Abstract
Endocrine treatment represents the cornerstone of endocrine-sensitive premenopausal early breast cancer. The estrogen blockade plays a leading role in the therapeutic management of hormone receptor-positive breast cancer together with surgery, radiotherapy, and selective antiestrogen treatments. For several years, selective estrogen receptor modulators, such as tamoxifen, have represented the mainstay of therapy. The role of amenorrhea has been extensively elucidated in the past year: the benefit observed with chemotherapy-induced amenorrhea has strengthened its therapeutic role. Luteinizing hormone-releasing hormone (LHRH) has been introduced in oncology practice to induce amenorrhea in order to increase the advantage obtained from endocrine treatment. Triptorelin is one of the most widely used LHRH analogs currently available in clinical practice. It was recently investigated in two major clinical trials that studied the role of complete estrogen blockade in the premenopausal setting. Both showed the clinical benefit due to ovarian suppression treatment, primarily in high-risk patients. Furthermore, triptorelin and other LHRH analogs have recently been investigated in the attempt to preserve the ovarian function in young patients. The medical treatment of early breast cancer is always evolving in the effort to search for safe and efficacious treatments. The role of LHRH analogs is actually well recognized as contributing to the improvement of the medical treatment of premenopausal women with early breast cancer.
Keywords:
Introduction
Luteinizing hormone-releasing hormone (LHRH) is a decapeptide hypo-physiotropic hormone produced by the hypothalamic neurons, which plays a central role in the endocrine regulation and the control of reproductive functions. It is secreted, in a pul-satile way, from the median eminence into the portal vein system, reaching the anterior pituitary gland inducing the release of the following two gonadotropin hormones: follicle-stimulating hormone (FSH) and luteinizing hormone (LH). The role of FSH and LH is crucial in the gametogenesis and steroid production. The gonadal steroids regulate the secretion of LHRH through the binding to specific receptors expressed on the hypothalamic neuronal cells and pituitary gland.Citation1 Since its discovery, LHRH has been studied for its potential activity in controlling the growth of endocrine sensitive cancer cells such as prostate, ovarian, endometrial, and breast cancers. The following two types of LHRH analogs have been developed: the LHRH agonists and the LHRH antagonists. LHRH agonists were introduced initially in the treatment of endocrine-sensitive cancers, such as prostate and premenopausal breast cancers. They represent the cornerstone of current endocrine treatments for both early and advanced disease. LHRH antagonists were developed some years later; their main application is in the management of prostate cancer.
LHRH agonists: biology and antitumoral effect
The LHRH agonists used in daily clinical practice are as follows: goserelin, tryptorelin, leuprorelide, and buserelin. They are decapeptides with an arginine in position 8 (Arg8) that is essential for the affinity to the mammalian receptor. The introduction of hydrophobic groups on the sixth amino acid further increases this bond with a major resistance to the enzymatic degradation.Citation2 The LHRH analogs operate as anticancer agents suppressing the pituitary gonadal functions, determining the fall of gonadal steroids levels, and reducing their mitogenic activity. Furthermore, it seems that LHRH analogs could have a direct antitumoral effect. In fact, the LHRH receptors are present in the cancer cells. The mRNA encoding for these receptors is similar to the pituitary receptors. An inhibition in cellular tumor growth has been observed in breast cancer.Citation3–Citation10 Physiologically, in pituitary gland, the gonadotropin receptor (GnRH) signaling is mediated through the G-protein αq. These proteins conduct the subsequent activation of phopholipase C (PLC) that catalyzes the hydrolysis of membrane phospholipids generating the liberation of intracellular Ca2+.Citation11,Citation12
The antiproliferative effect of LHRH analogs seems to be related to the signal transduction pathways involving the growth factor-induced mitogenic signaling, as the activity of MAPK and the c-fos expression. The GnRH receptors evident in the tumor activate phosphotyrosine phosphatase (PTP), resulting in the inhibition of mitogenic signal transduction and the reduction of cell proliferation.Citation13 Normally, estrogen induces gene transcription through nuclear receptor activation binding to the promoter of sensitive genes, but other unconventional transcriptional pathways could be involved as follows: steroidogenic factor-1 (SF-1),Citation14 specific factor-1 (Sp1),Citation15–Citation17 nuclear factor-Y (42), and activator protein-1 (AP-1).Citation18,Citation19 Furthermore, the MAPK pathway may be involved in a nongenomic stimulus, inducing the activation of proto-oncogene c-fos.Citation20
LHRH analogs and first evidence in early breast cancer
Chemical castration is the main reason for the clinical use of LHRH analogs in the endocrine-sensitive early breast cancer. Since the first evidence of efficacy of ovarian ablation in the treatment of breast cancer,Citation21 various methods were explored to induce the ovarian suppression.Citation22 Evidence from initial trials in metastatic breast cancer patients allowed the introduction of the possible use in the adjuvant setting for the endocrine-sensitive early breast cancer.
In clinical practice, the LHRH analogs have been added to the standard tamoxifen therapy due to the increased suppression of circulating estrogens achieved with the combination in previous studies.Citation23–Citation26 The question about the role of LHRH being added to chemotherapy, or compared to chemotherapy, has been evaluated in five randomized studies and a meta-analysis. These trials showed that the addition of LHRH to chemotherapy improves the outcome, but none of these trials contains an arm with tamoxifene alone or evaluates the estradiol (E2) levels after chemotherapy ().Citation27–Citation33 The Early Breast Cancer Trialists’ Collaborative Group (EBCTG) pointed out the value of ovarian suppression showing an improvement in recurrence-free interval and survival, across 2102 women treated in the clinical trials.Citation34–Citation36 In 2001, the EBCTG published an overview of the available randomized trials involving LHRH analogs in the premenopausal early breast cancer setting conducted before 1990s. This analysis pointed out the value of the addition of LHRH analogs to the standard hormone therapy, represented by tamoxifen.Citation37 Triptorelin was the LHRH analog used in one of the four studies examined in the review. In 2005, EBCTG produced a new overview in which LHRH analogs added to chemotherapy was the more advantageous possible therapeutic option, especially for patients younger than 40 years.Citation37 A meta-analysis of individual patient data, from 16 randomized adjuvant trials, has been conducted by Cuzick. Patient data of 11,906 women (9022 women were hormone receptor positive [HR+]) was included in the review. The addition of an LHRH agonist to tamoxifen, chemotherapy, or both significantly reduced the risk of recurrence, the death after recurrence, and any death. In women with HR+ breast cancer, the addition of LHRH agonists to tamoxifen, chemotherapy, or both reduces the risk of recurrence and death after recurrence and LHRH agonists are as effective as chemotherapy.Citation38 Thereafter, the National Institutes of Health (NIH) stated, in the consensus development conference statement, that ovarian ablation appears to produce a similar benefit to some chemotherapy regimens and estrogen deprivation can be achieved by the suppression of estrogen synthesis by LHRH agonists in pre-menopausal women. Ovarian suppression may be considered as an alternative treatment option, instead of chemotherapy, for node-negative endocrine-sensitive early breast cancer.Citation39–Citation41
Table 1 Randomized trials evaluating chemotherapy and chemotherapy ± ovarian suppression
Triptorelin from bench to bedside: the basis for the treatment of breast cancer
Triptorelin ([d-Ala-6, des-Gly-NH2-10]-LHRH ethylamide) has been synthesized in the late 1970s, and its antitumoral effect in endocrine-sensitive cancers has conducted to its utilization in the treatment of prostate cancerCitation42 and breast cancer.Citation43–Citation46 Triptorelin was shown to reduce the E2-related activation of c-fos with a subsequent reduction in the transcriptional activity and downregulation of cancer cell proliferation. This effect is observed both in LHRH receptor-positive and -negative cells, whereas it is not observed in the E2-induced pathway.Citation46,Citation47
The first clinical evidence for triptorelin efficacy was displayed, as monotherapy, in the treatment of endocrine-sensitive metastatic breast cancer.Citation48–Citation55 Searching more potent estrogen suppression, the association of triptorelin and formestane, first-generation aromatase inhibitors, was also evaluated, showing the feasibility of the treatment and E2 suppression.Citation56
Triptorelin: evolution in the treatment of early breast cancer
The role of E2 suppression induced by chemotherapy is known: chemotherapy-induced amenorrhea is associated with the reduction of relapse and increased survival outcomes.Citation57,Citation58 Patients with HR+ disease and at least 6 months of chemotherapy-related amenorrhea have a reduction in the risk of death or the recurrence of 24% (P=0.04) and 30% (P<0.001), respectively.Citation59 In order to explore the benefit of amenorrhea, a Phase III French studyCitation33 compared the hormonal treatment with tamoxifen and LHRH vs epirubicin-based chemotherapy, as adjuvant treatment, in premenopausal women with intermediate-risk HR + breast cancer (1–3 nodes involved and HR+ disease). A total of 333 patients were enrolled: 164 patients were randomly assigned to tamoxifen plus LHRH group and 169 patients assigned to chemotherapy group. Amenorrhea occurred in all patients treated with tamoxifen plus LHRH agonist triptorelin (and in 64% of patients receiving FEC50); after a 7-year follow-up, the study did not showed a difference between the two treatment arms in terms of disease-free survival (DFS) and overall survival (OS).Citation33
The prognostic role of treatment-induced amenorrhea (TIA) was evaluated in HER2-positive (HER2+) early breast cancer also. The ALTTO trial, a randomized Phase III study, conducted in patients with HER2+ early breast cancer patients, randomized the women in four adjuvant anti-HER2 arms. The exploratory analysis included 2863 premenopausal women at the time of randomization. This analysis showed that patients with HR+ disease and a TIA had an improvement in both DFS (HR 0.64; 95% confidence interval [CI] 0.52–0.79) and OS (HR 0.53; 95% CI 0.38–0.74). On the contrary, in hormone receptor-negative (HR−) disease, DFS and OS were similar between patients independently to TIA status. A cross-talk between HR+ and HER2+ signaling may exist, and its control may improve outcomes in HR+/HER2+ breast cancer. This information supports the use of ovarian suppression therapies in the adjuvant treatment of premenopausal women with HR+/HER2+ early breast cancer.Citation60
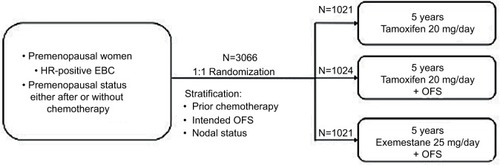
Two randomized Phase III trials (Tamoxifen and Exemestane Trial [TEXT] and Suppression of Ovarian Function Trial [SOFT]), involving premenopausal women with HR+ early breast cancer treated with hormonal therapy, recently evaluated the role of triptorelin. The TEXT enrolled 2672 patients and was designed to compare 5 years of exemestane plus triptorelin (3.75 mg every 28 days by intramuscular injection) with tamoxifen plus triptorelin (1338 and 1334 patients, respectively, enrolled in the two arms) ().Citation61 In SOFT, 3066 patients were randomly assigned to the following three different treatment groups: oral tamoxifen only (1021 patients), tamoxifen plus ovarian function suppression (OFS) (1024 patients), and oral exemestane plus OFS for 5 years (1021 patients) (). OFS was achieved by bilateral oophorectomy, bilateral ovarian irradiation, or using triptorelin 3.75 mg every 28 days.Citation61 In TEXT, the patients treated with chemotherapy received concurrent OFS (triptorelin) after randomization that was always used in the first 6 months after randomization. Afterward, patients could continue treatment with the LHRH analog or change to oophorectomy or ovarian radiation therapy, while in SOFT, patients who received chemotherapy (neoadjuvant or adjuvant), and who remained premenopausal, underwent randomization within 8 months from the end of chemotherapy.Citation61 For both, the primary endpoint was DFS. In SOFT, the addition of OFS to tamoxifen did not significantly improve DFS in the overall population: the 5-year DFS rate was 86.6% in the tamoxifen plus OFS group and 84.7% in the tamoxifen alone group (HR 0.83; 95% CI 0.66–1.04; P=0.10). Multivariate analysis reported a 22% reduction in the risk of disease progression in the tamoxifen plus OFS group (HR 0.78; 95% CI 0.62–0.98). Of note, the addition of OFS improved disease outcomes in women treated with adjuvant chemotherapy and in the younger ones ().Citation62 In the joint analysis of the two trials, which included 4690 premenopausal women with HR+ breast cancer, adjuvant endocrine therapy with exemestane plus OFS significantly improved DFS over tamoxifen plus OFS, showing a reduction of 34% in the risk of breast cancer recurrence with exemestane over tamoxifen (HR 0.66; 95% CI 0.55–0.80; P<0.001). No significant difference in OS was reported, and this could be due to the brevity of the follow-up to identify such difference.Citation63 The toxicity profile was similar between the two groups, and the DFS benefit was achieved without a detrimental effect of exemestane plus OFS on the quality of life, when compared with tamoxifen plus OFS.Citation64 A recent planned update at 8 years for the SOFT and at 9 years for the combined TEXT and SOFT showed that the addition of LHRH to tamoxifen or exemestane significantly improved the outcome compared to tamoxifen alone. It also confirms the efficacy of exemestane plus OFS over tamoxifen with a 4% absolute improvement in DFS at 8 years.Citation65,Citation66 A successive further analysis that included 4891 women who were enrolled in the two trials and evaluated breast cancer-free interval (BCFI) according to clinicopathologic features was performed. The results showed that the greater benefit derived from exemestane plus OFS is given to high recurrence-risk patients who may experience an improvement of 10–15% in 5-year BCFI. Therefore, not all premenopausal women should receive the combination, but a balance between risks, expected benefits, and toxicities is needed.Citation67 The basis for the efficacy of the association of aromatase inhibitors and LHRH analogs was explored in an Italian Phase III trial that compared the endocrine effects of 6 months of adjuvant treatment with tamoxifen and triptorelin or letrozole and triptorelin in 81 premenopausal women with early breast cancer.Citation68 The letrozole group has shown a major suppression of median E2 serum levels (P=0.0008) compared with tamoxifen; otherwise, FSH median levels were lower in patients receiving tamoxifen (P<0.0001). These results have led to the hypothesis that the greater efficacy of letrozole found in postmenopauseCitation69 could be confirmed also in premenopausal women. Of note, this greater suppression is related to a greater incidence of adverse effects (ie, osteoporosis, alteration of lipid metabolism, and sexual function impairment) that must be taken into consideration in younger women.Citation68
Table 2 Ovarian function suppression and outcome results
The possibility of incomplete estrogen suppression has been described in the SOFT-EST substudy. E2, estrone (E1), and E1 sulfate (E1S) levels were measured during the first year of monthly triptorelin plus exemestane or tamoxifen using a more specific and sensitive method (gas chromatography tandem mass spectrometry), among patients receiving exemestane plus triptorelin. Two-thirds of premenopausal patients treated with exemestane plus triptorelin showed a profound, persistent reduction in E2 levels during the first 12 months of treatment. This decrease was significantly lower than in the tamoxifen plus triptorelin group at all time points, although 17% of patients had an E2 level greater than the lower estimated level of 2.72 pg/mL at each time point. Interestingly, 34% (27/79) of patients, receiving exemestane plus triptorelin, had an E2 level greater than the predefined threshold and had at least one postbaseline E2 value >2.72 pg/mL. Baseline factors related to E2 level >2.72 pg/mL were as follows: no prior chemotherapy (P=0.06), higher body mass index (P=0.05), and lower FSH and LH (each P<0.01).Citation70
Figure 1 TEXT study description.
Abbreviations: EBC, early breast cancer; HR, hormone receptor; OFS, ovarian function suppression; TEXT, Tamoxifen and Exemestane Trial.
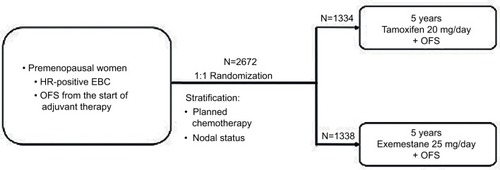
Figure 2 SOFT trial study description.
Abbreviations: EBC, early breast cancer; HR, hormone receptor; OFS, ovarian function suppression; SOFT, Suppression of Ovarian Function Trial.
Some reflections on the efficacy of LHRH analogs in the adjuvant setting could also be extrapolated from two trials conducted to explore the likelihood to preserve the ovarian function. The PROMISE-GIM6 trial was designed to evaluate the incidence of early menopause in young women with breast cancer treated with (neo)adjuvant chemotherapy plus temporary ovarian suppression obtained by the administration of triptorelin.Citation71 A post hoc extension of the study was conducted to evaluate long-term outcomes including long-term ovarian function, pregnancy, and DFS. Two hundred eighty patients were enrolled; >5-year DFS was 80.5% (95% CI 76.1–89.1%) in the LHRH analog group and 83.7% in the control group with an HR of 1.17 (95% CI 0.72–1.92; P=0.52), so the difference was not statistically significant. This increased risk appeared to be more prevalent in women with HR- cancer with a 5-year DFS of 62.1% in the experimental arm and 76.2% in the control arm, with an HR of 2.11 (95% CI 0.74–5.98) (). By contrast, in the HR+ patients, the difference between the two arms was not statistically significant, with an HR of 0.96 (95% CI 0.55–1.70); possibly, the lack of statistical significance could be related to the study being underpowered.Citation72 These findings are discordant with the results of the POEMS-SWOG SO230 study, which showed an advantage in 4-year DFS in 105 women treated with chemotherapy plus goserelin as compared with 113 women treated with chemotherapy alone (89 vs 78%, respectively, with an HR of 0.49; 95% CI 0.24–0.97; P=0.04) ().Citation73 A similar improvement was found in terms of OS with an HR of 0.43 (P=0.05). Of note, the trial enrolled only patients with ER-negative disease, confirming the safety of the concurrent administration of chemotherapy and LHRH agonist in this subset of patients.Citation73 The improvement in these outcomes was unexpected in this population, but it is concordant with preclinical evidence in the setting of triple-negative breast cancer suggesting the presence of high expression of LHRH receptors in this breast cancer subtype. Therefore, the use of LHRH analogs may be even related to cancer cells’ growth inhibition and reduction in metastatic spread.Citation74,Citation75
Triptorelin and preservation of ovarian function
Great interest rises up from the opportunity of LHRH analogs use in premenopausal women during chemotherapy treatment in order to preserve ovarian function. In the last few years, due to the improvement in the prognosis of cancer patients, growing attention has been given to the long-term consequences of the treatment, in particular to the fertility issue especially if we consider that 41% of breast cancers are diagnosed in patients younger than 50 years.Citation76 In young cancer patients, ovarian toxicity is a primary side effect of chemotherapy for those who often need and receive aggressive multimodality treatment; cancer survivors have reduced pregnancy rates when compared with the general population.Citation77 According to a big nationwide Norwegian study, published in 2011, female survivors have the lowest chance of subsequent pregnancy after a breast cancer diagnosis. This iŝ70% lower than the general population.Citation77 These data are even more important considering the percentage of women who wish to become pregnant. According to a study published by Letourneau et alCitation78 on cancer in 2012, 47% of young patients with breast cancer would like to get pregnant after treatment. The concerns about the possible loss of ovarian function and fertility can affect the treatment decisions of a significant percentage of young patients at the time of breast cancer diagnosis. In 2014, Ruddy et alCitation79 published the results of a survey as part of a prospective multicenter cohort study: 319 (51%) of the 620 women were concerned about becoming infertile after treatment. Due to fertility concerns, four (1%) women chose not to receive chemotherapy, 12 (2%) women chose one chemotherapy regimen over another, six (1%) women considered not receiving endocrine therapy, 19 (3%) women decided not to receive endocrine therapy, and 71 (11%) women considered receiving endocrine therapy for 5 years; 65 (10%) women used fertility preservation strategies. The population more concerned about fertility was women of a younger age, non-White race, childless, and who had to start chemotherapy.Citation79 These results are in accordance with the previous study published by Partridge et alCitation80 in 2004: 73% of women with BC were concerned about fertility, 57% of women were seriously concerned about sterility, and 29% of women did not comply with their treatment because of fertility issues. Premature ovarian failure (POF) is one possible effect of chemotherapy in premenopausal patients and even in the presence of resumed regular menses after treatment patients are still at risk of developing early menopause due to the damage of cytotoxic therapy to their ovarian reserve. The effects of chemotherapy on ovarian function are variable and related to the age of the woman, pre-existing ovarian reserve, and type and dose of chemotherapy.Citation81 Risk is particularly significant in those patients who are eligible to receive neoadjuvant or adjuvant chemotherapy with alkylating agents; HR+ disease implies adjuvant endocrine therapy for 5–10 years with a further delay in pregnancy and women older than 40 years.Citation82
Nowadays, major international guidelines recommend early discussion about fertility issues with young patients to help them make an informed decision and this process is an important component of quality oncology care.Citation83–Citation87 The clinicians should discuss the risks for infertility, fertility preservation, and the probability of successful pregnancies subsequent to the completion of BC therapy. In Italy, according to a survey published in 2015 by Biglia et al,Citation88 91% of oncologists considered it important to discuss the issue of fertility and 93% of them introduced this topic when the patient did not talk about it. At this current moment, the available options for premenopausal breast cancer patients are embryo or oocyte cryopreservation, ovarian tissue cryopreservation, and temporary menstrual suppression with LHRH analogs during chemotherapy; more than one technique can be used at the same time.
In order to evaluate the safest strategy to preserve fertility, two major trials have been conducted in breast cancer patients in the last years. The Prevention of Early Menopause Study (POEMS-SWOG)Citation73 showed that temporary ovarian suppression with goserelin during chemotherapy was associated with a significant reduction in the risk of treatment-related POF (8 vs 22%; OR 0.30; 95% CI 0.09–0.97) (). The updated results presented at San Antonio Breast Cancer Symposium (SABCS) 2017 after a median follow-up of 5.1 years showed higher pregnancy rates in the goserelin group compared with those in the standard group (22 vs 12%; OR 2.38; 95% CI 1.08–5.26, P=0.05); this has been associated with improved survival with a significant increase in both DFS and OS in the LHRH analog containing group.Citation89 The second one is the Italian Study (PROMISE-GIM6) conducted by Del Mastro et alCitation71 which showed a significant protective effect with the use of LHRH analog triptorelin in preserving ovarian function 1 year after the end of chemotherapy (9 vs 26%; OR 0.28; 95% CI 0.14–0.59) and also at long-term follow-up (). Furthermore, an increased pregnancy rate was reported by both the studies.Citation71,Citation73 This information was confirmed in a meta-analysis published in 2015 that included 12 randomized studies: the use of GHRH analogs was associated with a significant reduced risk of POF (OR 0.36; P<0.001) and a significantly increased number of pregnancies (33 vs 19 women; OR 1.83; P=0.041) with no apparent negative impact on patients’ prognosis ().Citation90 This finding seems to conclude the long debate behind the pharmacological protection for fertility preservation and in the light of this evidence; the last version of Italian guidelines regarding the issue of fertility recommends this strategy in all premenopausal patients undergoing chemotherapy.Citation87
Table 3 Triptorelin and ovarian function preservation
There is no complete agreement on the role of LHRH analogs on the preservation of fertility, according to the sec ond international consensus guidelines for breast cancer in young women (BCY2).Citation85 While in the St Gallen International Expert Consensus on the Primary Therapy of Early Breast CancerCitation91 and in National Comprehensive Cancer Network (NCCN) guidelines,Citation83 this strategy should be discussed with the patients; in the American Society of Clinical Oncology (ASCO)Citation86 and European Society for Medical Oncology (ESMO)Citation84 guidelines, the use of LHRH during chemotherapy is not recommended because it is considered as an experimental technique. The major reason for this difference is probably that the latest version of ASCO and ESMO guidelines were published in 2013 and an update is needed to encompass this new information.Citation84,Citation86 The recent result of the Phase III study (OPTIONCitation92) has shown that the use of LHRH analog (goserelin) provides some protection to the ovarian function during chemotherapy in women younger than 40 years. The effect seems to be uncertain for women who are older than 40 years (≤40 amenorrhea: 10 vs 25.4%, P=0.032, premature ovarian insufficiency (POI): 2.6 vs 20%, P=0.038; >40 amenorrhea: 42.9 vs 54.2%, P=0.376, POI: 42.3 vs 47.2%, P=0.798).Citation92 These results are in the same direction of two other studies and a meta-analysis. The results of the meta-analysis from five randomized clinical trials (PROMISE-GIM6,Citation71 POEMS/SWOG,Citation73 OPTION,Citation92 GBG 37 ZORO,Citation93 and Moffitt Cancer Center-led trialCitation94), in which premenopausal women with early breast cancer (EBC) were randomized to receive chemotherapy alone or with LHRH (437 or 436 women, respectively), have been recently presented by the Lambertini et al at the SABCC 2017. The POI rate was 14.1% in the LHRH group and 30.9% in the control group (adjusted OR 0.38; 95% CI 0.26–0.57; P<0.001), and the post-treatment pregnancy rate was 37 in the LHRH group vs 20 in the control group (incidence rate ratio 1.83; 95% CI 1.06–3.15; P=0.030). Similar DFS and OS were observed between groups regardless of the ER status.Citation95 According to some of the principal authors in this field, the puzzle on the protective role of temporary ovarian suppression with LHRH analogs during chemotherapy has been completed.Citation96
Conclusion
After several years of debate and studies, the role of LHRH analogs appears more definite to the adjuvant treatment of premenopausal women with endocrine-sensitive breast cancer. First, the adjuvant trials (TEXT/SOFT) point out the adequate length of LHRH treatment in the premenopausal setting and delineate the class of risk in which LHRH appears more beneficial; 5 years of LHRH treatment is an adequate treatment length in the high-risk setting, whereas it is not beneficial in the low-risk subset. The role of LHRH was also explored in the preservation of the ovarian function allowing the oncologist the possibility of offering a safe and effective treatment together with the other existing fertility preservation techniques. Triptorelin represents one of the LHRH analogs available in clinical practice worldwide; it has been extensively studied in various trials that have confirmed the magnitude of its effectiveness in the adjuvant treatment of early breast cancer in the premenopausal setting and represents a safe and successful treatment.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This review has been conducted following the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The objective of this review was to specifically evaluate the role of LHRH analog triptorelin in the management of early breast cancer. The types of studies selected in this review were as follows: randomized controlled clinical trials and their related updates, meta-analyses, and relevant published studies concerning the role of triptorelin in the breast cancer treatment. Also, the published trials and their related updates, concerning the role of triptorelin, and other LHRH analogs were selected to evaluate its role in the preservation of ovarian function in the early breast cancer setting.
References
- ShupnikMAGonadotropin gene modulation by steroids and gonado-tropin-releasing hormoneBiol Reprod19965422792868788177
- ConnPMCrowleyWFJrGonadotropin-releasing hormone and its analogsAnnu Rev Med1994453914058198390
- KakarSSGrizzleWENeillJDThe nucleotide sequences of human GnRH receptors in breast and ovarian tumors are identical with that found in pituitaryMol Cell Endocrinol19941061–21451497534732
- KottlerMLStarzecACarreMCLagardeJPMartinACounisRThe genes for gonadotropin-releasing hormone and its receptor are expressed in human breast with fibrocystic disease and cancerInt J Cancer19977145955999178813
- MillerWRScottWNMorrisRFraserHMSharpeRMGrowth of human breast cancer cells inhibited by a luteinizing hormone-releasing hormone agonistNature198531359992312332982100
- KériGBaloghASzökeBTeplánICsukaOGonadotropin-releasing hormone analogues inhibit cell proliferation and activate signal transduction pathways in MDA-MB-231 human breast cancer cell lineTumour Biol199112261671902971
- BlankesteinMAHenkelmanMSKlijnJGMDirect inhibitor effect of a luteinising hormone-releasing hormone agonist on MCF-7 human breast cancer cellsEur J Cancer Clin Oncol19852112149314993938397
- FeketeMWittliffJLSchallyAVCharacteristics and distribution of receptors for D-Trp6-luteinizing hormone, somatostatin. epidermal growth factor, and sex steroids in 500 biopsy samples of human breast cancerJ Clin Lab Anal1989331371472569034
- SrkalovicGSzendeBReddingTWGrootKSchallyAVReceptors for D-Trp6-luteinizing hormone-releasing hormone, somatostatin, and insulin-like growth factor I in MXT mouse mammary carcinomaProc Sac Exp Biol Med19891923209218
- MilovanovicSRRadulovicSSchallyAVEvaluation of binding of cytotoxic analogs of luteinizing hormone-releasing hormone to human breast cancer and mouse MXT mammary tumorBreast Cancer Res Treat19922421471588443402
- StojilkovicSSCattKJExpression and signal transduction pathways of gonadotropin-releasing hormone receptorsRecent Prog Horm Res1995501612057740156
- NaorZHarrisDShachamSMechanism of GnRH receptor signalling: combinatorial cross-talk of Ca2+ and protein kinase CFront Neuroendocrinol19981911199465287
- GründkerCVölkerPEmonsGAntiproliferative signaling of luteinizing hormone-releasing hormone in human endometrial and ovarian cancer cells through G-protein alpha(I)-mediated activation of phosphotyrosine phosphataseEndocrinology200114262369238011356684
- DreanYLLiuDWongAOXiongFHewCLSteroidogenic factor 1 and estradiol receptor act in synergism to regulate the expression of the salmon gonadotropin II beta subunit geneMol Endocrinol19961032172298833651
- XieWDuanRSafeSEstrogen induces adenosine deaminase gene expression in MCF-7 human breast cancer cells: role of estrogen receptor-Sp1 interactionsEndocrinology199914012192279886828
- LiCBriggsMRAhlbornTEKraemerFBLiuJRequirement of Sp1 and estrogen receptor alpha interaction in 17beta-estradiol-mediated transcriptional activation of the low density lipoprotein receptor gene expressionEndocrinology200114241546155311250935
- WangWDongLSavilleBSafeSTranscriptional activation of E2F1 gene expression by 17beta-estradiol in MCF-7 cells is regulated by NF-Y-Sp1/estrogen receptor interactionsMol Endocrinol19991381373138710446910
- PaechKWebbPKuiperGGDifferential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sitesScience19972775331150815109278514
- JakackaMItoMWeissJChienPYGehmBDJamesonJLEstrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathwayJ Biol Chem200127617136151362111278408
- WattersJJCampbellJSCunninghamMJKrebsEGDorsaDMRaid membrane effects of steroids in neuroblastoma cells: effects of estrogen on mitogen activated protein kinase signaling cascade and c-fos immediate early gene transcriptionEndocrinology19971389403040339275096
- BeatsonGTEdinMDOn the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment, with illustrative casesLancet18961483803162165
- PatersonRRussellMHClinical trials in malignant disease: part II breast cancer: value of irradiation of the ovariesJ Fac Radiol195910313013324546197
- WalkerKJWalkerRFTurkesAEndocrine effects of combination antioestrogen and LH-RH agonist therapy in premenopausal patients with advanced breast cancerEur J Cancer Clin Oncol19892546516542523808
- NicholsonRIWalkerKJMcClellandRADixonARobertsonJFBlameyRWZoladex plus tamoxifen versus Zoladex alone in pre- and peri-menopausal metastatic breast cancerJ Steroid Biochem Mol Biol19903769899952149510
- RobertsonJFWalkerKJNicholsonRIBlameyRWCombined endocrine effects of LHRH agonist (Zoladex) and tamoxifen (Nolva-dex) therapy in premenopausal women with breast cancerBr J Surg19897612126212652532556
- CohenITepperRFigerAFlexDShapiraJBeythYSuccessful co-treatment with LHRH-agonist for ovarian over-stimulation and cystic formation in premenopausal tamoxifen exposureBreast Cancer Res Treat199955211912510481939
- International Breast Cancer Study Group (IBCSG)Castiglione-GertschMO’NeillAAdjuvant chemotherapy followed by goserelin versus either modality alone for premenopausal lymph node-negative breast cancer: a randomized trialJ Natl Cancer Inst200395241833184614679153
- DavidsonNEO’NeillAMVukovAMChemoendocrine therapy for premenopausal women with axillary lymph node-positive, steroid hormone receptor-positive breast cancer: results from INT 0101 (E5188)J Clin Oncol200523255973598216087950
- ArriagadaRLêMGSpielmannMRandomized trial of adjuvant ovarian suppression in 926 premenopausal patients with early breast cancer treated with adjuvant chemotherapyAnn Oncol200516338939615677625
- KaufmannMGrafEJonatWA randomised trial of goserelin versus control after adjuvant, risk-adapted chemotherapy in premeno-pausal patients with primary breast cancer – GABG-IV B-93Eur J Cancer200743162351235817897821
- BaumMHackshawAHoughtonJAdjuvant goserelin in pre-menopausal patients with early breast cancer: results from the ZIPP studyEur J Cancer200642789590416545560
- Adjuvant Breast Cancer Trials Collaborative GroupOvarian ablation or suppression in premenopausal early breast cancer: results from the international adjuvant breast cancer ovarian ablation or suppression randomized trialJ Natl Cancer Inst200799751652517405996
- RochéHKerbratPBonneterreJComplete hormonal blockade versus epirubicin-based chemotherapy in premenopausal, one to three node-positive, and hormone-receptor positive, early breast cancer patients: 7-year follow-up results of French Adjuvant Study Group 06 randomised trialAnn Oncol20061781221122716731539
- Early Breast Cancer Trialists’ Collaborative GroupSystemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy: 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 womenLancet1992339878571851345869
- Early Breast Cancer Trialists’ Collaborative GroupOvarian ablation in early breast cancer: overview of the randomised trialsLancet19963489036118911968898035
- SharmaRHamiltonABeithJLHRH agonists for adjuvant therapy of early breast cancer in premenopausal womenCochrane Database Syst Rev20084CD00456218843661
- Early Breast Cancer Trialists’ Collaborative GroupEffects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trialsLancet200536594721687171715894097
- LHRH-Agonists in Early Breast Cancer Overview GroupCuzickJAmbroisineLUse of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trialsLancet200736995741711172317512856
- EifelPAxelsonJACostaJNational Institutes of Health Consensus Development conference statement: adjuvant therapy for breast cancer, November 1-3, 2000J Natl Cancer Inst2001931397998911438563
- GoldhirschAGlickJHGelberRDCoatesASSennHJMeeting highlights: International Consensus Panel on the treatment of primary breast cancer. Seventh International Conference on adjuvant therapy of primary breast cancerJ Clin Oncol200119183817382711559719
- BlameyRWfor EUSOMAGuidelines on endocrine therapy of breast cancer EUSOMAEur J Cancer200238561563411916542
- TolisGAckmanDStellosATumor growth inhibition in patients with prostatic carcinoma treated with luteinizing hormone-releasing hormone agonistsProc Natl Acad Sci U S A1982795165816626461861
- ReddingTWSchallyAVInhibition of mammary tumor growth in rats and mice by administration of agonistic and antagonistic analogs of luteinizing hormone-releasing hormoneProc Natl Acad Sci U S A1983805145914626219395
- SchallyAVReddingTWComaru-SchallyAMInhibition of the growth of some hormone dependent tumors by D-Trp6-LH-RHMed Oncol Tumor Pharmacother1984121091186242477
- NériCBlangyDSchatzBDrieuKBerebbiMMartinPMDirect inhibiting effects of [D-Trp6]gonadotropin-releasing hormone on the estrogen-sensitive progression of polyoma virus-induced mammary tumors in athymic miceCancer Res19905018589258972168284
- GründkerCGünthertARHellriegelMEmonsGGonadotropin-releasing hormone (GnRH) agonist triptorelin inhibits estradiol-induced serum response element (SRE) activation and c-fos expression in human endometrial, ovarian and breast cancer cellsEur J Endocrinol2004151561962815538941
- PasqualiniJRBlumberg-TickJNguyenBLEffect of triptorelin (Decapeptyl) combined with heparin on estradiol levels in MCF-7 mammary cancer cells after incubation with estrone sulfateActa Endocrinol (Copenh)199312932602628212992
- KlijnJGMDe JongFHTreatment with a luteinising hormone releasing hormone analogue (buserelin) in pre-menopausal patients with metastatic breast cancerLancet1982319828312131216
- KlijnJGDe JongFHLambertsSWBlankensteinMALHRH agonist treatment in clinical and experimental human breast cancerJ Steroid Biochem1985235B8678733935869
- PlowmanPNNicholsonRIWalkerKJRemission of postmenopausal breast cancer during treatment with the luteinising hormone releasing hormone agonist ICT 118630Br J Cancer1986549039092948537
- SchwartzLGuiochetNKeilingRTwo partial remissions induced by a LHRH analogue in two postmenopausal women with metastatic breast cancerCancer19886212249825002973369
- Nesković-KonstantinovićZBVuletićLBNikolić-StanojevićLITherapeutic and endocrine effects of Decapeptyl, synthetic LH-RH agonistic analogue in premenopausal women with metastatic breast cancer. A pilot phase II studyOncology1994511951018265112
- MariniLIacopinoFSchinzariGRobustelli della CunaFSMantovaniGSicaGDirect antiproliferative effect of triptorelin on human breast cancer cellsAnticancer Res1994145A188118857847821
- Sanchez-GarridoFComaru-SchallyAMSanchez del CuraGGonzales-EnriquezJSchallyAVClearance of lung metastases of breast carcinoma after treatment with triptorelin in postmenopausal womanLancet19953458953868
- Garcia-GiraltEBeuzebocPDierasVPhase II trial of decapeptyl (D-TRP-6), a potent luteinizing hormone-releasing hormone analogue in untreated advanced breast cancerAm J Clin Oncol19961954554588823473
- CelioLMartinettiAFerrariLPremenopausal breast cancer patients treated with a gonadotropin-releasing hormone analog alone or in combination with an aromatase inhibitor: a comparative endocrine studyAnticancer Res1999193B2261226810472341
- GoldhirschAGelberRDCastiglioneMThe magnitude of endocrine effects of adjuvant chemotherapy for premenopausal breast cancer patients: the International Breast Cancer Study GroupAnn Oncol1990131831882261364
- BiancoARDel MastroLGalloCPrognostic role of amenorrhoea induced by adjuvant chemotherapy in premenopausal patients with early breast cancerBr J Cancer19916357998032039706
- SwainSMJeongJHGeyerCEJrLonger therapy, iatrogenic amenorrhea, and survival in early breast cancerN Eng J Med20103622220532065
- LambertiniMCampbellCBinesJAdjuvant anti-HER2 therapy, treatment-induced amenorrhea (TIA) and survival in premenopausal patients (pts) with HER2-positive (HER2+) early breast cancer (EBC): analysis from the ALTTO trial (BIG 2-06) [abstract]Ann Oncol201728suppl5
- ReganMMPaganiOFlemingGFAdjuvant treatment of pre-menopausal women with endocrine-responsive early breast cancer: design of the TEXT and SOFT trialsBreast20132261094110024095609
- FrancisPAReganMMFlemingGFAdjuvant ovarian suppression in premenopausal breast cancerN Engl J Med2015372543644625495490
- PaganiOReganMMWalleyBAAdjuvant exemestane with ovarian suppression in premenopausal breast cancerN Engl J Med2014371210711824881463
- BernhardJLuoWRibiKAdjuvant exemestane versus tamoxifen in premenopausal women with early breast cancer undergoing ovarian suppression: patients reported outcomes in the TEXT and SOFT randomised trialsLancet Oncol201516784885826092816
- FlemingGFrancisPALángIRandomized comparison of adjuvant tamoxifen (T) plus ovarian function suppression (OFS) versus tamoxifen in premenopausal women with hormone receptor-positive (HR+) early breast cancer (BC): update of the SOFT trialOral presentation at: 2017 San Antonio Breast Cancer SymposiumDecember 5-9; 2017San Antonio, TX
- PaganiOReganMMFlemingGFRandomized comparison of adjuvant aromatase inhibitor exemestane (E) plus ovarian function suppression (OFS) vs tamoxifen (T) plus OFS in premenopausal women with hormone receptor positive (HR+) early breast cancer (BC): update of the combined TEXT and SOFT trialsOral presentation at: 2017 San Antonio Breast Cancer SymposiumDecember 5-9; 2017San Antonio, TX
- ReganMMFrancisPAPaganiOAbsolute benefit of adjuvant endocrine therapies for premenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative early breast cancer: TEXT and SOFT trialsJ Clinic Oncol2016341922212231
- RossiEMorabitoADe MaioEEndocrine effects of adjuvant letrozole+triptorelin compared with tamoxifen + triptorelin in premenopausal patients with early breast cancerJ Clin Oncol200826226427018086795
- Breast International Group (BIG)1-98 Collaborative GroupThürlimannBKeshaviahAA comparison of letrozole and tamoxifen in postmenopausal women with early breast cancerN Engl J Med2005353262747275716382061
- BelletMGrayKPFrancisPATwelve-month estrogen levels in premenopausal women with hormone receptor-positive breast cancer receiving adjuvant triptorelin plus exemestane or tamoxifen in the suppression of ovarian function trial (SOFT): the SOFT-EST substudy. [Abstract]J Clin Oncol201634141584159326729437
- Del MastroLBoniLMichelottiAEffect of the gonadotropin-releasing hormone analogue triptorelin on the occurence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trialJAMA2011306326927621771987
- LambertiniMBoniLMichelottiAOvarian suppression with triptorelin during adjuvant breast cancer chemotherapy and long-term ovarian function, pregnancies, and disease-free survival: a randomized clinical trialJAMA2015314242632264026720025
- MooreHCUngerJMPhilipsKAGoserelin for ovarian protection during breast-cancer adjuvant chemotherapyNEJM20153721092393225738668
- BuchholzSSeitzSSchallyAVTriple-negative breast cancers express receptors for luteinizing hormone-releasing hormone (LHRH) and respond to LHRH antagonist cetrorelix with growth inhibitionInt J Oncol200935478979619724914
- SchubertAHawighorstTEmonsGGründkerCAgonists and antagonists of GnRH-I and-II reduce metastasis formation by triple-negative human breast cancer cells in vivoBreast Cancer Res Treat2011130378379021279682
- AIOM-AIRTUM Working GroupThe number of Cancer in Italy [I numeri del Cancro in Italia]Il Pensiero Scientifico EditoreRome20167582
- StensheimHCvancarovaMMøllerBFossåSDPregnancy after adolescent and adult cancer: a population-based matched cohort studyInt J Cancer201112951225123621387311
- LetourneauJMSmithJFEbbelEERacial, socioeconomic, and demographic disparities in access to fertility preservation in young women diagnosed with cancerCancer2012118184579458822451228
- RuddyKJGelberSITamimiRMProspective study of fertility concerns and preservation strategies in young women with breast cancerJ Clin Oncol201432111151115624567428
- PartridgeAHGelberSPeppercornJWeb-based survey of fertility issues in young women with breast cancerJ Clin Oncol200422204174418315483028
- LeeSJSchoverLRPartridgeAHAmerican Society of Clinical Oncology recommendations on fertility preservation in cancer patientsJ Clin Oncol200624182917293116651642
- LambertiniMDel MastroLPescioMCCancer and fertility preservation: international recommendations from an expert meetingBMC Med2016141126728489
- National Comprehensive Cancer Network Clinical Practice Guidelines in OncologyBreast Cancer2017 Available from https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- PeccatoriFAAzimHAJrOrecchiaRCancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-upAnn Oncol201324suppl 616017022904238
- Paluch-ShimonSPaganiOPartridgeAHSecond international consensus guidelines for breast cancer in young women (BCY2)Breast201626879927017247
- LorenAWManguPBBeckLNFertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline updateJ Clin Oncol201331192500251023715580
- Italian Association of Medical Oncology (AIOM)Clinical Practice Guideline on Fertility Preservation in Cancer Patients2016 Available from http://www.aiom.it/professionisti/documenti-scientifici/linee-guida/2016_LGAIOM_Preserv_fertil.pdf
- BigliaNTorrisiRD’AlonzoMCodacci PisanelliGRotaSPeccatoriFAAttitudes on fertility issues in breast cancer patients: an Italian surveyGynecol Endocrinol201531645846425982361
- MooreHCFUngerJMPhillipsKAFinal analysis of SWOG S0230/Prevention of early menopause study (POEMS)Poster presentation at: 2017 San Antonio Breast Cancer SymposiumDecember 5-9; 2017San Antonio, TX
- LambertiniMCeppiMPoggioFOvarian suppression using luteinizing hormone releasing hormone agonists during chemotherapy to preserve ovarian function and fertility of breast cancer patients: a meta-analysis of randomized studiesAnn Oncol201526122408241926347105
- CoatesASWinerEPGoldhirschATailoring therapies-improving the management of early breast cancer: St Gallen International Expert Consensus on the primary therapy of early breast cancer 2015Ann Oncol20152681533154625939896
- LeonardRCFAdamsonDJABertelliGGnRH agonist for protection against ovarian toxicity during chemotherapy for early breast cancer: the Anglo Celtic Group OPTION trialAnn Oncol20172881811181628472240
- GerberBvon MinckwitzGStehleHEffect of luteinizing hormone-releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: the GBG 37 ZORO studyJ Clin Oncol2011292334234121537042
- MunsterPNMooreAPIsmail-KhanRRandomized trial using gonadotropin-releasing hormone agonist triptorelin for the preservation of ovarian function during (neo)adjuvant chemotherapy for breast cancerJ Clin Oncol201230553353822231041
- LambertiniMMooreHCFLeonardRCFPooled analysis of five randomized trials investigating temporary ovarian suppression with gonadotropin-releasing hormone analogs during chemotherapy as a strategy to preserve ovarian function and fertility in premenopausal early breast cancer patients [abstract]. San Antonio Breast Cancer Symposium; December 5-9, 2017; San Antonio, TXCancer Res2018784 Suppl abstract GS4-01
- Del MastroLLambertiniMGonadotropin releasing hormone analogs for ovarian function protection during chemotherapy in young early breast cancer patients: the last piece of the puzzle?Ann Oncol20172881683168528838207
- LambertiniMBoniLMichelottiALong-term outcome results of the phase III PROMISE-GIM6 study evaluating the role of LHRH analog (LHRHa) during chemotherapy (CT) as a strategy to reduce ovarian failure in early breast cancer (BC) patients [abstract]J Clin Oncol20143226 abstract105
- ElgindyEAEl-HaiegDOKhorshidOMGonadatrophin suppression to prevent chemotherapy-induced ovarian damage: a randomized controlled trialObstet Gynecol20131211788623262931
