Abstract
Objective
Chemerin was reported to regulate adipogenesis, metabolism, and immunity. But, its relation to cancer remains controversial. In breast cancer, chemerin expression has only been studied in serum, however, its expression in tissue, to our knowledge, has not been studied. The aim of this study was to investigate chemerin expression in breast cancer tissue in comparison to the adjacent normal tissue, and to assess its relationship to disease prognosis.
Methods
We examined chemerin expression in tissue with immunohistochemistry and analyzed the association of chemerin expression with the patients’ clinical and pathological characteristics to determine its role as a predictor of the disease and its relation to disease prognosis.
Results
We detected a significantly higher expression of chemerin in the malignant vs the non-cancerous tissue specimens in 30/53, (56%) patients, (P=0.001). Moreover, its expression was significantly higher in the metastatic lymph nodes in comparison to the tumor tissues, (P=0.01). Chemerin expression was significantly correlated with weight (r=0.256, P=0.04), body mass index (r=0.233, P=0.03), tumor size (r=0.235, P=0.03), lymph node metastasis (r=0.265, P=0.045), distant metastasis (r=0.267, P=0.02), and tumor grading, (r=0.421, P=0.004), while it was inversely significantly correlated with estrogen receptor and progesterone receptor expression in malignant breast tissues (P=0.038, r=−0.437, and P=0.047, r=–0.316), respectively. The area under the receiver operating characteristic curve for chemerin as a predictor of breast cancer was 0.82, (P<0.001, sensitivity 89%, and specificity 69%). The Kaplan–Meier survival curves revealed that patients with higher chemerin expression had worse overall survival in comparison to those with a lower chemerin expression, (P=0.001).
Conclusion
Our results revealed higher chemerin expression in malignant vs adjacent normal breast tissue and lend support to a presumable role of chemerin tissue expression as an independent predictor of poor prognosis in breast cancer patients.
Introduction
Breast cancer represents the most common female cancer in Egypt (32%),Citation1 affecting one in eight women, with nearly 700,000 deaths/year worldwide.Citation2,Citation3 Although the most important risk factor for breast cancer remains extended estrogen exposure by different etiologies, the exact pathophysiological mechanism has not been determined yet.
Obesity, a worldwide epidemic,Citation4 has been found to be associated with different kinds of malignancies.Citation5 Post-menopausal obesity, an estrogen excess condition, has been reported to cause a 63% increase in breast cancer riskCitation6 through many biologically active factors such as estrogen, insulin, IGF-I, leptin, and chemerin.Citation7–Citation10 The periadventitial fat stores have the capacity, in various manners, to produce bioactive peptides, adipokines.Citation11,Citation12 Through acting in an autocrine, paracrine, and endocrine manner, these adipokines have been shown to regulate several body functions and have been implicated in the pathophysiological mechanisms of clinical entities, including immunity, metabolism, and cancers.Citation4,Citation5,Citation13
The adipokine, chemerin, is synthesized as a 163-amino acid preproprotein (prochemerin), N-terminally cleaved with low activity.Citation14 Following secretion, prochemerin can be C-terminally cleaved by a variety of extracellular pro-teases, resulting in several chemerin isoforms with varying lengths, receptor affinity, and biological activity.Citation14 Its production from adipocytes is triggered by TNF-alpha.Citation15 Being a chemoattractant protein, chemerin acts as a ligand for the G-protein-coupled receptors; CMKLR1 (or ChemR23), GPR1, and CCRL2. Only the chemerin/CMKLR1 axis’ biological actions have been clearly described,Citation16 and are mainly expressed in adipocytes, immune cells, and in breast tissue.Citation17 It has been reported to be involved in various inflammatory diseases, as well as different malignancies.Citation18 Chemerin has been identified to regulate adipogenesis and is associated with metabolic syndrome, polycystic ovary syndrome, and obesity.Citation3,Citation14,Citation16,Citation19
Chemerin’s association with cancer is not clearly understood.Citation3 The conflicting reports about the link between chemerin and cancer have caused this relation to be controversial, with reported tumorigenic and antitumor effects. While chemerin/CMKLR1 showed a tumorigenesis effect in squamous cell carcinoma of the oral tongue, glioblastoma, squamous esophageal cancer, gastric cancerCitation20–Citation23 and was shown to be extensively produced by neuroblastoma cells (tumor cell growth could be impaired by inhibition of the chemerin/CMKLR1 axis in vitro and in vivo),Citation24 down-regulation or an antitumorigenic effect has been suggested in hepatocellular carcinoma, non-small-cell lung cancer, and melanoma.Citation25–Citation27 The expression of chemerin in inflamed tissues, its pro- and anti-inflammatory properties, and its chemotactic recruitment of macrophages and other cells expressing CMKLR1 receptor, elucidate its role in inflammatory states.Citation16,Citation28 High chemerin levels may have an initiating role in carcinogenesis by promoting angiogenesis through inducing matrix metalloproteinase secretion and activity.Citation29 Few studies have investigated chemerin expression in serum and tissue in gastric, colon, lung and esophageal cancers, and melanoma. In breast cancer, while chemerin expression has been extensively studied in serum, it has not been studied in malignant breast tissues.Citation3 This study was designed to determine chemerin expression in the malignant tissue of women suffering from breast cancer in comparison to the adjacent normal tissue, and to assess its relationship with disease prognosis.
Patients and methods
Tumor samples
After approval from the Faculty of Medicine Research Ethics Committee, Minia University, 53 Egyptian patients suffering from breast cancer who attended the Minia University hospital and the Minia Oncology center were chosen and signed written informed consent for participation in the study. The patients were investigated and followed from January 2012 to December 2016. Patients with history of diabetes mellitus (DM), metabolic syndrome, and those suffering from coexisting illnesses such as chronic liver and kidney disease were excluded.
All the participants were evaluated according to a standardized form which included medical history (focusing on menstrual history, reproductive history, menopausal history, history of DM, and family history of breast cancer) and physical examination. Height in meters and weight in kilograms were measured, and the body mass index (BMI) was calculated [weight (kg)/height (m)Citation2]. Paraffin blocks of the malignant tissues, the adjacent non-malignant tissues, and the lymph node (LN) metastasis were obtained from each case. The clinical and pathological data are given in .
Table 1 Demographic, clinical, and pathological characteristics of the female breast cancer patients
We performed immunohistochemical staining on 4 μm thick sections of the most representative paraffin blocks from each tumor. Slides were heated at 60°C and de-paraffinized in xylene. Subsequently, they were rehydrated by replacing graded alcohol with water. Heat-induced antigen retrieval was achieved by boiling sections in a citrate buffer, pH 6, in a microwave oven at 700 W for 20 minutes (four times for 5 minutes each). After boiling, sections were left to cool at room temperature and rinsed thoroughly with PBS for 5 minutes. Endogenous peroxidase was blocked with peroxidase block solution (provided in the EnVision kit, Dako Denmark A/S, Glostrup, Denmark) for 10 minutes and slides were rinsed with PBS. Sections were incubated for 30 minutes with primary mouse monoclonal anti-human chemerin antibody (Abcam, Cambridge, UK) using a 1:400 dilution. The same sections were processed without primary antibodies as negative control.
Tissue classification
Diagnoses were made based on paraffin-embedded 4 μm tissue sections after H&E staining. Tissues were classified according to histological subtypes as invasive ductal car cinoma of different grades (n=53). Invasive tumors were evaluated according to the Scarff-Bloom-Richardson grade classification modified by Elston et al.Citation30 Normal tissue adjacent to breast cancer was also analyzed as control (n=53). The staging of breast cancer was determined based on the TNM system based on definitions and recommendations of European Society of Medical Oncology, 2015.Citation31
Interpretation of immunohistochemistry staining
Positive signal for chemerin was located mainly in the cytoplasm and to a lesser extent the nucleus of the ductal cells and the score was semi-quantitatively estimated on the basis of both percentage and intensity of positively stained tumor cells. Five views were examined per slide, and 100 cells were counted per view at ×400 magnification. The intensity of chemerin staining was classified using the 4-point scale as 0 (no signal), 1 (weak staining), 2 (moderate staining), 3 (strong staining). Percentage scores were assigned as 0, no staining; 1, 1%–25%; 2, 26%–50%; 3, 51%75%; and 4, 76%–100%. Their multiplication (intensity score × area score) was calculated in each area. Thus, the score should range from 0 to 12. For statistical analysis, chemerin immunostaining scores of 0–4 were defined as weak or low expression, and scores of 5–12 were defined as high expression.
Statistical analysis
The statistical analyses were performed using SPSS version 24.0 (IBM Corporation, Armonk, NY, USA). Data on normal distribution were expressed as mean ± SD and were compared using independent samples t-test. Categorical variables were expressed as percentage and were compared using the chi-squared test or Fisher’s exact test, as appropriate. Nonparametric variables were analyzed using the Mann–Whitney U test. The Pearson correlation coefficients were used to study the correlation between different parametric variables. Spear-man rank correlation was used to quantify the association between continuous or ordered categorical variables. Receiver operating characteristic (ROC) curve analysis and the area under the curve (AUC) were calculated. Kaplan–Meier survival curves were calculated for disease-free survival. A multivariate analysis was performed using the Cox regression model to study the effects of different variables on survival. P-value <0.05 was considered as statistically significant.
Results
shows a summary of the clinical and pathological characteristics of the cases included in this study. A total of 53 patients with primary breast cancer were included. The mean patients’ age was 51.79±11.975 years at a range of (24–79), their mean BMI was 30.48±4.9157 (20.4–44.9). Their pathological diagnosis was invasive ductal carcinoma in 53 cases (100%). The mean tumor surface area was 10.591±7.3685 cm2 at a range of 1.0–30. LN metastasis was present in 86% of cases, the mean number of LN metastasis was 14.47±7.3685 at a range of 0–30. Distant metastasis was present in 45% of cases. Tumor grading was moderate in 43 cases (81.1%), and high in ten cases (18.9%).
The expression of chemerin in breast carcinomas versus normal breast tissue
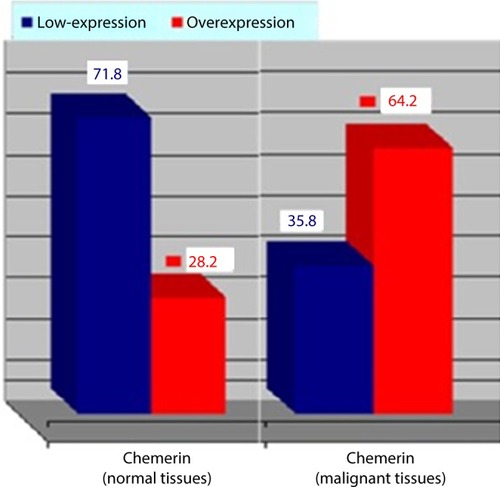
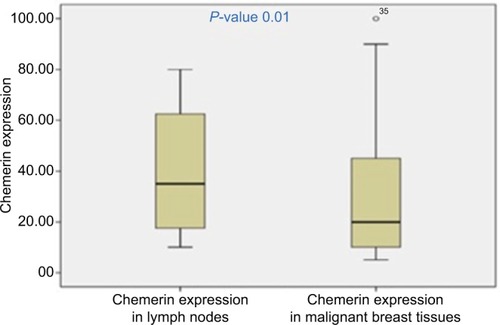
Immunohistochemistry was performed in 53 breast cancer specimens and their corresponding normal tissues. Of the 53 patients, 30 cases (56%) showed a high expression level of chemerin in malignant tissue vs non-cancerous tissue specimens (P=0.001). The chemerin protein appeared to be expressed mainly in the cytoplasm and to a lesser extent in the nuclei of tumor cells, and , also, the chemerin expression in the metastatic LNs was significantly higher than the malignant breast tissue ( and , (P=0.01).
Figure 3 Representative microphotographs of immunohistochemical analysis of chemerin antigen expression in human normal breast tissue and breast cancer tissue.
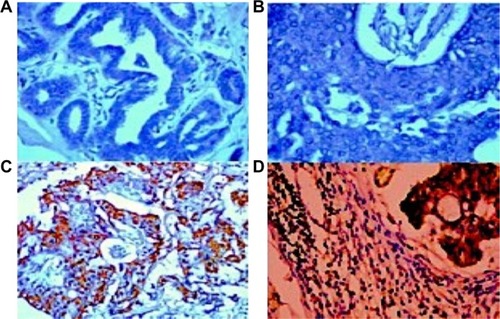
Table 2 The chemerin expression and distribution in malignant vs benign breast tissue
Correlations of chemerin expression with clinicopathologic variables
Chemerin expression was positively correlated with patients’ weight, BMI, tumor size, LN metastasis, distant metastasis, and tumor grading (r=0.256, P=0.04; r=0.233, P=0.03; r=0.235, P=0.03; r=0.265, P=0.045; r=0.267, P=0.02; r=0.421, P=0.004), respectively, and was inversely correlated with estrogen receptor (ER) and progesterone receptor (PR) expression in malignant breast tissues (r=−0.437, P=0.038), and (r=−0.316, P=0.047), respectively. Correlations of chemerin expression with clinicopathological variables were shown in .
Table 3 Associations of chemerin expression with clinical and pathological characteristics in women with breast cancer
Chemerin expression as predictor and prognostic factor of breast cancer
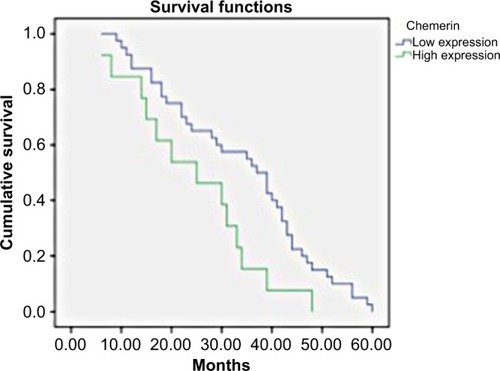
Chemerin expression was evaluated as a predictor of breast cancer, . The AUC was 0.82 (P<0.001), with a sensitivity of 89% and specificity of 69%. The 5-year disease-free survival rates among patients with higher expression were significantly worse than patients with low chemerin expression (P=0.001), . showed the Kaplan–Meier survival curves for the patients according to chemerin expression level. A significant difference in survival was observed between patients with high chemerin expression levels (n=30) in comparison to those with low chemerin expression levels (n=23). To assess other factors affecting 5-year disease-free survival, we used the Cox proportional hazard analysis. The factors considered were age, BMI, fasting blood sugar, tumor size, differentiation grade, histological type, and chemerin expression. The univariate Cox regression analysis indicated that TNM stage (HR 6.23, 95% CI 2.86–13.56; P=0.001), differentiation grade (HR 21.28, 95% CI 7.81–58; P=0.001), and chemerin expression level (HR 2.07, 95% CI 1.08–3.97; P=0.029) were independent factors affecting the 5-year disease-free survival rate.
Figure 4 Receiver operating characteristic (ROC) curves of chemerin expression differentiating malignant from adjacent non-tumor breast tissue.
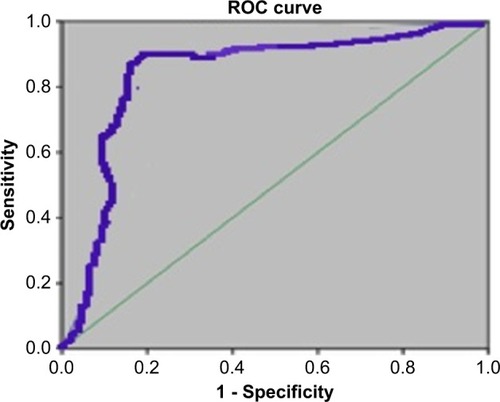
Figure 5 Kaplan–Meier survival curves of female patients with breast cancer according to chemerin expression levels.
Discussion
To the best of our knowledge, this is the first study which has addressed chemerin expression in breast cancer tissue. In this study, we found that chemerin expression was significantly higher in the cancer tissue in comparison to adjacent normal breast tissue. Moreover, chemerin expression was higher in the metastatic LNs in comparison to the tumor tissues. Its expression was strongly related to the tumor size, presence of LN or distant metastasis, and tumor grading. Similar to existing literature, chemerin levels were found to be strongly, positively correlated with weight and BMI. While chemerin levels was inversely correlated with ER and PR expression in the malignant breast tissue. Based on the ROC curve analysis, the AUC of chemerin was 0.82, with a sensitivity of 89% and specificity of 69%. The Kaplan–Meier survival curves revealed that patients with high expression of chemerin had unfavorable disease-free survival in comparison to those with low expression.
Several adipokines such as leptin, resistin, apelin, and visfatin have been implicated in cancer development through different pathophysiological mechanisms such as inflammation, insulin resistance, and sex hormones’ production. Their increased levels were reported to play a pro-carcinogenic role.Citation4 On the contrary, other adipokines such as adiponectin were reported to have an anti-carcinogenic role.Citation3 The recently discovered adipokine, chemerin (also known as TIG-2 or RARRES2), is a chemoattractant factor that was found to regulate adipocyte development, metabolic functions, and immunityCitation32,Citation33 and has been associated with obesity, metabolic syndrome, insulin resistance, inflammatory diseases, and cancer.Citation18,Citation20–Citation22,Citation25,Citation34–Citation38 Many reports have attempted to elucidate the pathophysiological mechanisms through which chemerin could induce its effects. Chemerin promotes tumorigenesis by triggering the production and activity of matrix metalloproteinase, which plays a crucial role in angiogenesis, while the antitumor role for high chemerin levels has been reported to be through recruitment of natural killer (NK) cells which would help the immune system to recognize and fight the cancer cells.Citation20–Citation23,Citation39 Also, chemerin could induce the initiation of the innate immune response and inflammatory processes by attracting the antigen presenting cells.Citation17,Citation25,Citation40 Therefore, with high chemerin levels, the immune system recognizes cancer cells and fights them. The inflammatory pathogenic nature of breast cancer may represent another mechanism that may link chemerin expression with breast cancer.Citation41,Citation42 Breast cancer stem cells’ biology has been reported to be affected by certain immune mediators.Citation43,Citation44 Chemerin has also been reported to be associated with numerous pro-inflammatory cytokines related to cytotoxic cell-mediated immunity such as IL-6, IL-8, and TNF-alpha,Citation45,Citation46 which represent inflammatory mediators in the breast.Citation47,Citation48
When compared to the adjacent Barrett’s tissue, the malignant tissues of esophageal adenocarcinoma were reported to have high chemerin expression,Citation49,Citation50 which confirmed an association between overexpression of chemerin and chemR23 and a more invasive esophageal squamous cell carcinoma. Recently, Xu et alCitation51 reported that chemerin expression in serum may be considered as a serum biomarker for diagnosis and prognosis in non-small-cell lung cancer patients.
Obesity, a metabolic disorder, is a well-established risk factor for the development of cancer, including breast cancer. Chemerin levels were reported to be significantly associated with obesity;Citation19 its plasma levels are significantly heritable and may be a stimulator of angiogenesis.Citation52 The effects of adipose-derived angiogenic factors in promotion of tumor growth have been demonstrated in experimental studies.Citation53,Citation54 Elevated chemerin levels secondary to obesity may further contribute to chemerin’s angiogenic properties through triggering the production and activity of the matrix metalloproteinase, formation of new capillary-like structures, and vascularization of endothelial cells.Citation52 In agreement with Catalan et al,Citation55 our study revealed that increased serum chemerin levels were correlated with BMI and obesity.
Although many studies have demonstrated the relationship between chemerin expression in serum and tissue and many types of tumors,Citation6–Citation11 for breast cancer, chemerin expression has only been studied in serum.Citation3 It has been reported that serum chemerin levels were not associated with tumor stage in breast cancer.Citation3 In the current study, chemerin expression in tissue was associated with unfavorable clinical and pathological variables, TNM staging, and LN and distant metastasis. Chemerin levels were higher in the metastatic LNs in comparison to malignant breast tissue, while they were negatively correlated with ER and PR expression. This was also proved by Kaplan–Meier survival curves and the ROC curve. Although further investigations are required, the results of this study may imply that increased chemerin levels might serve as a marker for diagnosis, progression, and prognosis of breast cancer patients.
This study has some limitations including, small sample size, all patients had grade II and III cancer, while no patient had grade I or IV, and lack of some investigations at our center such as Western blot analysis and verification of some upstream and downstream genes of chemerin in the tumor sections and the adjacent normal tissues. Therefore, our findings need further evaluation in prospective studies with larger sample sizes to determine a causal relationship of high chemerin expression. The expression of inflammatory and immunological markers, as the natural killer cells, in the malignant and adjacent benign tissue also, need to be clarified in further studies.
Conclusion
The higher chemerin expression in malignant in comparison to normal breast tissues and the increased expression in the LN metastatic tissue may introduce chemerin as an independent predictor of poor prognosis of women with breast cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
- IbrahimASKhaledHMMikhailNNBarakaHKamelHCancer incidence in egypt: results of the national population-based cancer registry programJ Cancer Epidemiol20142014118
- FerlayJSteliarova-FoucherELortet-TieulentJCancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012Eur J Cancer20134961374140323485231
- AkinSSerum Chemerin Level in Breast CancerInt J Hematol Oncol2017272127132
- CabiaBAndradeSCarreiraMCCasanuevaFFCrujeirasABA role for novel adipose tissue-secreted factors in obesity-related carcinogenesisObes Rev201617436137626914773
- BoothAMagnusonAFoutsJFosterMAdipose tissue, obesity and adipokines: role in cancer promotionHorm Mol Biol Clin Investig20152115774
- CalleEERodriguezCWalker-ThurmondKThunMJOverweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adultsN Engl J Med2003348171625163812711737
- CalleEEThunMJObesity and cancerOncogene200423386365637815322511
- GuastamacchiaERestaFTriggianiVEvidence for a putative relationship between type 2 diabetes and neoplasia with particular reference to breast cancer: role of hormones, growth factors and specific receptorsCurr Drug Targets Immune Endocr Metabol Disord200441596615032627
- GarofaloCSurmaczELeptin and cancerJ Cell Physiol20062071122216110483
- GunterMJHooverDRYuHInsulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal womenJ Natl Cancer Inst20091011486019116382
- RontiTLupattelliGMannarinoEThe endocrine function of adipose tissue: an updateClin Endocrinol2006644355365
- GustafsonBAdipose tissue, inflammation and atherosclerosisJ Atheroscler Thromb201017433234120124732
- KostopoulosCGSpiroglouSGVarakisJNApostolakisEPapadakiHHChemerin and CMKLR1 expression in human arteries and peri-adventitial fat: a possible role for local chemerin in atherosclerosis?BMC Cardiovasc Disord2014145624779513
- MatternAZellmannTBeck-SickingerAGProcessing B-SAG. Processing, signaling, and physiological function of chemerinIUBMB Life2014661192624446308
- ParleeSDErnstMCMuruganandanSSinalCJGoralskiKBSerum chemerin levels vary with time of day and are modified by obesity and tumor necrosis factor-{alpha}Endocrinology201015162590260220363880
- BondueBWittamerVParmentierMChemerin and its receptors in leukocyte trafficking, inflammation and metabolismCytokine Growth Factor Rev2011225–633133822119008
- WittamerVFranssenJDVulcanoMSpecific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluidsJ Exp Med2003198797798514530373
- GoralskiKBMccarthyTCHannimanEAChemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolismJ Biol Chem200728238281752818817635925
- BozaogluKBoltonKMcmillanJChemerin is a novel adipokine associated with obesity and metabolic syndromeEndocrinology2007148104687469417640997
- WangNWangQJFengYYShangWCaiMOverexpression of chemerin was associated with tumor angiogenesis and poor clinical outcome in squamous cell carcinoma of the oral tongueClin Oral Investig20141839971004
- KumarJDHolmbergCKandolaSIncreased expression of chemerin in squamous esophageal cancer myofibroblasts and role in recruitment of mesenchymal stromal cellsPLoS One201497e10487725127029
- WangCWuWKLiuXIncreased serum chemerin level promotes cellular invasiveness in gastric cancer: a clinical and experimental studyPeptides20145113113824274970
- YamaguchiYDuXYZhaoLMorserJLeungLLProteolytic cleavage of chemerin protein is necessary for activation to the active form, Chem157S, which functions as a signaling molecule in glioblastomaJ Biol Chem201128645395103951921949124
- TümmlerCSnapkovIWickströmMInhibition of chemerin/CMKLR1 axis in neuroblastoma cells reduces clonogenicity and cell viability in vitro and impairs tumor growth in vivoOncotarget2017856951359515129221117
- PachynskiRKZabelBAKohrtHEThe chemoattractant chemerin suppresses melanoma by recruiting natural killer cell antitumor defensesJ Exp Med201220981427143522753924
- ImaiKTakaiKHanaiTImpact of serum chemerin levels on liver functional reserves and platelet counts in patients with hepatocellular carcinomaInt J Mol Sci2014157112941130624968270
- ZhaoSLiCYeY-BinPengFChenQExpression of chemerin correlates with a favorable prognosis in patients with non-small cell lung cancerLab Med2011429553557
- RohSGSongSHChoiKCChemerin--a new adipokine that modulates adipogenesis via its own receptorBiochem Biophys Res Commun200736241013101817767914
- KaurJAdyaRTanBKChenJRandevaHSIdentification of chemerin receptor (ChemR23) in human endothelial cells: chemerin-induced endothelial angiogenesisBiochem Biophys Res Commun201039141762176820044979
- ElstonCWEllisIOPathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-upHistopathology19911954034101757079
- SenkusEKyriakidesSOhnoSPrimary breast cancer: ESMO Guidelines for diagnosis, treatment and follow-upAnn Oncol2015265v8v3026314782
- ErnstMCSinalCJChemerin: at the crossroads of inflammation and obesityTrends Endocrinol Metab2010211166066720817486
- ErnstMCIssaMGoralskiKBSinalCJChemerin exacerbates glucose intolerance in mouse models of obesity and diabetesEndocrinology201015151998200720228173
- LandgrafKFriebeDUllrichTChemerin as a mediator between obesity and vascular inflammation in childrenJ Clin Endocrinol Metab2012974E556E56422438234
- KuligPKantykaTZabelBARegulation of chemerin chemoattractant and antibacterial activity by human cysteine cathepsinsJ Immunol201118731403141021715684
- SchultzSSaalbachAHeikerJTProteolytic activation of prochemerin by kallikrein 7 breaks an ionic linkage and results in C-terminal rearrangementBiochem J2013452227128023495698
- BergVSveinbjornssonBBendiksenSBroxJMeknasKFigenschauYHuman articular chondrocytes express ChemR23 and chemerin; ChemR23 promotes inflammatory signalling upon binding the ligand chemerin (21-157)Arthritis Res Ther2010126R22821192818
- RourkeJLDranseHJSinalCJTowards an integrative approach to understanding the role of chemerin in human health and diseaseObes Rev201314324526223216632
- ParoliniSSantoroAMarcenaroEThe role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissuesBlood200710993625363217202316
- WittamerVBondueBGuillabertAVassartGParmentierMCommuniDNeutrophil-mediated maturation of chemerin: a link between innate and adaptive immunityJ Immunol2005175148749315972683
- BalkwillFMantovaniAInflammation and cancer: back to Virchow?Lancet2001357925553954511229684
- AriasJIAllerMAAriasJCancer cell: using inflammation to invade the hostMol Cancer200762917437633
- BoyleSTKochetkovaMBreast cancer stem cells and the immune system: promotion, evasion and therapyJ Mammary Gland Biol Neoplasia201419220321124997735
- JeongYJHkOParkSHBongJGAssociation between inflammation and cancer stem cell phenotype in breast cancerOncol Lett20181522380238629434947
- LeroithDRobertsCTThe insulin-like growth factor system and cancerCancer Lett2003195212713712767520
- BrahmkhatriVPPrasannaCAtreyaHSInsulin-like growth factor system in cancer: novel targeted therapiesBiomed Res Int201520155380192425866791
- GoldbergJESchwertfegerKLProinflammatory cytokines in breast cancer: mechanisms of action and potential targets for therapeuticsCurr Drug Target201011911331146
- SoriaGOfri-ShahakMHaasIInflammatory mediators in breast cancer: coordinated expression of TNFα & IL-1β with CCL2 & CCL5 and effects on epithelial-to-mesenchymal transitionBMC Cancer20111113021486440
- SomjaJDemoulinSRoncaratiPDendritic cells in Barrett’s esophagus carcinogenesis: an inadequate microenvironment for anti-tumor immunity?Am J Pathol2013201318203662168217923619476
- KumarJDKandolaSTiszlaviczLReiszZDockrayGJVarroAThe role of chemerin and ChemR23 in stimulating the invasion of squamous oesophageal cancer cellsBr J Cancer2016114101152115927092781
- XuCHYangYWangYCYanJQianLHPrognostic significance of serum chemerin levels in patients with non-small cell lung cancerOncotarget2017814224832248928160556
- BozaogluKCurranJEStockerCJChemerin, a novel adipokine in the regulation of angiogenesisJ Clin Endocrinol Metab20109552476248520237162
- LorinczAMSukumarSMolecular links between obesity and breast cancerEndocr Relat Cancer200613227929216728564
- PerrierSCaldefie-ChézetFVassonMPIL-1 family in breast cancer: potential interplay with leptin and other adipocytokinesFEBS Lett2009583225926519111549
- CatalánVGómez-AmbrosiJRodríguezAIncreased levels of chemerin and its receptor, chemokine-like receptor-1, in obesity are related to inflammation: tumor necrosis factor-α stimulates mRNA levels of chemerin in visceral adipocytes from obese patientsSurg Obes Relat Dis20139230631422154272