et al have further shown that Sema3B is a direct transcriptional target of GATA3 and suppresses breast cancer metastasis by interfering with the phosphorylation and activation of LIM kinases (LIMK1 and LIMK2).Citation48 The other members of class 3 semaphorins, such as Sema3C and Sema3E, are overexpressed in breast cancer cells and exhibit tumor-promoting function. Zhu et al have shown that siRNA-mediated knockdown of Sema3C in breast cancer cells abolishes cell proliferation and migration.Citation49 Interestingly, the p65-Sema3C fragment that is generated from cleavage of full-length Sema3C by FPPC shows tumor-promoting role. Full-length Sema3C shows inhibitory effect on lymphangiogenesis and metastasis in mice breast tumor xenografts ().Citation50 Nonetheless, the metalloprotease ADAMTS1 induces Sema3C cleavage from ECM and converts it to a soluble form, so that it diffuses and promotes tumor cell migration.Citation51 The role of Sema3C in regulation of tumor progression also depends on the type and nature of cancers. For example, Sema3C promotes pancreatic cancer progression through ERK1/2 signaling pathway.Citation52 However, the molecular mechanism by which Sema3C promotes breast cancer progression is unclear. Sema3E suppresses the apoptotic cell death in metastatic breast cancer cells by blocking plexin-D1–mediated NR4A1 pathway through binding and sequestering plexin-D1 ().Citation53 Cleaved p61-Sema3E promotes tumor metastasis through plexin-D1/ErbB2-dependent MAPK signaling ().Citation38 Other members of semaphorin family such as Sema4A, Sema4C, Sema4D, and Sema7A show tumor-promoting function in breast cancer. Sema4C promotes breast cancer cell proliferation and migration through plexin-B2/Met-dependent RhoA signaling axis. In addition, Sema4C induces migration and tube formation of lymphatic endothelial cells (LECs) and, thereby, lymphangiogenesis through plexin-B2/ErbB2-dependent RhoA signaling.Citation54 Tumor-derived Sema4D promotes bone metastasis by inhibiting bone deposition and, inducing osteoclastogenesis through the plexin-B1-dependant interleukin-8 secretion.Citation55 Effect of Sema4D/plexin-B1 signaling on tumor cell migration depends on their interaction with RTKs, ErbB2, and MET. Interaction of plexin-B1 with MET suppresses cellular migration, whereas interaction with ErbB2 increases the migration by activating the small GTPase, RhoA ().Citation56–Citation58 Sema7A promotes tumor growth and invasion in breast cancer through activation of integrin β1signaling.Citation59 Garcia-Areas et al have observed that cancer cell-derived Sema7A drives the macrophages toward tumor-promoting phenotype, and these macrophages promote angiogenesis by producing proangiogenic molecules such as CXCL1, CXCL2, and matrix metalloproteinase-9 ().Citation60
Figure 2 Semaphorin signaling in breast cancer.
Abbreviations: ERK1/2, extracellular signal-regulated kinases1/2; FPPC, furin-like proprotein convertase; MMP, matrix metalloproteinase; PTEN, phosphatase and tensin homolog; VEGFR, vascular endothelial growth factor receptor.
Role of semaphorins in breast cancer progression
Semaphorins regulate various hallmarks of cancer by binding to different types of receptors. Various pathophysiological functions regulated by semaphorins in breast cancer are listed in .
Table 1 Semaphorins, their receptors, and pathologic functions in breast cancer
Role of semaphorins in breast tumor angiogenesis
Angiogenesis is a process in which sprouting of new blood vessels takes place in order to supply nutrients to rapidly growing tumors. As the tumor grows rapidly, the core part of solid tumor undergoes O2 and nutrient deprivation, a phenomenon known as hypoxia. Hypoxia is the major driving force in inducing tumor angiogenesis by regulating the expression of proangiogenic genes such as VEGF, HIF-1, and so on and enriching cancer stem-like phenotype in the tumor microenvironment.Citation61,Citation62 It has also been reported that breast cancer stem-like cells undergo transdifferentiation to endothelial cells to support angiogenesis, which is termed as vasculogenic mimicry.Citation63 Various growth factors and cytokines are known to regulate angiogenesis. Semaphorins play crucial roles in angiogenesis directly or indirectly by regulating VEGF/VEGFR axis.Citation12–Citation14 Some members of semaphorins, such as Sema4D and Sema7A, are the positive regulators of angiogenesis, whereas the members of class 3 semaphorins are shown to have an antiangiogenic role in breast cancer.Citation8,Citation60 Especially, Sema3A, Sema3B, Sema3E, and Sema3F exhibit antiangiogenic properties and thereby inhibit tumor progression.Citation8,Citation9 It has been reported that class 3 semaphorins inhibit angiogenesis by competing with angiogenic factors, such as members of VEGF family, for binding to neuropilins.Citation12,Citation13 Recently, Mishra et al have reported the mechanism by which Sema3A attenuates tumor growth and angiogenesis by inducing the expression of tumor suppressor gene, MelCAM, in breast cancer model. Their studies have shown that Sema3A induces the expression of MelCAM through NRP1-mediated PTEN-dependent FOXO 3a activation.Citation11 Casazza et al have shown that overexpression of Sema3A inhibits the vessel formation and increases tumor hypoxia and necrosis in an in vivo mice model.Citation64 Earlier reports have suggested that cleaved Sema3C (p65-Sema3C) is formed from full-length Sema3C by the action of FPPC. Cleaved Sema3C (p65-Sema3C) is required for the survival of NRP2-expressing tumor cells, whereas furin cleavage-resistant Sema3C (FR-Sema3C) is shown to inhibit lymphangiogenesis and metastasis.Citation50 Cole-Healy et al have found the positive correlation between Sema3C expression and microvessel density (CD31) by immunohistochemical analysis. These studies have shown that the expression of Sema3C is more in endothelial cells of premalignant tissues, suggesting the role of Sema3C in angiogenesis during tumor development.Citation16 Jiang et al have revealed that downregulation of Sema4D decreases the tumor growth and angiogenesis.Citation65 Tumor-associated macrophages are the major stromal cells that secrete Sema4D in the tumor microenvironment. Tumor-associated macrophage–derived Sema4D contributes to breast cancer angiogenesis and tumor progression.Citation66 Another member of semaphorin family, Sema7A, is shown to be upregulated by the proangiogenic molecule, COX-2. Sema7A mediates COX-2-induced lymphangiogenesis by activating β1-integrin signaling.Citation59 In addition, Sema7A has been found to induce macrophages to produce proangiogenic molecules such as CXCL2/MIP-2 in an orthotopic breast cancer model.Citation60 These reports imply the possible role of semaphorins in tumor–stroma interaction in breast cancer.
Role of semaphorins in the invasion and metastasis of breast cancer
Metastasis is mostly responsible for the cancer-related deaths in different types of cancers.Citation67 Cancer cells disseminate to various parts of the body through the blood circulation upon acquiring mesenchymal phenotype by a phenomenon known as EMT. Epithelial cancer cells lack the motility and invasion potentials. Hence, the epithelial cells undergo EMT in order to acquire mesenchymal stem-like phenotype and obtain migration and invasion potentials.Citation68 Breast cancer cells are highly metastatic to lungs and bone, depending on the subtype and the hormone receptor status.Citation67 Semaphorins are known to play a key role in breast cancer cell dissemination. A recent report has revealed that Sema3C expression is associated with breast cancer cell proliferation and migration. The study has demonstrated that silencing Sema3C using siRNA resulted in suppression of proliferation and migration of estrogen receptor (ER)+ve breast cancer cells, MCF-7.Citation49 Malik et al have found that Sema3C downregulation reduces the cell adhesiveness and the invasion of human breast cancer cells such as MCF-7 and MDA-MB-231.Citation6 It has shown that hypoxia downregulates the expression of Sema3A. Hypoxia-regulated Sema3A is known to be involved in the regulation of osteoblast differentiation.Citation69 It has been reported that VEGF promotes the migration of cancer cells, whereas Sema3F inhibits this effect owing to the competitive binding of VEGF and Sema3F to the NRP1.Citation70 Retinoid orphan nuclear receptor alpha (RORα), a member of the orphan nuclear factor family, inhibits breast cancer cell invasion by enhancing Sema3F expression at the transcriptional level by binding to its promoter. Nuclear levels of RORα are correlated with Sema3F expression in human breast cancer. Moreover, higher grades of breast cancer are mostly double negative for RORα and Sema3F, compared to lower grades. Kaplan–Meier log-rank analyses of breast cancer tissue microarray containing >400 patients’ samples have revealed that patients with lower RORα and Sema3F have shorter survival rates.Citation71 Another member of class 3 semaphorin, Sema3E, is important for tumor progression and metastasis. Mouse mammary carcinoma cell line, 168FARN, gains the ability to metastasize to lungs upon overexpression of Sema3E. Conversion of full-length Sema3E into p61-Sema3E isoform is required for invasion, migration, and lung metastasis. p61-Sema3E isoform is also required for the activation of ERK signaling in endothelial cells.Citation72 Garcia-Areas et al have studied the role of Sema7A in tumor growth and metastasis using in vivo mouse model. In this study, they found that downregulation of Sema7A using shRNA decreases the proliferation and reduces the migration and invasion potential of 4T1 cells.Citation73 Reduction in breast cancer cell adhesion, invasion, and motility was observed upon silencing the expression of Sema7A in MCF10DCIS cells.Citation59 Allegra et al have studied the role of Sema7A in regulation of EMT as this process is involved in acquisition of metastatic potential. Their reports have shown that downregulation of Sema7A expression by the Ets2-repressor factor represses the EMT program in Ras-dependent mammary epithelial cells.Citation15 These reports indicate that Sema7A increases the migration of breast cancer cells by inducing EMT. Members of Sema4, such as Sema4C and Sema4D, are shown to be positive regulators of metastasis. Chen et al have evaluated the expression of Sema4C in 45 breast tumor specimens and identified higher Sema4C expression in lymph node metastatic specimens as compared to non-metastatic ones. Moreover, they have observed higher expression of Sema4C in metastatic breast cancer cell lines, MDA-MB-231 and MDA-MB-435S, as compared to low-metastatic breast cancer cells, MCF-7.Citation74 Secretory Sema4C enhances the migration of breast cancer cells and promotes tube formation and migration of LECs and thereby enhances the lymphangiogenesis and metastasis in breast cancer.Citation54 Wu et al have isolated the normal LECs and tumor-associated LECs from normal breast and cancer tissues using LCM after detecting these cells in tissue sections by rapid immunohistochemistry. Differentially expressed genes in these cells were analyzed by microarray, and it was found that Sema4C is highly expressed in tumor-associated LECs as compared to normal LECs.Citation75 It was shown that miR-125b has an important role in regulating paclitaxel resistance–induced EMT in breast cancer cells by targeting Sema4C. Downregulation of miR-125b and upregulation of Sema4C were observed in paclitaxel-resistant breast cancer cells.Citation76 It has been shown that knocking down the expression of Sema4D in MDA-MB-468 and MDA-MB-231 using shRNA reduces the proliferation, invasion, and migration of these cells. Downregulation of Sema4D also increases the apoptosis in these cells.Citation65 Furthermore, it has also been observed that Sema4D inhibits bone formation through interaction with plexin-B1 and stimulates osteoclastogenesis through the induction of interleukin-8 expression and thereby induces bone metastasis of breast cancer cells.Citation55 These results suggest the prometastatic role of Sema4D in breast cancer. Evans et al have shown that the expression of Sema4D is higher at the invasive margins of breast tumor where it influences the infiltration of monocytes and leukocytes into tumor microenvironment. Blocking Sema4D using mouse monoclonal antibody, MAb67, leads to tumor rejection in ErbB2+ve murine breast cancer model. Thus, targeting Sema4D in human breast cancer might be beneficial for the treatment of breast cancer. VX15/2503, a humanized monoclonal antibody to Sema4D, is in Phase I clinical trial for the treatment of solid tumors.Citation77 Various reports have shown that Sema3B is a tumor suppressor in several cancers including breast cancer. GATA3-induced Sema3B suppresses the breast tumor progression and metastasis by abrogating the phosphorylation and activation of LIMK1 and LIMK2.Citation48 Based on the above findings, semaphorins are proved to be crucial targets for the management of metastatic breast cancer.
Clinical relevance of semaphorin expression in breast cancer
Several reports on clinical studies suggested that semaphorin expression is correlated with disease progression, indicating the prognostic significance of semaphorins in breast cancer. Some members of semaphorin family are downregulated, whereas other members are overexpressed during breast cancer progression. It has been reported that the expression levels of Sema3A, Sema3B, and Sema3F are high in normal breast tissues as compared to invasive breast tumors, suggesting the tumor suppressor role of these semaphorins. Similarly, the expression of semaphorin receptor, plexin-A3, is also decreased in invasive breast cancer.Citation5 Studies on 119 human breast tumor specimens have revealed that the expression of plexin-B1, a receptor of Sema4D, is inversely correlated with the aggressiveness of this cancer. Moreover, plexin-B1 expression is positively correlated with the ER status of breast cancer.Citation78 Microarray dataset of 1086 breast cancer patients has revealed that plexin-B1 has a prognostic value in ER+ve breast cancer. The loss of plexin-B1 is associated with an increased expression of ErbB2 and the proliferation marker, Ki67, in ER+ve breast cancer. In addition, the loss of plexin-B1 expression is associated with poor prognosis in ER+ve breast cancer.Citation79 These reports emphasize the prognostic significance of plexin-B1 in ER+ve breast cancer. In another study, the data revealed that reduced expression of plexin-B1 is associated with poor disease-free survival in ErbB2−ve cancer, whereas in ErbB2-overexpressing patients, low plexin-B1 expression levels are associated with high disease-free survival.Citation58 VEGF and semaphorins have been found to exhibit contrasting functions in breast cancer progression. In support of this, meta-analyses of 2656 breast tumor samples have revealed high VEGF and low secreted semaphorin levels in 60% of total TNBC specimens. Moreover, in non-TNBC patients, higher expression of VEGF and lower expression of semaphorins are associated with low survival rates.Citation17 A recent report has suggested that reduced levels of Sema4D are associated with poor clinical outcomes and decreased disease-free survival in breast cancer. It was also shown that reduced expression of Sema4D is associated with bone metastasis.Citation18 Malik et al have observed that the transcript level of Sema3C is higher in breast cancer tissues as compared to adjacent normal mammary tissues, using real-time polymerase chain reaction analysis of 106 breast tumors and 28 adjacent normal breast specimens.Citation6 The data have shown that triple-negative breast tumors have higher Sema3C levels as compared to ER+ve breast tumors, using tissue microarray analysis of 343 breast tumor samples. The data also suggested that Sema3C expression is high in ER-ve, PR-ve, and HER2+ve breast tumors.Citation16 Expression of Sema6D in human breast cancer was analyzed using the human cancer genome atlas database, and it was found that the expression of Sema6D was correlated with overall survival in TNBC patients, and this might be helpful in predicting the progression of this subtype of breast cancer.Citation80 These studies suggest that semaphorins and their receptors are promising prognostic factors for the prediction of breast cancer progression.
Conclusion
Some of the members of the semaphorin family exhibit antitumorigenic effect, whereas others show protumorigenic effect in breast cancer by regulating tumor growth, angiogenesis, and metastasis. Semaphorin expression is dysregulated during tumor progression. Differential expression of semaphorins is associated with the disease progression of different subtypes of breast cancer. Higher expression levels of Sema3C and Sema3E are associated with increased tumor aggressiveness and poor prognosis in breast cancer patients. Lower expression levels of Sema3A, Sema3B, and Sema3F are associated with increased tumor progression and decreased survival of patients. Because of the clinical relevance of semaphorin expression in breast cancer, these genes might be used as a prognostic factor for the prediction of breast cancer progression. Thus, targeting semaphorins, their receptors, and downstream signaling molecules with therapeutic drugs might be useful in inhibiting angiogenesis and metastasis for the management of breast cancer.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We thank Anuradha Bulbule for critically reading the manuscript.
References
- SiegelRLMillerKDJemalACancer statistics, 2016CA Cancer J Clin201666173026742998
- Nwabo KamdjeAHSeke EtetPFVecchioLMullerJMKramperaMLukongKESignaling pathways in breast cancer: therapeutic targeting of the microenvironmentCell Signal201426122843285625093804
- BaskinYYigitbasiTClinical proteomics of breast cancerCurr Genomics201011752853621532837
- SørlieTPerouCMTibshiraniRGene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implicationsProc Natl Acad Sci USA20019819108691087411553815
- StatonCAShawLAValluruMExpression of class 3 semaphorins and their receptors in human breast neoplasiaHistopathology201159227428221884206
- MalikMFSatherleyLKDaviesELYeLJiangWGExpression of semaphorin 3C in breast cancer and its impact on adhesion and invasion of breast cancer cellsAnticancer Res20163631281128626977026
- KolodkinALMatthesDJGoodmanCSThe semaphorin genes encode a family of transmembrane and secreted growth cone guidance moleculesCell1993757138913998269517
- CapparucciaLTamagnoneLSemaphorin signaling in cancer cells and in cells of the tumor microenvironment–two sides of a coinJ Cell Sci2009122111723173619461072
- NeufeldGKesslerOThe semaphorins: versatile regulators of tumour progression and tumour angiogenesisNat Rev Cancer20088863264518580951
- MishraRKumarDTomarDChakrabortyGKumarSKunduGCThe potential of class 3 semaphorins as both targets and therapeutics in cancerExpert Opin Ther Targets201519342744225434284
- MishraRThoratDSoundararajanGSemaphorin 3A upregulates FOXO 3a-dependent MelCAM expression leading to attenuation of breast tumor growth and angiogenesisOncogene201534121584159524727891
- AppletonBAWuPMaloneyJStructural studies of neuropilin/antibody complexes provide insights into semaphorin and VEGF bindingEMBO J200726234902491217989695
- ParkerMWLinkugelADVander KooiCWEffect of C-terminal sequence on competitive semaphorin binding to neuropilin-1J Mol Biol2013425224405441423871893
- Castro-RiveraERanSThorpePMinnaJDSemaphorin 3B (SEMA3B) induces apoptosis in lung and breast cancer, whereas VEGF165 antagonizes this effectProc Natl Acad Sci USA200410131114321143715273288
- AllegraMZaragkouliasAVorgiaESemaphorin-7a reverses the ERF-induced inhibition of EMT in Ras-dependent mouse mammary epithelial cellsMol Biol Cell201223193873388122875994
- Cole-HealyZVerganiPHunterKBrownNJReedMWStatonCAThe relationship between semaphorin 3C and microvessel density in the progression of breast and oral neoplasiaExp Mol Pathol2015991192425910410
- BenderRJMac GabhannFExpression of VEGF and semaphorin genes define subgroups of triple negative breast cancerPloS One201385e6178823667446
- MalikMFYeLJiangWGReduced expression of semaphorin 4D and plexin-B in breast cancer is associated with poorer prognosis and the potential linkage with oestrogen receptorOncol Rep20153421049105726035216
- GoodmanCSKolodkinALLuoYPüschelAWRaperJAUnified nomenclature for the semaphorins/collapsinsCell199997555155210367884
- GherardiELoveCAEsnoufRMJonesEYThe sema domainCurr Opin Struct Biol200414666967815582390
- KoppelAMFeinerLKobayashiHRaperJAA 70 amino acid region within the semaphorin domain activates specific cellular response of semaphorin family membersNeuron19971935315379331346
- TakahashiTStrittmatterSMPlexina1 autoinhibition by the plexin sema domainNeuron200129242943911239433
- Kong-BeltranMStamosJWickramasingheDThe Sema domain of Met is necessary for receptor dimerization and activationCancer Cell200461758415261143
- TamagnoneLEmerging role of semaphorins as major regulatory signals and potential therapeutic targets in cancerCancer Cell201222214515222897846
- ChangKKarnadAZhaoSFreemanJWRoles of c-Met and RON kinases in tumor progression and their potential as therapeutic targetsOncotarget201563507351825784650
- BorkPDoerksTSpringerTASnelBDomains in plexins: links to integrins and transcription factorsTrends Biochem Sci199924726126310390613
- BasileJRHolmbeckKBuggeTHGutkindJSMT1-MMP controls tumor-induced angiogenesis through the release of semaphorin 4DJ Biol Chem200728296899690517204469
- MessinaAGiacobiniPSemaphorin signaling in the development and function of the gonadotropin hormone-releasing hormone systemFront Endocrinol20134133
- HeZTessier-LavigneMNeuropilin is a receptor for the axonal chemorepellent Semaphorin IIICell19979047397519288753
- TamagnoneLArtigianiSChenHPlexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebratesCell1999991718010520995
- JanssenBJRobinsonRAPérez-BrangulíFStructural basis of semaphorin-plexin signallingNature201046773191118112220877282
- OinumaIIshikawaYKatohHNegishiMThe Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-RasScience2004305568586286515297673
- KolodkinALGintyDDSteering clear of semaphorins: neuropilins sound the retreatNeuron1997196115911629427240
- SokerSTakashimaSMiaoHQNeufeldGKlagsbrunMNeuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factorCell19989267357459529250
- Pellet-ManyCFrankelPJiaHZacharyINeuropilins: structure, function and role in diseaseBiochem J2008411221122618363553
- YazdaniUTermanJRThe semaphorinsGenome Biol20067321116584533
- TakahashiTFournierANakamuraFPlexin-neuropilin-1 complexes form functional semaphorin-3A receptorsCell1999991596910520994
- CasazzaAFinisguerraVCapparucciaLSema3E–Plexin D1 signaling drives human cancer cell invasiveness and metastatic spreading in miceJ Clin Invest201012082684269820664171
- MasudaKFuruyamaTTakaharaMFujiokaSKurinamiHInagakiSSema4D stimulates axonal outgrowth of embryonic DRG sensory neuronesGenes Cells20049982182915330859
- DengSHirschbergAWorzfeldTPlexin-B2, but not Plexin-B1, critically modulates neuronal migration and patterning of the developing nervous system in vivoJ Neurosci200727236333634717554007
- ArtigianiSConrottoPFazzariPPlexin-B3 is a functional receptor for semaphorin 5AEMBO Rep20045771071415218527
- PanHWanamiLSDissanayakeTRBachelderREAutocrine sema - phorin3A stimulates alpha2 beta1 integrin expression/function in breast tumor cellsBreast Cancer Res Treat2009118119720518787945
- GehlerSCompereFVMillerAMSemaphorin 3A increases FAK phosphorylation at focal adhesions to modulate MDA-MB-231 cell migration and spreading on different substratum concentrationsInt J Breast Cancer20172017961973428182100
- Guttmann-RavivNShraga-HeledNVarshavskyAGuimaraes-SternbergCKesslerONeufeldGSemaphorin-3A and semaphorin-3F work together to repel endothelial cells and to inhibit their survival by induction of apoptosisJ Biol Chem200728236262942630517569671
- AcevedoLMBarillasSWeisSMGöthertJRChereshDASemaphorin 3A suppresses VEGF-mediated angiogenesis yet acts as a vascular permeability factorBlood200811152674268018180379
- ShenWWChenWGLiuFZBreast cancer cells promote osteoblastic differentiation via Sema 3A signaling pathway in vitroInt J Clin Exp Pathol2015821584158325973043
- Castro-RiveraERanSBrekkenRAMinnaJDSemaphorin 3B inhibits the phosphatidylinositol 3-kinase/Akt pathway through neuropilin-1 in lung and breast cancer cellsCancer Res200868208295830318922901
- ShahiPWangCYChouJGATA3 targets semaphorin 3B in mammary epithelial cells to suppress breast cancer progression and metastasisOncogene201736405567557528581515
- ZhuXZhangXYeZSilencing of semaphorin 3C suppresses cell proliferation and migration in MCF-7 breast cancer cellsOncol Lett20171455913591729113226
- MumblatYKesslerOIlanNNeufeldGFull-length semaphorin-3C is an inhibitor of tumor lymphangiogenesis and metastasisCancer Res201575112177218625808871
- EsselensCMalapeiraJColoméNThe cleavage of semaphorin 3C induced by ADAMTS1 promotes cell migrationJ Biol Chem201028542463247319915008
- XuXZhaoZGuoSIncreased semaphorin 3c expression promotes tumor growth and metastasis in pancreatic ductal adenocarcinoma by activating the ERK1/2 signaling pathwayCancer Lett2017397122228315433
- LuchinoJHocineMAmoureuxMCSemaphorin 3E suppresses tumor cell death triggered by the plexin D1 dependence receptor in metastatic breast cancersCancer Cell201324567368524139859
- WeiJCYangJLiuDTumor-associated lymphatic endothelial cells promote lymphatic metastasis by highly expressing and secreting SEMA4CClin Cancer Res201723121422427401250
- YangYHBuhamrahASchneiderASemaphorin 4d promotes skeletal metastasis in breast cancerPLoS One2016112e015015126910109
- SwierczJMWorzfeldTOffermannsSErbB-2 and met reciprocally regulate cellular signaling via plexin-B1J Biol Chem200828341893190118025083
- Ch’ngESKumanogohARoles of Sema4D and Plexin-B1 in tumor progressionMol Cancer20109125120858260
- WorzfeldTSwierczJMLoosoMStraubBKSivarajKKOffermannsSErbB-2 signals through Plexin-B1 to promote breast cancer metastasisJ Clin Invest201212241296130522378040
- BlackSANelsonACGuruleNJFutscherBWLyonsTRSemaphorin 7a exerts pleiotropic effects to promote breast tumor progressionOncogene201635395170517827065336
- Garcia-AreasRLibrerosSAmatSSemaphorin7A promotes tumor growth and exerts a pro-angiogenic effect in macrophages of mammary tumor-bearing miceFront Physiol201451724550834
- SchneiderBPMillerKDAngiogenesis of breast cancerJ Clin Oncol20052381782179015755986
- ConleySJGheordunescuEKakaralaPAntiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxiaProc Natl Acad Sci USA201210982784278922308314
- LiuTJSunBCZhaoXLCD133+ cells with cancer stem cell characteristics associates with vasculogenic mimicry in triple-negative breast cancerOncogene201332554455322469978
- CasazzaAFuXJohanssonISystemic and targeted delivery of semaphorin 3A inhibits tumor angiogenesis and progression in mouse tumor modelsArterioscler Thromb Vasc Biol201131474174921205984
- JiangHChenCSunQThe role of semaphorin 4D in tumor development and angiogenesis in human breast cancerOnco Targets Ther201695737575027729799
- SierraJRCorsoSCaioneLTumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophagesJ Exp Med200820571673168518559453
- SeyfriedTNHuysentruytLCOn the origin of cancer metastasisCrit Rev Oncog2013181–2437323237552
- SinghASettlemanJEEMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancerOncogene201029344741475120531305
- XuZChenWGSunJChenPChuTWHypoxia inhibits the expression of semaphorin 3A in breast cancer cells and regulates the differentiation of preosteoblast cellsTumor2016361212981306
- NasarrePConstantinBRouhaudLSemaphorin SEMA3F and VEGF have opposing effects on cell attachment and spreadingNeoplasia200351839212659673
- XiongGWangCEversBMZhouBPXuRRORα suppresses breast tumor invasion by inducing SEMA3F expressionCancer Res20127271728173922350413
- ChristensenCAmbartsumianNGilestroGProteolytic processing converts the repelling signal Sema3E into an inducer of invasive growth and lung metastasisCancer Res200565146167617716024618
- Garcia-AreasRLibrerosSSimoesMSuppression of tumor-derived Semaphorin 7A and genetic ablation of host-derived Semaphorin 7A impairs tumor progression in a murine model of advanced breast carcinomaInt J Oncol20175151395140429048670
- ChenYZhangLLiuWExpression of semaphorin4C (Sema4C) in breast cancer, endometrial cancer and prostate cancer and its clinical significanceChin J Clin Oncol2010379507511
- WuMHanLShiYDevelopment and characterization of a novel method for the analysis of gene expression patterns in lymphatic endothelial cells derived from primary breast tissuesJ Cancer Res Clin Oncol2010136686387219936789
- YangQWangYLuXMiR-125b regulates epithelial-mesenchymal transition via targeting Sema4C in paclitaxel-resistant breast cancer cellsOncotarget2015653268327925605244
- EvansEEJonasonASBusslerHAntibody blockade of semaphorin 4D promotes immune infiltration into tumor and enhances response to other immunomodulatory therapiesCancer Immunol Res20153668970125614511
- RodyAHoltrichUGaetjeRPoor outcome in Estrogen receptor–positive breast cancers predicted by loss of Plexin B1Clin Cancer Res20071341115112217317819
- RodyAKarnTRuckhäberleELoss of Plexin B1 is highly prognostic in low proliferating ER positive breast cancers–results of a large scale microarray analysisEur J Cancer2009453405413
- ChenDLiYWangLJiaoKSEMA6D expression and patient survival in breast invasive carcinomaInt J breast cancer2015201553972125973277
