Abstract
Breast cancer (BC) is a highly prevalent disease, accounting for the second highest number of cancer-related mortalities worldwide. The anthracycline doxorubicin (DOX), isolated from Streptomyces peucetius var. caesius, is a potent chemotherapeutic drug that is successfully used to treat various forms of liquid and solid tumors and is currently approved to treat BC. DOX exerts its effects by intercalation into DNA and inhibition of topoisomerases I and II, causing damage to DNA and the formation of reactive oxygen species (ROS), resulting in the activation of caspases, which ultimately leads to apoptosis. Unfortunately, DOX also can cause cardiotoxicity, with patients only allowed a cumulative lifetime dose of 550 mg/m2. Efforts to decrease cardiotoxicity and to increase the blood circulation time of DOX led to the US Food and Drug Administration (FDA) approval of a PEGylated liposomal formulation (L-DOX), Doxil® (known internationally as Caelyx®). Both exhibit better cardiovascular safety profiles; however, they are not currently FDA approved for the treatment of metastatic BC. Here, we provide detailed insights into the mechanism of action of L-DOX and its most common side effects and highlight results of its use in clinical trials for the treatment of BC as single agent and in combination with other commonly used chemotherapeutics.
Introduction
Breast cancer (BC) is the second most frequent cause of cancer-related deaths in women worldwide. It is a heterogeneous disease composed of multiple subtypes with distinct pathological features and clinical implications. Although men are affected, to a lesser degree, the most significant risk factors are gender (women) and older age. Other risk factors include obesity, estrogen exposure, alcohol consumption, and a family history.Citation1 Over the past 2 decades, accumulating evidence, both clinical and experimental, has suggested that BCs with different histopathological and biological features exhibit distinct behaviors that lead to different treatment responses and, therefore, should be given different therapeutic strategies.Citation2 On this basis, at diagnosis, BC patients are systematically tested for the presence of receptors, including estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), in order to explore tailored treatment options with molecularly targeted therapies. However, for patients who are triple negative (ER-, PR-, and HER2-), those who have innate or acquired resistance to targeted therapies, and patients whose disease has metastasized, traditional treatment options including surgery, radiotherapy, and chemotherapy are favored.
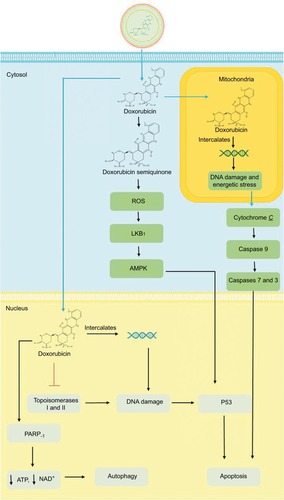
Anthracycline-based chemotherapy with doxorubicin (DOX) is one of the most efficacious anticancer agents for both early- and late-stage BCs.Citation3 DOX’s mechanism of action () on cancer cells begins with its passive diffusion through the phospholipid bilayer membrane of malignant cells into the cytoplasm, where DOX is converted into a semiquinone and generates reactive oxygen species (ROS), causing free radical formation and oxidative stress. In the cytosol, DOX enters the mitochondria causing DNA damage and energetic stress. As a result, the mitochondria release the cytochrome C protein, triggering the caspase cascade leading to cell death. From the cytosol, DOX translocates into the nucleus where it intercalates between double-stranded DNA helices and inhibits the enzymes topoisomerases I and II. The resulting damage to DNA leads to free radical generation, alkylation, and activation of the p53 pathway, hence inhibiting cell proliferation and inducing apoptosis. DOX can also hyperactivate the nuclear enzyme poly ADP ribose polymerase (PARP)-1, hence depleting the cell’s energy, thereby resulting in autophagy.Citation4–Citation6
Figure 1 Mechanism of action of DOX and L-DOX.
Abbreviations: DOX, doxorubicin; L-DOX, liposomal DOX; PARP, poly ADP ribose polymerase; ROS, reactive oxygen species.
However, the potential therapeutic benefits of DOX are limited by the risk of cardiotoxicity, which has been evidently related to its lifetime cumulative dose.Citation7–Citation9 To overcome this hurdle, the liposomal DOX (L-DOX) formulation was developed in order to reduce DOX-associated cardiotoxicity while preserving its antitumor efficacy.Citation10 The L-DOX formulation encapsulates DOX within a phospholipid bilayer that is coated with methoxypolyethylene glycol (). The PEGylation protects the liposomes from recognition by the mononuclear phagocyte system (MPS) and allows a longer circulation time in the bloodstream while reducing the exposure of free DOX circulating in the plasma. Biodistribution studies have shown that L-DOX has the ability to deposit and/or penetrate tumors and release DOX.Citation11 Although the mechanism of release of DOX from its liposomes is still unknown, a 10-fold higher exposure of L-DOX than DOX is observed in metastatic BC tissue as compared to healthy breast tissue,Citation12 which is explained by the enhanced permeability retention effect.Citation13 This indicates that a therapeutic approach with L-DOX is more targeted compared to DOX. A differential pharmacokinetic (PK) characteristic between the two formulations includes a decreased clearance (CL), a smaller volume of distribution, and a longer half-life for L-DOX compared to DOX as a result of the sequestration of liposomes, due to their increased size, in the sinusoidal lumen of the liver, which limits their flow from the fenestrations of the lumen into the hepatocytes as compared to free DOX. This is also believed to contribute to reduced hepatic extraction of L-DOX compared to DOX as a single agent.Citation14 The latter being primarily metabolized in the liver to the major cytotoxic metabolites: doxorubicinol and cytotoxic aglycones. The PK properties of L-DOX as compared to DOX are summarized in .
Figure 2 PEGylated liposomal DOX (Doxil®).
Abbreviation: DOX, doxorubicin.
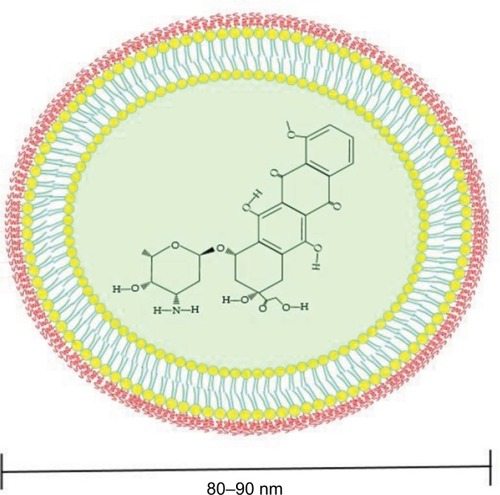
Table 1 Pharmacokinetic properties of DOX and L-DOX
The use of DOX as a stand-alone treatment has shown effectiveness in overall survival and response rate and time to disease progression.Citation15 Its limitations are seen in its effect on healthy cells and the resulting adverse effects, with the most significant being cardiotoxicity. The comparison of safety profiles between DOX and L-DOX is summarized in with the most common side effect for L-DOX being palmar–plantar erythrodysesthesia, a skin toxicity that can be managed with supportive care, unlike cardiotoxicity, which is significantly more prevalent for DOX.Citation16 Although the mechanism of DOX-induced cardiotoxicity is not fully understood, its administration is dose limited. It was shown that the risk of DOX-induced cardiotoxicity increases with the increase in its cumulative lifetime dose to becoming irreversible (ie, cardiomyocytes death) when the latter reaches 450–550 mg/m2.Citation17 The other major cardiotoxic effects of DOX are the occurrence of congestive heart failure (CHF) in >20% of the treated patients.Citation8 Additional but less detrimental adverse reactions include nausea, vomiting, gastrointestinal problems, neurological symptoms, and cutaneous injuries at the site of injection.Citation4
Table 2 Comparison of use, efficacy, and safety profiles of DOX and L-DOX
While L-DOX has proven to be advantageous over DOX as a single agent, it is only indicated in the treatment of metastatic BC in combination with docetaxel,Citation18 although it is frequently utilized as adjuvant therapy in metastatic BC.Citation19 The comparative anticancer efficacies of these two drugs in addition to their use in combination with other anticancer agents in BC continue to be investigated.Citation20 summarizes the main findings of trials assessing regimens containing L-DOX. These trials evaluated the efficacy of several combinations in varied patient populations including both locally advanced and metastatic BCs as well as the elderly and in patients with previously treated BCs. Overall, L-DOX was effective and well tolerated in the majority of trials, making it a feasible treatment option when combined with other chemotherapeutics. In the present work, we sought to investigate L-DOX’s efficacy on BC and its potential associated cardiac toxicity when combined with other chemotherapeutic or targeted therapy, with the goal of showcasing the beneficial effect of L-DOX over DOX on BC therapy and cardiac function.
Table 3 Clinical trials with L-DOX in combination with other chemotherapeutics in breast cancer
Methods
A literature search was conducted on PubMed, Google Scholar, and ClinicalTrials.gov using the following main keywords: liposomal DOX, Doxil® and BC, Caelyx® and BC, and Doxil® or Caelyx® and combinations in BC, to obtain relevant publications evaluating the safety and efficacy of L-DOX in the treatment of breast tumors. Additionally, information regarding the PK and safety profiles of L-DOX and conventional DOX was acquired mainly from drug monographs. Publications assessed in this review included Phases II and III clinical trials of patients with BC, ranging from early to metastatic stages. No particular preference was given in regard to BC subtype, and the only criteria that needed to be met for combinations were the use of L-DOX in at least one arm with at least one additional agent.
Results
and highlight the disparity in PK parameters and toxicity profiles between L-DOX and DOX. These can be attributed to differences in the formulation of the two agents, with the encapsulation of free DOX into a phospholipid bilayer and exterior PEGylation of the liposomes providing improvements in terms of increasing the drug’s half-life (T 1/2), decreasing both the volume of distribution (V d) and plasma CL, and reducing the severity of toxicity associated with the use of anthracyclines. L-DOX’s decreased CL (~ 0.7 vs 324–809 mL/ min/m2 for DOX) and increased T 1/2 (55±4.8 vs 20–48 h for DOX) may be attributed to decreased metabolism by the liver and MPS. L-DOX liposomes are ~80–90 nm in diameter, although some references state that the size of the molecule is ≥100 nm, a characteristic that impedes their passage across hepatic sinusoidal epithelial fenestrations and decreases their metabolism by hepatocytes. In addition, PEGylation of liposomes decreases their opsonization by immunoglobulin/ complement proteins and their uptake by phagocytic cells of the MPS (eg, Kupffer cells and splenic macrophages), thus prolonging the agent’s plasma circulation time.
Another advantage of L-DOX is its extremely small volume of distribution in comparison to that of DOX (2.72±0.12 vs 809–1,214 L/m2). While DOX’s large V d indicates that it can effectively distribute into all compartments of the body, its lack of selectivity for tumors means that it can cause a wide range of toxicities. In contrast, the small volume of distribution of L-DOX indicates that the drug is mostly confined into the vascular space, with little free DOX available, as the drug is contained within the liposomes and does not distribute freely to healthy tissues. The small size of L-DOX allows it to extravasate more selectively across fenestrations in the epithelium of blood vessels supplying tumors, where it releases DOX, meaning that generally the use of L-DOX is associated with milder side effects.Citation5,Citation14 The classic adverse effect associated with DOX use is cardiotoxicity that can range in severity from an acute form that develops shortly after exposure to DOX to a more severe late form where patients may experience decreases in left ventricular ejection fraction (LVEF) and a subsequent diagnosis of DOX induced CHF. Furthermore, the use of DOX is limited by a cumulative lifetime dose limit of up to 550 or 450 mg/m2 if a patient received previous mediastinal radiation. In contrast, the more common toxicities from L-DOX use include palmar–plantar erythrodysesthesia, nausea, and alopecia.Citation16,Citation21–Citation25
Since DOX is considered one of the most effective chemotherapy drugs available, it is often added to regimens for localized or metastatic BC as first- or second-line therapy, as a part of a neoadjuvant therapy prior to surgery or as a salvage therapy. Although it is an effective agent, the risks of cardiotoxicity, particularly when combined with other medications associated with the development of CHF, such as trastuzumab and cyclophosphamide, can limit its use.Citation26 In the case of L-DOX, the decreased rates of cardiotoxicity due to the formulation/PK differences described in the preceding paragraph allows its inclusion in regimens where free DOX would have a high risk of cardiotoxicity. summarizes several trials where L-DOX has been combined with other chemotherapeutics or targeted therapies. Of note, a study combining L-DOX, trastuzumab, and cyclophosphamide was one of the most effective, with an overall survival of 34.2 months and the progression-free survival (PFS) of 12 months.Citation27 In terms of toxicity, eight of the 48 included patients experienced asymptomatic decreases in LVEF and all but one recovered; of the affected patients, six patients had prior exposure to anthracyclines. As for the other trials included in , a majority did not find any significant changes to LVEF or high incidences of clinically relevant cardiotoxicity; however, in several instances where mild-to-moderate cardiotoxicity was reported, it was often in patients who either had prior anthracycline exposure or were concurrently being treated with trastuzumab.Citation27–Citation31
Discussion
The decreased risk for the cardiotoxicity of L-DOX combined with its comparable efficacy to DOX in the treatment of BC has made it a suitable alternative therapy in treatment regimens that traditionally utilized conventional DOX.Citation16 In the in vivo setting, the prolonged systemic circulation of L-DOX due to its relatively long half-life,Citation32 along with its selective delivery to the tumor site due to its extravasation through leaky tumor vasculature,Citation33 results in a higher tumor accumulation as compared to normal tissues. In addition, circulating free-drug concentrations in plasma are reduced due to the highly stable L-DOX formulation, leading to lower cardiac tissue exposure of free-DOX, as compared to tumor tissue. Thus, the use of L-DOX would not only be able to alleviate cardiotoxicity but also to retain significant cytotoxic activity against target tumor cells, due to differences in exposure as well as relative potency of DOX in both tissue types. This is in agreement with results from a Phase III study,Citation16 where L-DOX was shown to be as efficacious as DOX, with significantly reduced cardiotoxicity and other adverse events, in patients with metastatic BC.
summarizes all clinical trials for combinatorial effects of L-DOX with other chemotherapeutics and targeted agents. It is noted that practically all trials are Phase II, and the cardiotoxic events observed were either very low or not existent. In most cases where patients experienced mild-to-moderate cardiotoxicity, they were reported to have received prior anthracycline therapy or were on regimens that included trastuzumab, which is known to augment cardiotoxicity caused due to DOX.Citation34 Nevertheless, the cardiotoxicity observed in the case of L-DOX was significantly lower than that observed with DOX, thus establishing the cardiac safety of this formulation and supporting its clinical use.
In this work, we sought to discuss the therapeutic use of L-DOX in BC. A review of available Phase II and III trials in BC patients has demonstrated that the use of L-DOX generally causes very little cardiotoxicity, while retaining efficacy when used in combination with other chemotherapeutics. Together, this information suggests that L-DOX should continue to be evaluated in further Phase II and III trials in BC, as it remains an effective agent when combined with other chemotherapeutics and is a reasonable agent to substitute in the place of conventional DOX, particularly in patients who are at higher risk for cardiotoxicity.
Disclosure
The authors report no conflicts of interest in this work.
References
- BreastCancer.Org [webpage on the Internet]US Breast Cancer Statisctics2016 Available from: http://www.breastcancer.org/symptoms/understand_bc/statisticsAccessed December 11, 2016
- NakadaHNakagomiHHirotsuYA study of tumor heterogeneity in a case with breast cancerBreast Cancer201724348348927687626
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG)Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trialsLancet200536594721687171715894097
- TacarOSriamornsakPDassCRDoxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systemsJ Pharm Pharmacol201365215717023278683
- GabizonAAPatilYLa-BeckNMNew insights and evolving role of pegylated liposomal doxorubicin in cancer therapyDrug Resist Updat2016299010627912846
- OttMRobertsonJDGogvadzeVZhivotovskyBOrreniusSCytochrome c release from mitochondria proceeds by a two-step processProc Natl Acad Sci U S A20029931259126311818574
- ShanKLincoffAMYoungJBAnthracycline-induced cardiotoxicityAnn Intern Med1996125147588644988
- SwainSMWhaleyFSEwerMSCongestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trialsCancer200397112869287912767102
- BriaECupponeFFornierMCardiotoxicity and incidence of brain metastases after adjuvant trastuzumab for early breast cancer: the dark side of the moon? A meta-analysis of the randomized trialsBreast Cancer Res Treat2008109223123917638068
- LaoJMadaniJPuértolasTLiposomal doxorubicin in the treatment of breast cancer patients: a reviewJ Drug Deliv2013201345640945641223634302
- JanssenCaelyx Product Monograph2011 Available from: https://www.janssen.com/canada/sites/www_janssen_com_canada/files/prod_files/live/caelyx_cpm.pdfAccessed August 20, 2018
- MinchintonAITannockIFDrug penetration in solid tumoursNat Rev Cancer20066858359216862189
- NicholsJWBaeYHEPR: evidence and fallacyJ Control Release201419045146424794900
- HilmerSNCoggerVCMullerMLe CouteurDGThe hepatic pharmacokinetics of doxorubicin and liposomal doxorubicinDrug Metab Dispos200432879479915258103
- RiveraELiposomal anthracyclines in metastatic breast cancer: clinical updateOncologist20038suppl 239
- O’BrienMEWiglerNInbarMCAELYX Breast Cancer Study GroupReduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancerAnn Oncol200415344044914998846
- Doxil - Dosing for progressed or recurrent ovarian cancer [webpage on the Internet]Janssen Products, LP2018 Available from: https://www.doxil.com/hcp/progressed-or-recurrent-ovarian-cancer/Accessed August 20, 2018
- FDA Approves Supplemental New Drug Application for DOXIL [webpage on the Internet]ManchesterNews Medical Life Sciences2009 Available from: http://www.news-medical.net/news/20090910/FDA-approves-Supplemental-New-Drug-Application-for-DOXIL.aspxAccessed August 20, 2018
- EspelinCWLeonardSCGerettiEWickhamTJHendriksBSDual HER2 Targeting with trastuzumab and liposomal-encapsulated doxorubicin (MM-302) demonstrates synergistic antitumor activity in breast and gastric cancerCancer Res20167661517152726759238
- RafiyathSMRasulMLeeBWeiGLambaGLiuDComparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: a meta-analysisExp Hematol Oncol2012111023210520
- FribergLEKarlssonMOMechanistic models for myelosuppressionInvest New Drugs200321218319412889739
- FribergLEHassanSBLindhagenELarssonRKarlssonMOPhar-macokinetic-pharmacodynamic modelling of the schedule-dependent effect of the anti-cancer agent CHS 828 in a rat hollow fibre modelEur J Pharm Sci200525116317315854812
- MinamiHSasakiYSaijoNIndirect-response model for the time course of leukopenia with anticancer drugsClin Pharmacol Ther19986455115219834043
- FribergLEBrindleyCJKarlssonMODevlinAJModels of schedule dependent haematological toxicity of 2’-deoxy-2’-methylidenecytidine (DMDC)Eur J Clin Pharmacol200056856757411151746
- OutomuroDGranaDRAzzatoFMileiJAdriamycin-induced myocardial toxicity: new solutions for an old problem?Int J Cardiol2007117161516863672
- BovelliDPlataniotisGRoilaFESMO Guidelines Working GroupCardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO clinical practice guidelinesAnn Oncol201021suppl 5v277v28220555097
- MartínMSánchez-RoviraPMuñozMGEICAMPegylated liposomal doxorubicin in combination with cyclophosphamide and trastuzumab in HER2-positive metastatic breast cancer patients: efficacy and cardiac safety from the GEICAM/2004-05 studyAnn Oncol201122122591259621421542
- ChiaSClemonsMMartinLAPegylated liposomal doxorubicin and trastuzumab in HER-2 overexpressing metastatic breast cancer: a multicenter phase II trialJ Clin Oncol200624182773277816682726
- ArdavanisAMavroudisDKalbakisKPegylated liposomal doxorubicin in combination with vinorelbine as salvage treatment in pretreated patients with advanced breast cancer: a multicentre phase II studyCancer Chemother Pharmacol200658674274816718470
- StickelerEKlarMWatermannDPegylated liposomal doxorubicin and trastuzumab as 1st and 2nd line therapy in her2/neu positive metastatic breast cancer: a multicenter phase II trialBreast Cancer Res Treat2009117359159819156515
- TorrisiRCardilloACancelloGPhase II trial of combination of pegylated liposomal doxorubicin, cisplatin, and infusional 5-fluorouracil (CCF) plus trastuzumab as preoperative treatment for locally advanced and inflammatory breast cancerClin Breast Cancer201010648348821147693
- GabizonACataneRUzielyBProlonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomesCancer Res19945449879928313389
- SolomanRGabizonAAClinical pharmacology of liposomal anthracyclines: focus on pegylated liposomal doxorubicinClin Lymphoma Myeloma200881213218501085
- RomondEHJeongJHRastogiPSeven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancerJ Clin Oncol201230313792379922987084
- Doxorubicin full prescribing information [webpage on the internet]DAILYMED Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1fd148fb-0fbc-4b6f-b790-23546fb46a71#section-11.2Accessed August 20, 2018
- Doxil full prescribing information [webpage on the internet]DAILYMED Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=21d9c619-7e94-49e2-ac41-31e9ea96554a#section-11.2Accessed August 20, 2018
- RochlitzCRuhstallerTLerchSSwiss Group for Clinical Cancer Research (SAKK)Combination of bevacizumab and 2-weekly pegylated liposomal doxorubicin as first-line therapy for locally recurrent or metastatic breast cancer. A multicenter, single-arm phase II trial (SAKK 24/06)Ann Oncol2011221808520595448
- IrvinWJOrlowskiRZChiuWKPhase II study of bortezomib and pegylated liposomal doxorubicin in the treatment of metastatic breast cancerClin Breast Cancer201010646547021147690
- ColleaRPKruterFWCantrellJEPegylated liposomal doxorubicin plus carboplatin in patients with metastatic breast cancer: a phase II studyAnn Oncol201223102599260522431702
- OvermoyerBSilvermanPHolderLWTripathyDHendersonICPegylated liposomal doxorubicin and cyclophosphamide as first-line therapy for patients with metastatic or recurrent breast cancerClin Breast Cancer20056215015716001993
- KurtzJERousseauFMeyerNPhase II trial of pegylated liposomal doxorubicin-cyclophosphamide combination as first-line chemotherapy in older metastatic breast cancer patientsOncology2007733–421021418424884
- RauKMLinYCChenYYPegylated liposomal doxorubicin (Lipo-Dox®) combined with cyclophosphamide and 5-fluorouracil is effective and safe as salvage chemotherapy in taxane-treated metastatic breast cancer: an open-label, multi-center, non-comparative phase II studyBMC Cancer20151542325994543
- Gil-GilMJBelletMMoralesSPegylated liposomal doxorubicin plus cyclophosphamide followed by paclitaxel as primary chemotherapy in elderly or cardiotoxicity-prone patients with high-risk breast cancer: results of the phase II CAPRICE studyBreast Cancer Res Treat2015151359760625981896
- TuxenMKColdSTangeUBBalslevENielsenDLPhase II study of neoadjuvant pegylated liposomal doxorubicin and cyclophosphamide ± trastuzumab followed by docetaxel in locally advanced breast cancerActa Oncol201453101440144524991893
- de La FouchardièreCLargillierRGoubelyYDocetaxel and pegylated liposomal doxorubicin combination as first-line therapy for metastatic breast cancer patients: results of the phase II GINECO trial CAPYTTOLEAnn Oncol200920121959196319556321
- WolffACWangMLiHPhase II trial of pegylated liposomal doxorubicin plus docetaxel with and without trastuzumab in metastatic breast cancer: Eastern Cooperative Oncology Group trial E3198Breast Cancer Res Treat2010121111112020333545
- SparanoJAMakhsonANSemiglazovVFPegylated liposomal doxorubicin plus docetaxel significantly improves time to progression without additive cardiotoxicity compared with docetaxel monotherapy in patients with advanced breast cancer previously treated with neoadjuvant-adjuvant anthracycline therapy: results from a randomized phase III studyJ Clin Oncol200927274522452919687336
- FabiAFerrettiGPapaldoPPegylated liposomal doxorubicin in combination with gemcitabine: a phase II study in anthracycline-naïve and anthracycline pretreated metastatic breast cancer patientsCancer Chemother Pharmacol200657561562316163541
- RiveraEValeroVArunBPhase II study of pegylated liposomal doxorubicin in combination with gemcitabine in patients with metastatic breast cancerJ Clin Oncol200321173249325412947059
- AdamoVLorussoVRosselloRPegylated liposomal doxorubicin and gemcitabine in the front-line treatment of recurrent/metastatic breast cancer: a multicentre phase II studyBr J Cancer200898121916192118493232
- ArtioliGGraziaAMocellinSPhase II study of neoadjuvant gemcitabine, pegylated liposomal doxorubicin, and docetaxel in locally advanced breast cancerAnticancer Res20103093817382120944176
- PircherMMlineritschBFridrikMALapatinib-plus-pegylated liposomal doxorubicin in advanced HER2-positive breast cancer following trastuzumab: a phase II trialAnticancer Res201535151752125550597
- RossiDBaldelliAMCasadeiVNeoadjuvant chemotherapy with low dose of pegylated liposomal doxorubicin plus weekly paclitaxel in operable and locally advanced breast cancerAnticancer Drugs200819773373718594216
- VorobiofDARapoportBLChasenMRFirst line therapy with paclitaxel (taxol) and pegylated liposomal doxorubicin (caelyx) in patients with metastatic breast cancer: a multicentre phase II studyBreast200413321922615177425
- ChristodoulouCKostopoulosIKalofonosHPStudy of the Hellenic Cooperative Oncology GroupTrastuzumab combined with pegylated liposomal doxorubicin in patients with metastatic breast cancer. Phase II Study of the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluationOncology200976427528519262067
- ChowLWYipAYLangBHA phase II trial of vinorelbine and pegylated liposomal doxorubicin in patients with pretreated metastatic breast cancerAm J Clin Oncol200730213313817414461
- MartinMGarcía-DonasJCasadoAPhase II study of pegylated liposomal doxorubicin plus vinorelbine in breast cancer with previous anthracycline exposureClin Breast Cancer20045535335715585072
- ViciPColucciGGiottaFA multicenter prospective phase II randomized trial of epirubicin/vinorelbine versus pegylated liposomal doxorubicin/vinorelbine as first-line treatment in advanced breast cancer. A GOIM studyJ Exp Clin Cancer Res2011303921481280
