Abstract
In normal cell cycle progression, transition of G0/G1 phase to synthesis (S) phase for breast and other cells is regulated by association of cyclin D and cyclin-dependent kinases 4 and 6 (CDK4/6) that leads to phosphorylation of retinoblastoma (Rb) protein. Imbalance of this cyclin D-CDK4/6-inhibitors of CDK4/6-Rb phosphorylation pathway is associated with tumorigenesis of hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) breast cancers. Despite effective first-line endocrine therapy, HR+/HER2− metastatic breast cancers remain still incurable. Currently, advances in understanding of cell cycle checkpoints are evolving as promising strategy to target in treatment of various types of cancers including breast cancer. Therapies that target this cell cycle machinery in HR+/HER2− breast cancers are getting approval by the US Food and Drug administration (FDA) including ribociclib (LEE011). Ribociclib got the first FDA approval in March 13, 2017, as an initial therapy for HR+/HER2− advanced or metastatic breast cancer in combination with an aromatase inhibitor. This review, therefore, addresses the role of selective CDK4/6 inhibitors in advanced or metastatic breast cancer with a specific focus on ribociclib. Some findings of clinical trials involving ribociclib found pivotal benefits of ribociclib in HR+/HER2− metastatic breast cancer in terms of prolonging progression-free survival and objective response rates. Daily dosage range of the drug for such benefits is 50–900 mg with common daily doses of 400 or 600 mg and 600 mg in early and advanced breast cancer therapies, respectively. Along with its therapeutic benefits, however, more incident but manageable dose-limiting grade 3 or 4 toxicities, primarily hematologic adverse events, are common in patients treated with ribociclib. Generally, there are several active clinical trials undergoing to investigate the clinical efficacy and toxicity profile of the drug in various cancerous conditions other than breast cancer and will likely benefit patients with other cancer types.
Introduction
Overview of cell cycle pathways and cyclins/cyclin-dependent kinases
To keep homeostasis, cellular multiplication processes and associated programmed cell death (apoptosis) need to be regulated. However, improper signal passed on to cell cycle regulators (e.g., cyclins, cyclin-dependent kinases [CDKs], and endogenous CDK inhibitors) as a result of mutation and other related factors is associated with tumorigenesis of many cancersCitation1–Citation5 including breast cancer.Citation2 This means that normal cyclins and CDKs are deregulated and/or apoptosis is inappropriately regulated in the cancers accounting for unrestrained cellular duplication as hallmark of cancer cells.Citation4,Citation6–Citation8 Therefore, understanding the normal cellular progression and development machineries is critical to effective or targeted treatment of cancers including breast cancer.
Majority of normal human cells reside in a detained cell cycle state called G0 phase.Citation8,Citation9 The detained state can be either transient or permanent. The transient (G0 phase) cells can be potentiated to reenter the cell cycle by various factors that include CDKs and their respective regulatory subunits called cyclins.Citation1,Citation4,Citation8,Citation9 More specifically, most of the factors, through activation of cascades of intracellular signaling pathways, cause CDK4 and CDK6 to instigate the cell cycle progression from G0/G1 transition state to synthesis (S) phase.Citation9 In G1 phase, association of cyclin D with CDK4 and/or CDK6 forms a complex that results in the activation of CDK4/6.Citation10–Citation12 In turn, the activated complex of cyclin-CDK4/6 can phosphorylate a signaling protein called retinoblastoma (Rb).Citation8,Citation10 The later process leads to dictation of genes required for G1/S transition and move on to cell cycle progression.Citation10 At this stage, targeted inhibition of the regulators of G1/S transition checkpoint can arrest the cellular cycle from progressing to S phase.Citation13 Likewise, the necessary instigation for cellular progression form G1/S transition and S phase of cell cycle to subsequent phases is regulated by cyclin E-CDK2 and cyclin A-CDK2, respectively. Similar pathways occur at G2 and mitosis (M) phases being regulated by cyclin A-CDK1 and cyclin B-CDK1, respectively.Citation4,Citation8,Citation10 For more detail understanding, the aforementioned descriptions of cellular processes and regulatory pathways are clearly portrayed in .
Figure 1 Illustrated description of cell cycle progression and potential pathways for cancer therapy.
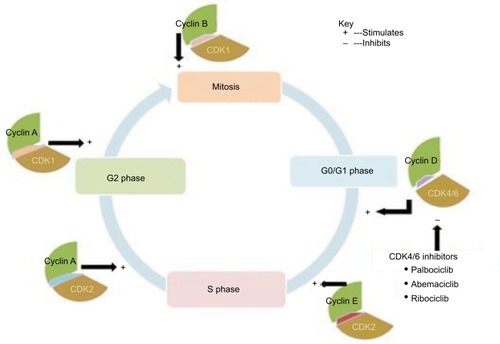
In normal cells, the activities of CDKs are controlled positively by associating primarily with the “D cyclins” (D1, D2, and D3) and ‘cyclins A, B, and E’; this move on pathways are blocked by endogenous inhibitors of CDK (INK) such as p16INK4A, p15INKB, p18INK4C, and p19INK4D family proteins.Citation9 Moreover, besides the regulation of cell cycle progression, CDKs in the presence of their respective cyclins can form families of heterodimeric kinases, which play pivotal roles in key life processes like metabolic function, dictation of genetic material, and neuronal delineation.Citation4
Methods
In our literature search strategy, we employed Boolean operators (AND, OR, NOT) for various combinations of key search terms: “cell cycle*”, “cyclin-dependent kinase 4/6”, “HR+/HER2−”, “metastatic breast cancer”, “CDK4/6 inhibitors”, “Ribociclib”, and “LEE011”. Truncation was applied to increase the probability of getting key and other related articles for the present topic. Accordingly, searches for the topic in indexing services and databases involving PubMed, PubMed Central, MEDLINE, Scopus, and Pro-Quest and other additional data sources (Google scholar and WorldCat) resulted in a retrieval of 925 articles. Next to these searches, authors employed in-depth screening of articles to remove duplicate articles, titles and abstracts not related to the current topic, abstracts with limited access to full texts, and full-text articles that lack sufficient data for the required information. Lastly, 79 references were included for the study among which 29 original articles were critically reviewed to sum up the current therapeutic benefits of selective CDK4/6 inhibitors with special focus on ribociclib. With regard to data extraction, general information linked to the roles of cyclins/CDKs in cell cycle progression both in normal and cancerous conditions including breast cancer; current challenges to treatment of metastatic breast cancer; and the role of selective CDK4/6 inhibitors in hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) metastatic breast cancer and other cancer types were overviewed. Concerning to drug of interest, ribociclib, data pertaining to primary or secondary endpoints of preclinical and clinical studies, its pharmacokinetics and toxicity profiles were considered. Moreover, relevant information about the undergoing clinical trials involving ribociclib in various cancerous conditions was also reviewed via a separate visit to official clinical trial website of the National Library of Medicine (www.clinicaltrials.gov). The articles were searched (collected) during July–August 2017.
Current challenges to treatment of advanced breast cancer
Approximately up to 60%–75% of breast cancers are HR+ and respond well to first-line endocrine therapy (ET).Citation14,Citation15 Yet HR+ breast cancers may become recurrent despite the effective first-line ET as a result of various factors related to metastasis and/or resistance, among others. Over-expression of CDK6, for instance, was found to mediate shedding of both estrogen receptor (ER) and progesterone receptor. This process can reduce responsiveness of the ER to blockage by ET, ultimately leading to increased resistance.Citation16 Similarly, a deficiency of activated (phosphorylated) Rb in conjunction with elevated CDKN2A and CCNE1 levels can also drive resistance to ET.Citation17 Moreover, the resistance to ET in metastatic breast cancer is also associated with genomic alterations and mutational signatures.Citation18
Advanced knowledge of the cell cycle checkpoints connected with regulation of instigation and succession of many cancers including breast cancer has attributed to the discovery of new drug targets for therapy.Citation2,Citation16,Citation19,Citation20 Consequently, findings from various preclinical and clinical settings advocate targeting the cell cycle pathways as an attractive strategy to arrest cancer progression.Citation21 Although a dramatic clinical shift was achieved with targeted therapies of patients with estrogen receptor-positive (ER+) and HER2− breast cancer, metastasis and resistance to the therapies made the cancer to remain still deadly and incurable.Citation20,Citation21
Currently, first-line therapy for hormone (estrogen or progesterone) receptor-positive (HR+), HER2− (HR+/HER2−) advanced or metastatic breast cancer is ET.Citation22–Citation24 However, since resistance and metastasis connected with the HR+/HER2− advanced breast cancer occurred even during ET, therapies targeting regulators of cell cycle are evolving to augment the ET.Citation1,Citation22,Citation25 The resistance to ET is correlated with cyclin D-CDK4/6–phosphorylation of Rb pathway. Hence, effectiveness of the ET can potentially be improved by the CDK4/6 inhibitors that can arrest the resistance pathway at the cyclin D-CDK4/6 checkpoint.Citation5,Citation26 For this reason, targeted therapies with specific progression or resistance pathway inhibition, with different modes of action, and with additive or synergistic inhibition of tumorigenesis are among key current recommendations pertaining to the treatment of HR+/HER2− advanced or metastatic breast cancer.Citation23 The selective CDK4/6 inhibitors and their associated cyclins are among the key targeted therapies that have shown clinical benefit in HR+/HER2− metastatic breast cancer.Citation22,Citation27 There fore, this review aims to address the current advances in selective CDK4/6 inhibitors as potential therapeutic targets for treatment of metastatic breast cancer with a specific focus on the newly approved CDK4/6 inhibitor called ribociclib (LEE011).
Role of selective CDK4/6 inhibitors in advanced breast cancer
Contrary to traditional antineoplastic agents, which kill dividing cells by interfering with DNA replication (S phase) or mitosis (M phase) during the cell cycle, CDK4/6 inhibitors arrest tumorigenesis through the G1 phase. This activity promotes transient cell cycle withdrawal to enter into the G0 phase or permanent multiplicative arrest.Citation28 Palbociclib (PD0332991) is the first selective CDK4/6 inhibitor that got approval by the US Food and Drug Administration (FDA) in 2015. It acts by binding to adenosine trisphosphate pockets with high selectivity for cyclin D1-CDK4, cyclin D3-CDK4, and cyclin D2-CDK6.Citation29 Besides, abemaciclib and ribociclib are the other selective CDK4/6 inhibitors that obtained first FDA approval very recently; they are also under clinical development for the treatment of advanced or metastatic cancers.Citation5,Citation23,Citation30–Citation33
The aforementioned targeted drugs (palbociclib, abemaciclib, and ribociclib) are highly selective CDK4/6 inhibitorsCitation34 with primary promising indications in postmenopausal women with HR+/HER2− advanced or metastatic breast cancer.Citation35 Sensitivity of luminal androgen receptor subtype of triple negative breast cancer to palbociclib and ribociclib is also reported.Citation36 A promising report with regard to palbociclib activity on metastatic luminal breast cancer in combination with ET also supplements the role of selective CDK4/6 inhibitors in other subtypes of breast cancer.Citation37 There are several effectiveness data linked to CDK4/6 inhibition in advanced breast cancer. This evidence may warrant clinical trial of CDK4/6 inhibition in other cancer types that will probably benefit patients.Citation38 However, extending the use of CDK4/6 inhibitors beyond HR+ breast cancer will likely require the use of biomarkers to predict and optimize their response.Citation39
On the top of efficacy in breast cancers, the selective CDK4/6 inhibitors are also similar in some of their adverse event (AE) profiles. In the group, hematologic toxicities like neutropenia and leukopenia are the leading but manageable AEs. As a result, their dosing is based mostly on 3 weeks-on/1 week-off schedule to allow patients recover from such toxicities.Citation34 Moreover, the CDK4/6 inhibitors are used mainly in combination with ET (e.g., aromatase inhibitors) to optimally lengthen effectiveness of the ET in breast cancer therapy.Citation40 For these and other relevant reasons, palbociclib, abemaciclib, and ribociclib are now getting to clinical practice in combination with endocrine-based therapy ().Citation41
Table 1 Overview of common FDA-approved/investigational CDK4/6 inhibitors and their potential treatment profile
Owing to different reasons, monotherapy of the selective CDK4/6 inhibitors is of limited efficacy against advanced cancers. First, the cell cycle regulation that can be brought about by the CDK4/6 inhibitors per se would not be complete as there are other pathways regulating the cell cycle progression. Second, there is also premature adaptation to the CDK4/6 inhibitors that can terminate effectiveness of the drugs.Citation27 Overall, palbociclib, abemaciclib, and ribociclib are under extensive clinical development and got the FDA approval for recurrent or metastatic breast cancers mainly in combination with endocrine-based therapy. Accordingly, it is recommended that optimal use of the CDK4/6 inhibitors in metastatic breast cancer should be in combination with another agent (s).Citation23,Citation27,Citation42
Despite the various similarities among the CDK4/6 inhibitors with regard to their efficacy in treating advanced breast cancer, their dosing schedule, and superiority of their combined use as already mentioned, they also have differences. For instance, fatigue and gastrointestinal toxicities are more common with abemaciclib treatment than with ribociclib and palbociclib treatments.Citation43 Major dose-limiting toxicities (DLTs) with palbociclib and ribociclib treatments are hematologic, while these are fatigue and/or gastrointestinal toxicities with the abemaciclib treatment.Citation43,Citation44 Abemaciclib has an established central nervous system penetration for the treatment of certain primary or malignant cancers in brain,Citation43,Citation45 while the brain distribution of ribociclib and palbociclib is limited.Citation43 However, in vitro sensitivity to palbociclib in studies involving patient derived glioblastoma (the most frequent malignant form of brain cancer); glioblastoma multiforme; brainstem glioma of genetically engineered mouse model; and rodents and the reach of unbound brain levels for the drug contradict its limited distribution to the central nervous system.Citation45–Citation48 Generally, the selective CDK4/6 inhibitors share similar efficacy in the treatment of advanced cancers including breast cancer, but biomarker-based trials are necessary to identify patients for whom the CDK4/6 inhibition is cost-effective or not.
In next sections, we would like to review research evidences pertaining to chemistry, pharmacology (pharmaco-kinetics), toxicity profiles, and clinical trials of ribociclib focusing mainly on its current advances in metastatic breast cancer. However, it would be logical to highlight the other core selective CDK4/6 inhibitors prior to the ribociclib discussion.
Palbociclib
Palbociclib is the first class of CDK4/6 inhibitor that got initial FDA approval in the year 2015.Citation49,Citation51,Citation52 In ER+ breast cancers, estrogen provokes CDK4 and CDK6 activity leading to excessive phosphorylation of Rb and promotion of cell cycle progression.Citation61,Citation62
In a Phase 3 study of patients with HR+/HER− metastatic breast cancer (Palbociclib: Ongoing Trials in the Management of Breast Cancer–3 [PALOMA-3]), the addition of palbociclib to a treatment with fulvestrant significantly increased the median progression-free survival (PFS) of the patients to 9.2 months from 3.8 months of PFS with fulvestrant alone.Citation61–Citation63 In addition, greater clinical benefit rate (CBR) and overall objective response rate (ORR) were also reported among the palbociclib-fulvestrant group than the placebo-fulvestrant group (10.4% and 34.0% for palbociclib vs. 6.3% and 19.0% for placebo) ().Citation62
Table 2 Selected completed clinical trials of CDK4/6 inhibitors in HR+/HER2− metastatic breast cancer
In a similar manner, in Phase 2 study (PALOMA-2) of palbociclib and letrozole compared to placebo and letrozole, a significant benefit of palbociclib was reported in terms of increasing median months of PFS (24.8 months vs. 14.5 months), ORR (55.3% vs. 44.4%), and CBR (84.3% vs. 70.8%) ().Citation14 A perspective also reports Phase 1 trial finding of the drug as striking in which palbociclib combined with letrozole had a significantly prolonged PFS compared to letrozole alone among women with ER+/HER2− metastatic breast cancer.Citation28
Abemaciclib
Abemaciclib (Lilly) is another dual selective CDK4 and CDK6 inhibitor under extensive clinical trials that involve combination of the drug with other pathway inhibitors aimed mainly for treatment of various types and subtypes of cancers.Citation28 On September 28, 2017, the FDA approved abemaciclib in combination with fulvestrant for women with HR+/HER2− advanced or metastatic breast cancer with disease progression following ET.Citation33 This approval was based on finding of a single trial and it may not be full approval as the same organization has also granted priority review of the drug as both monotherapy and combination therapy.Citation64 Different from the other selective CDK4/6 inhibitors, abemaciclib has a promising effectiveness as monotherapy and its hematologic toxicities are less frequent than hematologic AEs of palbociclib and ribociclib.Citation65
In a 12-month analysis for Phase 2 study of abemaciclib as a single arm (MONARCH-1), striking results primarily in terms of median months of PFS (6 months), overall ORR (19.7%), CBR (42.4%), and overall survival rate (17.7 months) were reported among women with HR+/HER2− metastatic breast cancer who had progressed on or after prior ET and had 1 or 2 chemotherapy regimens in the metastatic setting.Citation53 Similarly, in a Phase 3 study (MONARCH-2) comparing efficacy of abemaciclib-fulvestrant and placebo-fulvestrant, the abemaciclib group had a significantly extended median month of PFS compared to placebo (16.4 months vs. 9.3 months; hazard ratio [HR] 0.553; 95% confidence interval [95% CI], 0.449–0.681; p<0.001). Moreover, ORR and CBR found among patients treated with abemaciclib-fulvestrant versus fulvestrant alone, respectively, were 48.1% (95% CI, 42.6%–53.6%; p<0.001) and 73.3% (95% CI, 68.4%–78.1%; p<0.001) versus 21.3% (95% CI, 15.1%–27.6%) and 51.8% (95% CI, 44.2%–59.5%) ().Citation57
Ribociclib: chemistry, pharmacology, clinical trials of efficacy, and toxicity profile
Chemistry
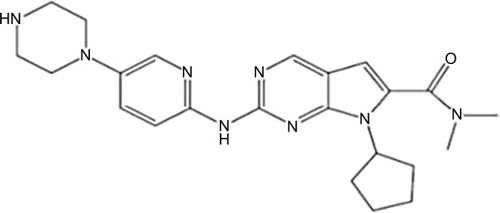
Ribociclib (LEE011; 7-cyclopentyl-N,N-dimethyl-2-<5-(piperazin-1-yl)pyridin-2-yl]amino}-7H-pyrrolo[2,3-d] pyrimidine-6-carboxamide) got the first FDA approval on March 13, 2017, as initial endocrine-based therapy for the treatment of postmenopausal women with HR+/HER2− advanced or metastatic breast cancer in combination with an aromatase inhibitor. It is a small sized molecule with molecular formula of C23H30N8O and molar mass of 434.55 g/mol ().Citation31,Citation59
Pharmacology
Ribociclib is an orally bioavailable drug that has a selective inhibitory activity on cyclin D1-CDK4 and cyclin D3-CDK6 complexes of the CDK target proteins through which it plays its G1 arrest role against cancer cell proliferation.Citation66 Because of its small size and high selectivity to the regulators of cellular pathways, it is becoming a key partner in strategies to improve efficacy of other anticancer drugs.Citation67 Thus, various preclinical studies witness its effectiveness as a monotherapy and/or a combination with other drugs in various cancer types (HR+ breast cancer, luminal breast cancer, metastasized solid tumors, neuroblastoma, and lymphoma)Citation68–Citation71 and other non- tumor conditions like acute kidney injury.Citation72 In addition, the drug’s efficacy and tolerability of its toxicities have also been shown in several trials (Phase I, II, and III) involving patients with various cancer types and subtypes.Citation15,Citation60,Citation73–Citation75
In a Phase 1 dose escalation trial of ribociclib monotherapy among 132 patients with advanced breast cancer or lymphoma at a starting dose of 50 mg/day orally on 3-weeks-on/1-week-off schedule or continuous oral dosing of 600 mg/day, the drug was rapidly absorbed with median time to maximum plasma concentration (Tmax) ranging from 1 to 5 hours.Citation74 After repeated daily dosing, plasma concentrations of the drug accumulated to approximately 2- to 3-fold at 21 days of first administration and steady state concentration was reached approximately on eighth day of initiation.Citation60,Citation74 In the plasma, ribociclib circulates approximately 70% by binding to plasma proteins.Citation76 It has an average accumulated dose half-life (t1/2) of approximately 32 hours.Citation43,Citation77 This long t1/2 enabled a once-daily dosing for the drug.Citation67 In addition, dose proportionality analyses over the dose range of 50–1,200 mg/day revealed that plasma concentration of ribociclib raised with the administered dose, with both peak concentration and area under the curve increasing slightly more than the proportion of dose increment.Citation74 Accordingly, on the 3-weeks-on/1-week-off schedule, maximum tolerated dose (MTD) of 900 mg/day and a recommended Phase 2 dose (RP2D) of 600 mg/day were established.Citation43 Parent ribociclib is primarily responsible for its desired effects and it is cleared either as metabolite or unchanged. Its metabolism is based mostly on the metabolic effects of cytochrome P450 (CYP) enzymes.Citation76,Citation77 Consequently, drugs with inhibitory effects against CYP1A2 and CYP3A4 can affect excretion of the drug.Citation77
Similarly, a Phase I study in pediatric patients by Geoerger et al on the treatment of ribociclib revealed that the drug was rapidly absorbed after oral administration and Tmax reaches in between 2 and 4 hours across dose levels (280, 350, or 470 mg/m2). Its bioavailability was dose-dependent and this was optimum for a dose range between 350 and 470 mg/m2 (adult equivalent range is 600–900 mg). Consistent to adult patients, steady state for the drug was achieved approximately on 8th day of repeated dosing among pediatric patients. On day 15 of dosing, the overall accumulation of ribociclib was 2- to 3-fold and the median effective t1/2 across the dose levels ranged from 30 to 41 hours.Citation78
Clinical trials of efficacy
In a Phase 3 study (Mammary Oncology Assessment of LEE011’s (Ribociclib’s) Efficacy and Safety–2 [MON-ALEESA-2]) report, on comparing the efficacy of ribociclib-letrozole versus placebo-letrozole, it was found that the ribociclib group had more prolonged PFS (95% CI, 19.3 – not reached vs. 14.7 months [95% CI, 13.0–16.5]), greater ORR (52.7% vs. 37.1%), and higher CBR (80.1% vs. 71.8%) than the placebo group. Consequently, the PFS rate in the ribociclib group versus placebo group was 63.0% (95% CI, 54.6–70.3) vs. 42.2% (95% CI, 34.8%–49.5%) by interim analysis at 18 months and median PFS was not reached during the follow-up period ().Citation15
Differently, a study by Curigliano et al also assessed responsiveness of early breast cancer measured by Ki-67 level reduction among patients treated with ribociclib (400 or 600 mg/day)-letrozole (2.5 mg/day) versus letrozole alone. Accordingly, average decreases in the Ki-67-positive cell fraction were 69% (38%–100% in patients treated with letrozole 2.5 mg/day; n=2), 96% (78%–100% in patients treated with ribociclib 400 mg/day and letrozole; n=6), and 92% (75%–100% among patients treated with ribociclib 600 mg/day and letrozole; n=3).Citation60 Evidence of ribociclib efficacy was also measured using other markers which involve decreased quantities of phosphorylated Rb and genes expressed for CDK4, CDK6, CCND2, CCND3, and CCNE1.Citation60
Similarly, a relatively prolonged median month of PFS was also reported in a subgroup analysis for elderly women (n=295) with HR+/HER2− advanced breast cancer (MONA-LEESA-2) treated with ribociclib-letrozole (95% CI; 19.3 months – not reached) compared to those elderly women treated with placebo-letrozole (95% CI; 15.0 months – not reached).Citation75
Besides breast cancer therapy, ribociclib has activity on other solid tumors. Disease stabilization was considered as a meaningful treatment outcome in a Phase I study by Geoerger et al on the use of ribociclib at intermittent dosing schedule of ≥280 mg/m2 (equivalent adult dose of ≥400 mg) among pediatric patients with malignant rhabdoid tumors (MRT), neuroblastoma, and other solid tumors. Accordingly, 7 patients with neuroblastoma and 2 patients with primary central nervous system MRT treated with ribociclib got the best overall response of stable disease and they were able to receive ribociclib for more than 4 cycles (prolonged disease stabilization).Citation78
Furthermore, several clinical trials are underway to investigate the safety and efficacy of ribociclib (LEE011) alone or in combination with other medications for the treatment of various cancers of different histologic conditions that involve neuroblastoma, glioblastoma, metastatic sarcoma, advanced malignant solid neoplasm, lymphomas, malignant neoplasms, MRT, teratoma and ovarian, fallopian tube, gastrointestinal, neuroendocrine, breast and prostate cancers (). As shown in , in most trials, primary outcomes for efficacy measurement are PFS, overall response, 50% reduction in biomarker, and CBR, while measures of safety are DLTs, incidence of AEs, MTD, recommended dose for expansion, and RP2D (www.clinicaltrials.gov).
Table 3 Pivotal undergoing clinical trials of ribociclib or LEE011 for the treatment of various cancers (www.clinicaltrials.gov)
Toxicity profile
Along with the added benefits of ribociclib in advancing the current therapy of metastatic breast cancers, dose-limiting AEs, more specifically grade 3 or 4 events, need to be critically monitored in clinical practice. Grade 3 or 4 AEs refer to toxicities that are incident in greater than 5% of the patients receiving the drug. This section, therefore, addresses all grades and/or grade 3/4 AEs of the ribociclib.
Many studies have reported various AEs without regard to grades of the events (all grades) and/or with specific focus on grade 3 and 4.Citation15,Citation74,Citation78 In a study by Hortobagyi et al, the most common all grade or grade 3/4 AEs were found to be more frequent in patients treated with the regimen containing ribociclib than those patients treated with letrozole alone. Accordingly, all grade toxicity profiles of treatments by ribociclib versus placebo were led mostly by hematologic AEs like neutropenia (74.3% vs. 5.2%), leukopenia (32.9% vs. 3.9%) along with non-hematologic AEs such as nausea (51.5% vs. 28.5%), infections (50.3% vs. 42.4%), fatigue (36.5% vs. 30.0%), diarrhea (35.0% vs. 22.1%), alopecia (33.2 % vs. 15.5%), vomiting (29.3% vs. 15.5%), increased alanine aminotransferase (ALT) level (15.6% vs. 3.9%), increased aspartate aminotransferase (AST) level (15.0% vs. 3.6%), and decreased appetite (18.6% vs. 15.2%).Citation15 From among the aforementioned AEs, grade 3 or 4 events encountered by patients treated with ribociclib versus placebo were neutropenia (59.3.0% vs. 0.9%), leukopenia (21.0% vs. 0.6%), increased ALT levels (9.3% vs. 1.2%), and increased AST levels (5.7% vs. 1.2%) ().Citation15,Citation79
Table 4 Most common adverse event profiles of ribociclib in adult and pediatric patients
Similarly, a Phase 1 dose escalation study of ribociclib by Infante et al also reported toxicity profile based mostly on hematologic AEs. As per the result, all grade AEs such as neutropenia (46%), leukopenia (43%), lymphopenia (30%), thrombocytopenia (24%), fatigue (45%), nausea (42%), anemia (26%), and prolonged electrocardiographic QTc (11%) were among the most incident events reported. Grade 3/4 AEs noted were also neutropenia (27%), leukopenia (17%), lymphopenia (16%), and thrombocytopenia (8%) ().Citation74 Moreover, in elderly women with HR+/HER2− advanced breast cancer treated by ribociclib versus placebo, grade 3/4 neutropenia (63% vs. 0%) and leucopenia (21% vs. 1%) were the frequent hematologic AEs.Citation75
Parallel to adult study findings already mentioned, hematologic toxicities were the most prevalent AEs in a Phase I study of ribociclib in pediatric patients. Accordingly, neutropenia (72%), leucopenia (63%), thrombocytopenia (44%), anemia (44), and lymphopenia (38%) were the frequent hematologic toxicities reported. Non-hematologic AEs such as vomiting (38%), fatigue (25%), nausea (25%), prolonged QTc (22%), decreased appetite (19%), and increased AST (16%) were also noted ().Citation78
Conclusion
The present review addressed recent advances of the selective CDK4/6 inhibitors as potential therapies in the treatment of HR+/HER2− metastatic breast cancer or other cancer types with similar tumorigenesis pathways. Ribociclib is key among the selective CDK4/6 inhibitors that obtained the FDA designation as initial treatment of postmenopausal women with HR+/HER2− metastatic breast cancer in combination with ET. Its potential therapeutic value in breast cancer and other cancer types hinges highly on its clinical benefits based mostly on prolongation of median months of PFS and CBR in patients treated with ribociclib versus placebo or monotherapy of ribociclib. The drug has a long t1/2, enabling a once-daily dosing. Despite its clinical benefits in advanced and other breast cancer subtypes, the drug is not without DLTs. Hematologic AEs like neutropenia and leucopenia are the most frequent AEs of the drug but they are manageable. Consequently, a dosing of 3-weeks-on/1-week-off schedule for the drug administration is commonly recommended for ease of managing its AEs. Generally, ribociclib is a promising drug for both early and advanced breast cancers. To this end, clinical trials of the drug in other subtypes of breast cancer and/or tumor types with the expression of cyclin D-CDK4/6-Rb pathways involving the use of biomarkers as measures of response will likely be expected to undergo for optimal benefit of patients with various cancer types.
Author contributions
Both authors designed the study, collected scientific literature, critically screened individual articles for inclusion, wrote the review article, and drafted the manuscript for publication. They also read and approved the final manuscript for publication.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The authors would like to thank Mr Fekede Asefa for his comments on an early draft of this piece and his editorial assistance, and Mrs Turu Rabira for her overall assistance.
References
- MikhailSAlbaneseCPishvaianMJCyclin-dependent kinase inhibitors and the treatment of gastrointestinal cancersAm J Pathol20151851185119725747534
- BeckJTPotential role for mammalian target of rapamycin inhibitors as first-line therapy in hormone receptor-positive advanced breast cancerOnco Targets Ther201583629363826675495
- HanahanDWeinbergRAHallmarks of cancer: the next generationCell2011144564667421376230
- PeyressatreMPrévelCPelleranoMMorrisMCTargeting cyclin-dependent kinases in human cancers: from small molecules to peptide inhibitorsCancers (Basel)20157117923725625291
- HamiltonEInfanteJRTargeting CDK4/6 in patients with cancerCancer Treat Rev20164512913827017286
- MalumbresMBarbacidMCell cycle, CDKs and cancer: a changing paradigmNat Rev Cancer20099315316619238148
- HanahanDWeinbergRAThe hallmarks of cancerCell20001001577010647931
- Suárez-ArroyoIJLoperena-AlvarezYRosario-AcevedoRMartínez-MontemayorMMGanoderma spp.: a promising adjuvant treatment for breast cancerMedicines (Basel)201741129
- OttoTSicinskiPCell cycle proteins as promising targets in cancer therapyNat Rev Cancer20171729311528127048
- JingYWangGGeYXuMTangSGongZAA-PMe, a novel asiatic acid derivative, induces apoptosis and suppresses proliferation, migration, and invasion of gastric cancer cellsOnco Targets Ther201691605162127073325
- LaPakKMBurdCEThe molecular balancing act of p16INK4a in cancer and agingMol Cancer Res201412216718324136988
- SherrCJCancer cell cyclesScience19962745239167216778939849
- VuHLAplinAETargeting mutant NRAS signaling pathways in melanomaPharmacol Res201610711111626987942
- FinnRSMartinMRugoHSPalbociclib and letrozole in advanced breast cancerN Engl J Med2016375201925193627959613
- HortobagyiGNStemmerSMBurrisHARibociclib as first-line therapy for HR-positive, advanced breast cancerN Engl J Med2016375181738174827717303
- YangCLiZBhattTAcquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependenceOncogene2017362255226427748766
- RaspéECoulonvalKPitaJMCDK4 phosphorylation status and a linked gene expression profile predict sensitivity to palbociclibEMBO Mol Med2017981052106628566333
- LefebvreCBachelotTFilleronTMutational profile of metastatic breast cancers: a retrospective analysisPLoS Med201613e100220128027327
- XuYQinLSunTTwist1 promotes breast cancer invasion and metastasis by silencing Foxa1 expressionOncogene2017361157116627524420
- WarburtonAJBooneDNInsights from global analyses of long non-coding RNAs in breast cancerCurr Pathobiol Rep201751233428616363
- CoronaSPRavelliACretellaDCDK4/6 inhibitors in HER2-positive breast cancerCrit Rev Oncol Hematol201711220821428325261
- BoérKFulvestrant in advanced breast cancer: evidence to date and place in therapyTher Adv Med Oncol20179746547928717399
- BrufskyAMDelaying chemotherapy in the treatment of hormone receptor–positive, human epidermal growth factor receptor 2-negative advanced breast cancerClin Med Insights Oncol2015913714726793013
- MalorniLPiazzaSCianiYA gene expression signature of retinoblastoma loss-of-function is a predictive biomarker of resistance to palbociclib in breast cancer cell lines and is prognostic in patients with ER positive early breast cancerOncotarget2016742680126802227634906
- SelliCDixonJMSimsHAAccurate prediction of response to endocrine therapy in breast cancer patients: current and future biomarkersBreast Cancer Res201618111827903276
- ReinertTBarriosCHOptimal management of hormone receptor positive metastatic breast cancer in 2016Ther Adv Med Oncol20157630432026557899
- Herrera-AbreuMTPalafoxMAsgharUEarly adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancerCancer Res20167682301231327020857
- SherrCJA new cell-cycle target in cancer — inhibiting cyclin D-dependent kinases 4 and 6N Engl J Med20163752019201923 Perspective27959598
- RoccaASchironeAMaltoniRProgress with palbociclib in breast cancer: latest evidence and clinical considerationsTher Adv Med Oncol2017928310528203301
- ParrishKEPokornyJMittapalliRKBakkenKSarkariaJNElmquistWFEfflux transporters at the blood-brain barrier limit delivery and efficacy of cyclin-dependent kinase 4/6 inhibitor palbociclib (PD-0332991) in an orthotopic brain tumor modelJ Pharmacol Exp Ther2015355226427126354993
- FDA. The U.S. Food and Drug Administration approved ribociclib (KISQALI, Novartis Pharmaceuticals Corp.), a cyclin-dependent kinase 4/6 inhibitor, in combination with an aromatase inhibitor as initial endocrine-based therapy for the treatment of postmenopausal women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer. ID: 4068375. Accessed March 13, 2017.
- RubovszkyGHorváthZRecent advances in the neoadjuvant treatment of breast cancerJ Breast Cancer201720211913128690648
- FDAFDA approves abemaciclib for HR+, HER2− breast cancerCancer Discov Epub2017104
- SpringLBardiaAModiSTargeting the cyclin D–cyclin-dependent kinase (CDK)4/6–retinoblastoma pathway with selective CDK 4/6 inhibitors in hormone receptor-positive breast cancer: rationale, current status, and future directionsDiscov Med201621113657426896604
- KwapiszDCyclin-dependent kinase 4/6 inhibitors in breast cancer: palbociclib, ribociclib, and abemaciclibBreast Cancer Res Treat20171661415428741274
- AsgharUSBarrARCuttsRSingle-cell dynamics determines response to CDK4/6 inhibition in triple negative breast cancerClin Cancer Res201723185561557228606920
- GampenriederSPRinnerthalerGGreilRCDK4/6 inhibition in luminal breast cancerMemo20169768127429659
- LallemandLDuhouxFPCDK 4/6 inhibitors in the treatment of advanced breast cancerBelg J Med Oncol201711234241
- O’LearyBFinnRSTurnerNCTreating cancer with selective CDK4/6 inhibitorsNat Rev Clin Oncol2016137418430
- SuhDHKimMKimKKimHJLeeKKimJWMajor clinical research advances in gynecologic cancer in 2016: 10-year special editionJ Gynecol Oncol2017283e4528382802
- TossAVenturelliMPeterleCPiacentiniFCascinuSCortesiLMolecular biomarkers for prediction of targeted therapy response in metastatic breast cancer: trick or treat?Int J Mol Sci2017181E8528054957
- LeeMSHelmsTLFengNEfficacy of the combination of MEK and CDK4/6 inhibitors in vitro and in vivo in KRAS mutant colorectal cancer modelsOncotarget2016726395953960827167191
- Barroso-SousaRShapiroGITolaneySMClinical development of the CDK4/6 inhibitors ribociclib and abemaciclib in breast cancerBreast Care (Basel)201611316717327493615
- SpringLMZangardiMLMoyBBardiaAClinical management of potential toxicities and drug interactions related to cyclin-dependent kinase 4/6 inhibitors in breast cancer: practical considerations and recommendationsOncologist20172291039104828706010
- RaubTJWishartGNKulanthaivelPBrain exposure of two selective dual CDK4 and CDK6 inhibitors and the antitumor activity of CDK4 and CDK6 inhibition in combination with temozolomide in an intracranial glioblastoma xenograftDrug Metab Dispos20154391360137126149830
- SchröderLBMcDonaldKLCDK4/6 inhibitor PD0332991 in glioblastoma treatment: does it have a future?Front Oncol2015525926649278
- BartonKLMisuracaKCorderoFPD-0332991, a CDK4/6 inhibitor, significantly prolongs survival in a genetically engineered mouse model of brainstem gliomaPLoS One2013810e7763924098593
- MichaudKSolomonDAOermannEPharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenograftsCancer Res20107083228323820354191
- IBRANCE® (palbociclib) capsules [prescribing information]New YorkPfizer Inc2015
- IBRANCE® (palbociclib). Fact sheetNew YorkPfizer Inc2015
- EMAAnnex 1: summary of product characteristicsEuropean Medicines Agency2015
- BeaverJAAmiri-KordestaniLCharlabRFDA approval: palbociclib for the treatment of postmenopausal patients with estrogen receptor–positive, HER2-negative metastatic breast cancerClin Cancer Res201521214760476626324739
- DicklerMNTolaneySMRugoHSMONARCH 1, a Phase 2 study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2− metastatic breast cancerClin Cancer Res201723175218522428533223
- KondoSYamamotoNTamuraKPhase 1 study of abemaciclib, a CDK 4 and 6 inhibitor, as a single agent for Japanese patients with advanced cancerEur J Cancer201551Suppl 3S59
- Eli Lilly and CompanyLilly Announces Phase 3 MONARCH 2 Breast Cancer Study of Abemaciclib Met Primary Endpoint of Progression-Free Survival Available from https://investor.lilly.com/releasedetail.cfm?ReleaseID=1017952Accessed August 16, 2017
- Eli Lilly and CompanyLilly Builds Upon Body of Data for Abemaciclib with Phase 3 MONARCH 2 Data Demonstrating Superior Progression-Free Survival in Advanced Breast Cancer Available from https://www.newswire.com/news/lilly-builds-upon-body-of-data-for-abemaciclib-with-phase-3-monarch-2-dataAccessed August 16, 2017
- SledgeGWJrToiMNevenPMONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapyJ Clin Oncol201735252875288428580882
- KISQALI® (ribociclib) tablets [prescribing information]East Hanover, NJNovartis Pharmaceuticals Corporation2017
- ChoyMPharmaceutical approval updateP T201742636637128579722
- CuriglianoGGómez PardoPMeric-BernstamFRibociclib plus letrozole in early breast cancer: a presurgical, window-of-opportunity studyBreast20162819119827336726
- CareyLAPerouCMPalbociclib — taking breast-cancer cells out of gearN Engl J Med2015373327327426176385
- TurnerNCRoJAndréFPALOMA3 Study GroupPalbociclib in hormone-receptor-positive advanced breast cancerN Engl J Med2015373320921926030518
- TurnerNCHuang BartlettCCristofanilliMPalbociclib in hormone-receptor–positive advanced breast cancerN Engl J Med20153731716721673
- Eli Lilly and CompanyFDA Grants Priority Review for Lilly’s Abemaciclib for the Treatment of Advanced Breast Cancer2017 Available from https://investor.lilly.com/releasedetail.cfm?ReleaseID=1032523Accessed October 18, 2017
- WolffACCDK4 and CDK6 inhibition in breast cancer — a new standardN Engl J Med2016375201993199527959592
- MurphyCGDicklerMNThe role of CDK4/6 inhibition in breast cancerOncologist201520548349025876993
- TripathyDBardiaASellersWRRibociclib (LEE011): mechanism of action and clinical impact of this selective cyclin-dependent kinase 4/6 inhibitor in various solid tumorsClin Cancer Res201723133251326228351928
- WoodACKrytskaKRylesHTDual ALK and CDK4/6 inhibition demonstrates synergy against neuroblastomaClin Cancer Res201723112856286827986745
- RaderJRussellMRHartLSDual CDK4/CDK6 inhibition induces cell-cycle arrest and senescence in neuroblastomaClin Cancer Res201319226173618224045179
- HartLSRaderJRamanPPreclinical therapeutic synergy of MEK1/2 and CDK4/6 inhibition in neuroblastomaClin Cancer Res20172371785179627729458
- ZhangYXSicinskaECzaplinskiJTAntiproliferative effects of CDK4/6 inhibition in CDK4-amplified human liposarcoma in vitro and in vivoMol Cancer Ther20141392184219325028469
- PablaNGibsonAABuegeMMitigation of acute kidney injury by cell-cycle inhibitors that suppress both CDK4/6 and OCT2 functionsProc Natl Acad Sci U S A2015112165231523625848011
- O’SullivanCCOvercoming endocrine resistance in hormone-receptor positive advanced breast cancer – the emerging role of CDK4/6 inhibitorsInt J Cancer Clin Res201524 pii:029
- InfanteJRCassierPAGerecitanoJFA Phase I study of the cyclin-dependent kinase 4/6 inhibitor ribociclib (LEE011) in patients with advanced solid tumors and lymphomasClin Cancer Res201622235696570527542767
- SonkeGSHartLLCamponeMEfficacy and safety of ribociclib (LEE011) + letrozole in elderly patients with hormone receptor-positive (HR+), HER2-negative (HER2−) advanced breast cancer (ABC) in MONALEESA-2Eur J Cancer201772Suppl 1S1S2
- SyedYYRibociclib: first global approvalDrugs201777779980728417244
- O’SullivanCCCDK4/6 inhibitors for the treatment of advanced hormone receptor positive breast cancer and beyond: 2016 updateExpert Opin Pharmacother201617121657166727322766
- GeoergerBBourdeautFDuBoisSGA Phase I study of the CDK4/6 inhibitor ribociclib (LEE011) in pediatric patients with malignant rhabdoid tumors, neuroblastoma, and other solid tumorsClin Cancer Res201723102433244128432176
- López-TarruellaSJerezYMárquez-RodasIEchavarriaIMartinMRibociclib for the treatment of advanced hormone receptor-positive, HER2-negative breast cancerFuture Oncol201713242137214928758424