Abstract
Background
Black cohosh (BC) is an herbal remedy often used by women to treat symptoms associated with menopause. Research has shown that the molecular activity of BC is associated with estrogen receptor alpha (ER-α) regulation. Progesterone receptor (PR) expression is found to be consistent with ER expression and mutations in the BRCA1 gene, a tumor-suppressor gene, are known to be responsible for about 40%–45% of hereditary breast cancers.
Purpose
The objective of this study was to determine the effects of BC alone, as well as in combination with hormones and antihormones, on cell viability and expression of ER-α, PR, and BRCA1 in both T-47D and MCF-7 cell lines.
Methods
Cells were cultured in charcoal-stripped serum prior to their treatment and subsequent protein extraction. Western blot analyses were performed following a Bio-Rad Bradford protein assay and SDS-PAGE gel electrophoresis, with ECL luminescence and Image Studio Lite software. Cellular viability assays were performed using propidium iodine (PI) staining, and the distribution of fluorescent structures was evaluated through confocal microscopy. RT-qPCR analysis was performed on extracted cellular RNA. All statistical analyses were performed using SPSS software, and data was subjected to Kruskal-Wallis testing, followed by post-hoc analysis using the Mann-Whitney U-test to determine the statistical significance of all findings.
Results
Western blot analysis displayed significant alterations of ER-α, PR, and BRCA1 protein levels after 24-hour treatment with 80–500 μM BC. BC displayed a concentration-dependent decrease on ER-α and BRCA1 expression, with an 87% reduction of ER-α expression and a 43% of BRCA1 expression in T-47D cells compared to control. After six days of treatment with 400 μM BC, a 50% decrease in cell proliferation was observed. Following 24 hours of co-treatment with 400 μM BC and 10 nM E2, ER-α was downregulated by 90% and BRCA1 expression was reduced by 70% compared to control. The expression of PR, following the same treatment, exhibited similar effects. The proliferative effect of E2 was reduced in the presence of BC.
Conclusion
Black Cohosh demonstrates substantial anti-cancer properties, and this study may significantly aid in the understanding of the molecular effects of BC on ER-α, PR, and BRCA1 in breast cancer cells.
Introduction
Breast cancer is one of the most common causes of cancer death for women in the USA but its impact is worldwide, calling for progressive research, treatment, and prevention strategies. In 2012 alone, over 1.7 million people were diagnosed with breast cancer, and it remains in the top three of most common cancers in the world.Citation1,Citation2 Breast cancer mortality rates have decreased in both North America and the European Union over the past several years, perhaps partly due to an increase in research of the mechanisms of breast cancer molecular targets and potential therapeutic agents.Citation1
Estrogen is the hormone primarily responsible for the development and regulation of female reproductive organs. The most prevalent form of estrogen in the female body is known as 17β-estradiol, or E2. This steroid hormone has been shown in previous laboratory studies, as well as studies from our own laboratory,Citation3 to exert proliferative effects on the hormone-dependent T-47D and MCF-7 breast cancer cell lines.Citation3–Citation6
The primary receptor for E2 is known as estrogen receptor alpha, or ER-α. Only 10% of normal breast epithelium is positive for this receptor, but it is present in approximately 50%–80% of breast tumors; it is this presence that drastically increases the mitotic activity of breast tumors in patients with mutated tumor-suppressor genes.Citation7,Citation8 Studies have shown that abnormal ER-α expression is involved in the development and progression of hormone-dependent breast cancer.Citation8,Citation9 Alterations in the progesterone receptor (PR), the receptor for the female hormone progesterone, have also been shown to be involved in the progression of hormone-dependent breast cancer. These PR alterations are often consistent with those of ER-α.Citation9
Abnormalities in BRCA1, a tumor-suppressor gene, are shown to be associated with many hereditary breast cancers. The BRCA1 gene is found in the wild-type form in both T-47D and MCF-7 cell lines; it is, therefore, valuable to examine the effects of alternatives for hormone replacement therapy on this gene and its corresponding protein.Citation10,Citation11
The T-47D cell line used in these experiments was isolated from ductal carcinoma in situ (DCIS) of the breast of a 54-year-old woman.Citation12,Citation13 The progression to invasive breast carcinoma (IBC) involves several stages; DCIS is one of the later stages of IBC. DCIS refers to an intraductal neoplastic proliferation of epithelial cells that has not yet penetrated the basement membrane of the breast stroma. The other cell line used in these studies, MCF-7, was also isolated from infiltrating ductal carcinoma of the breast from a postmenopausal woman; MCF-7 cells are also sensitive to estrogen.Citation6,Citation14,Citation15
Black cohosh (BC) is a herbal remedy used by menopausal women to alleviate hot flashes, emotional fluctuations, sleep disturbances, and other complaints associated with menopause.Citation16 BC is isolated from the rhizomes of Cimicifuga racemosa, a plant native to North America. It has been known to demonstrate astringent, diuretic, antidiarrheal, and antioxidant properties. BC has been used across cultures for centuries as a remedy for a variety of health complaints including as an anti-inflammatory agent by Native Americans.Citation17–Citation20
Conventional hormone replacement therapy, used to treat menopausal symptoms, has recently been found to increase the risk of developing breast cancer or endometrial cancer. Therefore, herbal alternatives such as BC are now more commonly used as menopausal treatments.Citation17–Citation20
Recent studies have demonstrated the growth-inhibitory effects of BC on human breast cancer cells.Citation21 This project attempts to help determine whether BC has estrogenic or antiestrogenic effects, as well as verify its potential as a breast cancer treatment. It also examines the effects of BC on PR and BRCA1. The chemical structure of BC is found to have carbohydrate-based modifications called triterpene glycosides; the BC used in these studies has a 2.5% triterpene-glycoside composition (NatureX, Avignon, France). This study will contribute to a better understanding of the effects of herbal remedies, which are becoming more commonly used as a menopausal treatment, on breast cancer cells.
Materials and methods
Cell culture and treatment
The hormone-dependent T-47D and MCF-7 breast cancer cell lines (ATCC® HTB-133 TM; ATCC, Manassas, VA, USA) were regularly cultured at 37°C with 5% CO2, which simulates the optimum conditions of the human body. The T-47D cells were cultured in a media (RPMI 1640) (Hyclone, Logan, UT, USA) supplemented with 10% FBS containing growth factors and exogenous steroids. This RPMI media contained 2 mM L -glutamine, 25 mM HEPES, 24 mM sodium bicarbonate, 0.5% 100× nonessential amino acids, 100 U/mL penicillin, 0.1 mg/mL streptomycin, 0.25 mg/mL amphotericin B (Hyclone), and 0.14 IU/mL insulin (Sigma, St. Louis, MO, USA) with 10% (v/v) FBS (Hyclone). The media are also known as 10% whole serum, and it assisted with cell growth and cellular proliferation. The MCF-7 cells were cultured in Eagle’s minimum essential medium (Corning Cellgro), which contained 1.5 g/L sodium bicarbonate, nonessential amino acids, L-glutamine, and sodium pyruvate. The cells of each cell line were then plated in six-well plates (200,000 cells for T-47D and 240,000 cells for MCF-7) and the medium was changed to a 5% dextran-coated charcoal-stripped fetal bovine serum (DCC-FBS) (5% stripped serum) to avoid cellular exposure to endogenous steroids in the FBS. This stripped serum was used in order to deplete the cells of endogenous steroids and growth factors, thus allowing the cells to be at their basal metabolic rate at the time of treatment, confirming that any effects seen on the cells and the cells’ proteins are caused exclusively by the treatments they receive and not any other components of the culturing media. The cells were cultured in this charcoal-stripped serum for 6 days. On the sixth day, the cells in the six-well plates were treated with 2 μL of ligands for 24 hours.Citation22 Control groups were treated with dimethyl sulfoxide (DMSO), the solvent used for BC treatments. Varying concentrations (80–500 μM) of BC were used in the concentration-dependent studies, while estrogens and antiestrogens were combined with 400 μM BC for the hormone studies. For estrogen treatments 10 nM E2 was used. As a pure estrogen antagonist treatment 1 μM ICI (ICI 182, 780) was used, and 1 μM TAM (Tamoxifen) was used as a selective estrogen receptor modulator treatment.Citation22,Citation23
Protein extraction and quantification
The cellular proteins were extracted by the addition of radioimmunoprecipitation assay buffer obtained from Santa Cruz Biotechnology (Dallas, TX, USA). The cells were then centrifuged at a speed of 15,000 × g for 15 minutes at 4°C. After centrifugation, the protein supernatant was separated and used to prepare a protein assay based on the Bradford method (Bio-Rad Kit; Bio-Rad, Hercules, CA, USA). The Bradford method (Bio-Rad Kit) allowed for the quantification and normalization of the protein in each extracted sample by use of a spectrophotometer.Citation24
SDS-PAGE and Western blot analyses
The extracted proteins were subjected to SDS-PAGE. The protein of interest was then isolated using Western blot analysis. To denature the sample to its primary structure, the protein supernatant was heated for 3 minutes at 85°C. Equal amounts of protein were then loaded into a 7.5%–12.5% polyacrylamide gel. Proteins that were run on these gels were transferred to an Immobilon polyvinylidene fluoride membrane (Millipore, Bedford, MA, USA) by the process of electroblotting. To begin probing, 5% nonfat dry milk was used to block nonspecific proteins. The membranes were then probed with the corresponding primary and secondary antibodies for each protein of interest. For ER-α, anti-ER monoclonal antibody (1:2000) from Santa Cruz Biotechnology (Santa Cruz, CA, USA) was used. To detect the primary antibody, secondary goat-anti-mouse IgG2a antibody (1:2000) was used. Actin bands were probed by anti-actin (monoclonal antibody clone C4) (Millipore, Bedford, MA, USA). ER-α, PR-A/B, and BRCA-1 levels were normalized to protein levels of the evolutionarily conserved actin-protein according to the manufacturer’s protocol. Anti-PR (monoclonal) (1:2000) and anti-BRCA1 (monoclonal) (1:2000) primary antibodies were obtained from Cell Signaling (Danvers, MA, USA) and detected by secondary goat-anti-rabbit antibodies. All secondary antibodies were obtained from Jackson Laboratories (Bar Harbor, ME, USA). The specific band for each protein of interest was then visualized by the enhanced chemiluminescence method according to the instructions from Amersham (GE Healthcare Biosciences, Piscataway, NJ, USA). The protein bands were visualized using the Chemi-Doc XRS + imaging system (Bio-Rad). The Western blots were subjected to quantification of the protein band density using the Image Studio Lite program, version 3.1 (LI-COR Biosciences, Lincoln, NE, USA).Citation24
Cell viability assay
A cell viability assay shows the number of live cells in a total population after treatments with ligands, at varying concentrations, for 6 days, with treatments administered every 2 days. The cell viability studies were cultured in 12-well plates (30,000 cells per well). Throughout the experiment, the medium was replenished every 48 hours. The plates were fed with 10% whole serum for the first 2 days to ensure confluency. The cells were then incubated in the presence of stripped serum for 6 days and quantified on the sixth day using the Cellometer Vision CBA software (Nexcelom Bioscience LLC). This was done by propidium iodide staining, which fluorescently tags dead cells. The number of dead cells versus total cells was quantified in order to calculate cell viability.Citation24
Immunofluorescence and confocal microscopy
Cells were plated as described under the “Cell viability assays” section. Immunolabeling was performed for BRCA1 in T-47D cells. The distribution of three-dimensional fluorescent structures was analyzed using a Nikon Digital Eclipse C1 plus confocal microscope and differential interference contrast (DIC) images were taken in parallel. NIS Elements AR software (Nikon Instruments, Melville, NY, USA) was used for image analysis.Citation24
Reverse transcription quantitative real-time PCR (RT-qPCR)
Total RNA was extracted from T-47D and MCF-7 cells using “Trizol” reagent (Invitrogen, Life Technologies, Waltham, MA, USA) per manufacturer’s protocol. The gDNA-free total RNA was reverse transcribed using iScript cDNA Synthesis Kit according to Life Sciences Technology. Prior to RT-qPCR analysis, cDNA was diluted tenfold in PCR-grade water. RT-qPCR was performed in a 96-well format in the Bio-Rad CFX384 Real Time System. The assay included NTC, RT(-), and NRT controls to detect reagent contamination and presence of genomic DNA. For data analysis, the quantification cycle (Cq) value was determined and specific gene expression normalized to endogenous control using ΔΔCq method. Expressions of ACTB and HPRT1 were set as the reference genes. The data were analyzed using Bio-Rad CFX Manager. ESR1 mRNA levels were determined by RT-qPCR. The cells were treated in the presence or absence of 100 μM BC, E2, and ICI for 24 hours. Results are shown as the mean ± standard error of the mean (SEM) of at least three independent experiments with three replicates in each experiment.Citation24
Statistical analyses
The results are expressed as mean ± SEM. Statistical significance was determined by the Kruskal–Wallis test, followed by post hoc analysis using the Mann–Whitney U test. Differences are considered significant at P <0.05. In all figures: *P<0.05; **P<0.01; ***P<0.001. Statistical analyses were carried out using SPSS for Windows version 11.5 (SPSS Inc., Chicago, IL, USA).Citation24
Results
Concentration-dependent effects of BC on ER-α, PR, and BRCA1 protein expression
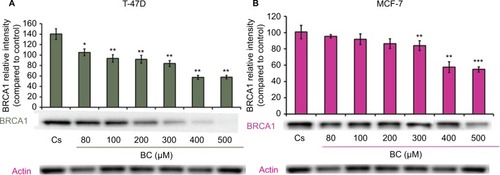
Western blot analysis revealed a concentration-dependent decrease in ER-α protein expression with treatments of 80–500 μM BC in both cell lines. Actin, a housekeeping protein consistently expressed under both normal and pathologic conditions, was probed as a control to confirm equivalence in protein loading among wells. An 87% decrease in ER-α expression in T-47D cells was observed with treatment of 400 μM BC compared to control (), and a 49% decrease in ER-α expression with treatment of 400 μM BC compared to control in MCF-7 cells (). Similar effects were observed with both PR and BRCA1 expression in both cell lines, also displaying 400 μM as the optimal concentration. In T-47D cells, 400 μM BC showed a 20% reduction in PR expression compared to control (), and a 50% reduction in BRCA1 expression compared to control (). In MCF-7 cells, the same BC concentration of 400 μM showed a 29% reduction in PR expression compared to control (), and a 43% reduction in BRCA1 expression compared to control ().
Figure 1 (A) BC downregulates ER-α protein expression in T-47D cells. (B) BC downregulates ER-α in MCF-7 cells.
Abbreviations: BC, black cohosh; ER-α, estrogen receptor alpha.
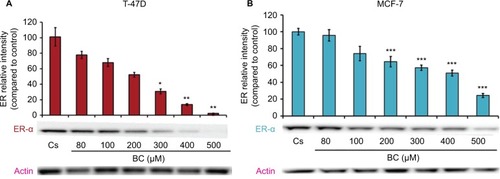
Figure 2 (A) Black cohosh downregulates PR protein expression in T-47D cells. (B) BC downregulates PR protein expression in MCF-7 cells.
Abbreviations: BC, black cohosh; PR, progesterone receptor.
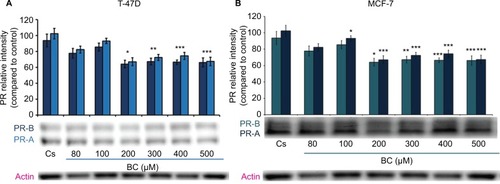
Figure 3 (A) BC downregulates BRCA1 protein expression in T-47D cells. (B) BC downregulates BRCA1 protein expression in MCF-7 cells.
Abbreviation: BC, black cohosh.
Effects of BC in combination with estrogens and antiestrogens on ER-α, PR, and BRCA1 protein expression
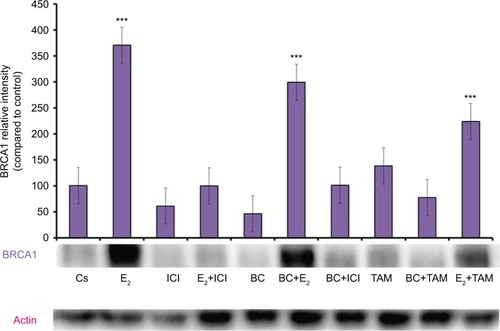
Treatments of E2 (10 nM) show an expected decrease in ER-α expression in both the T-47D () and MCF-7 () cell lines. Similar effects are seen with ICI, as well as the combination of E2 and ICI. A synergistic effect was seen on the downregulation of ER-α expression when BC (400 uM) was combined with E2 or ICI. A 90% decrease in ER-α expression was observed following cotreatment of BC and E2 in T-47D cells, with a 31% decrease compared to the control in MCF-7 cells following this same treatment. Combined treatment with BC and ICI showed an additive effect on the downregulation of PR compared to ICI alone in both T-47D () and MCF-7 cells (). Treatment of BC in combination with ICI and in combination with TAM showed a downregulation in BRCA1 expression in both cell lines ( and ).
Figure 4 The effects of hormones and antihormones in combination with BC on ER-α expression in T-47D cells.
Abbreviations: BC, black cohosh; ER-α, estrogen receptor alpha.
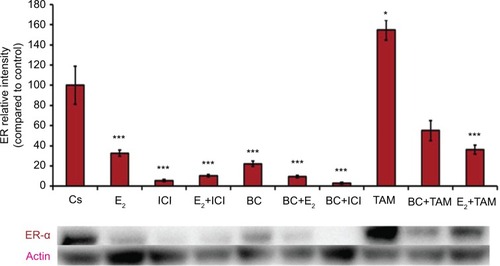
Figure 5 The effects of hormones and antihormones in combination with BC on ER-α expression in MCF-7 cells.
Abbreviations: BC, black cohosh; ER-α, estrogen receptor alpha.
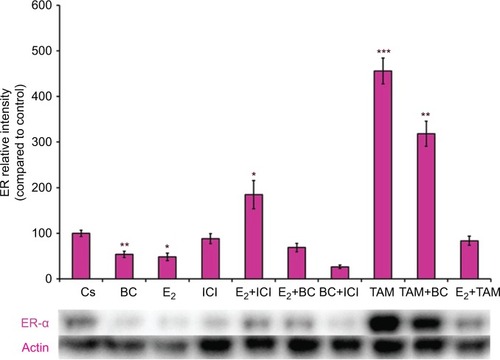
Figure 6 The effects of hormones and antihormones in combination with BC on PR expression in T-47D cells.
Abbreviations: BC, black cohosh; PR, progesterone receptor.
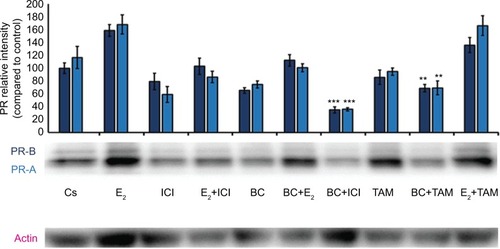
Figure 7 The effects of hormones and antihormones in combination with BC on PR expression in MCF-7 cells.
Abbreviations: BC, black cohosh; PR, progesterone receptor.
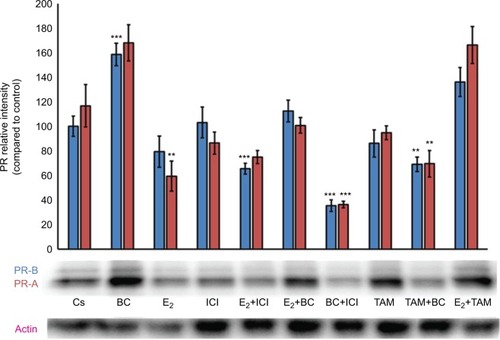
Figure 8 The effects of hormones and antihormones in combination with BC on BRCA1 expression in T-47D cells.
Abbreviation: BC, black cohosh.
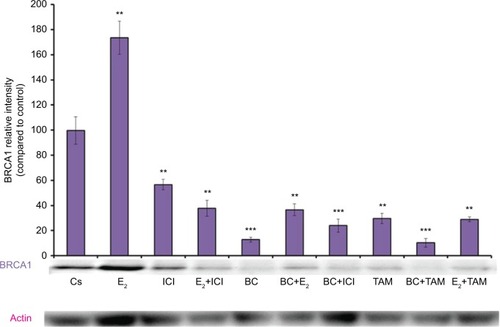
Figure 9 The effects of hormones and antihormones in combination with BC on BRCA1 expression in MCF-7 cells.
Abbreviation: BC, black cohosh.
Concentration-dependent effects of black cohosh on cell viability
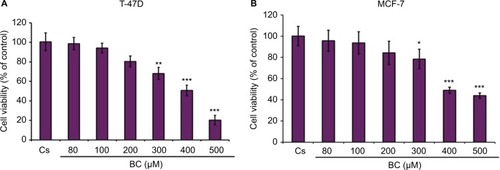
BC treatments of varying concentrations (80–500 μM) demonstrated a concentration-dependent decrease in both T-47D and MCF-7 cell viability, consistent with the decrease in ER-α, PR, and BRCA1 protein expression (). The previously determined optimal concentration of BC (400 μM) showed a 50% decrease in T-47D viability compared to control, and a 48% decrease in MCF-7 viability compared to control.
Figure 10 (A, B) BC decreases T-47D and MCF-7 cell viability.
Abbreviation: BC, black cohosh.
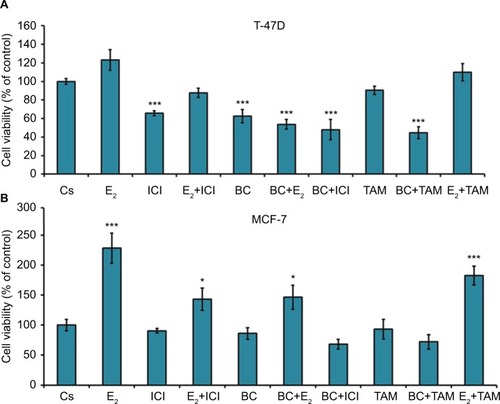
Effects of BC in combination with estrogens and antiestrogens on cell viability
Treatment with E2 displayed a proliferative effect on both T-47D and MCF-7 cell viability, which is to be expected. This effect of E2 was reversed by BC and ICI alone, which is seen by the combination of both treatments with E2 in T47-D (). Treatments of BC alone and in combination with E2, ICI, and TAM all demonstrate a significant decrease in cell viability in T47-D compared to control. In MCF-7, treatment with E2 alone and in combination with TAM demonstrated a significant increase in cell viability ().
Figure 11 (A, B) The effects of hormones and antihormones in combination with BC on T-47D and MCF-7 cell viability.
Abbreviation: BC, black cohosh.
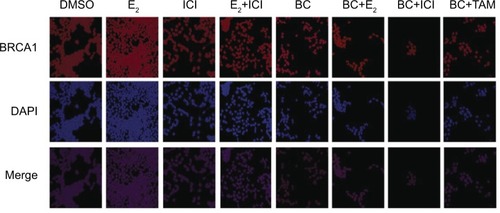
Effects of BC on the cellular localization of BRCA1
Confocal images show that BRCA1 is localized in the control (DMSO), as expected (). Treatment of cells with 10 nM E2 significantly increased BRCA1 nuclear intensity compared to control. The addition of 1 μM ICI combined with E2 significantly reduced the intensity of immunolocalization of BRCA1 as compared to the E2 treatment. Treatment with ICI, TAM, and BC reduced the BRCA1 levels compared to control, which may also indicate a possible ER-α-dependent mechanism.
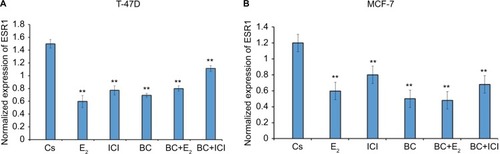
Effects of BC, E2, and ICI alone and in combination on ESR1 levels in T-47D and MCF-7 cells
RT-qPCR was utilized to study the effects of BC on the levels of ER-α under various conditions. ESR1 gene expression was normalized to reference genes, HPRT-1 and ACTB, in T-47D and MCF-7 cells exposed to treatment conditions for 24 hours. These results, depicted in , showed that ESR1 expression was reduced with 10 nM E2 and with 400 μM BC, as well as in combination with E2 and BC when compared to the control. The effects observed by BC and ICI combined induced a 30% decrease in ESR1 expression levels as compared to the DMSO-treated control.
Figure 13 (A, B) The effects of BC, E2, and ICI alone and in combination on ESR1 levels in T-47D and MCF-7 breast cancer cells.
Abbreviations: BC, black cohosh; SEM, standard error of the mean.
Discussion
Breast cancer is a leading cause of death among women in the USA and continues to have an impact around the world. The research of hormone-dependent breast cancer in particular is important in delineating the role of potential therapeutic mechanisms that act on steroid hormone receptors. BC, which is becoming more commonly used as an herbal alternative to conventional hormone-replacement therapy after menopause, is currently being investigated as possibly having this therapeutic potential. These studies may contribute to the understanding of its mechanism of action in hormone-dependent breast cancer cells.
Overall, Western blot analyses revealed a decrease in ER-α expression when the optimal concentration of BC (400 μM) was present. This is consistent with the findings of the cell viability experiments, which show a corresponding decrease in cell viability at this same optimal concentration. There was an approximate 80% reduction in ER-α expression compared to the control and an analogous 50% decrease in T-47D cell viability after treatment with 400 μM BC. In the MCF-7 cell line, treatment with the optimal BC concentration of 400 μM resulted iñ50% reduction in ER-α expression as compared to the control and an analogous 48% decrease in MCF-7 cell viability. This may be indicative of a relationship between the two effects.
Mutations in the tumor-suppressor gene BRCA1 have been known to play a significant role amid high-risk factors for breast cancer development. Research has shown that there may be a possible interaction between the BRCA1 protein and ER-α. It has previously been demonstrated that BRCA1-related breast cancer patients have a higher risk of developing cancer in estrogen-sensitive tissues.Citation25 It is suggested that ER-α and BRCA1 signal together through crosstalk mechanisms and these interactions may contribute to breast tumorigenesis and/or growth in the presence of estrogen. Interactive pathways such as these may begin to provide an explanation as to why BC and other anticancer compounds decrease the production of tumor-suppressor proteins such as BRCA1 and p53.Citation4,Citation5 Currently, however, it is unclear whether the growth-inhibitory effects of BC cause an appropriate decreased cellular viability for tumor-suppressor gene expression or if other cellular mechanisms lead to this decreased expression. Therefore, the correlational effects of BC on ER-α and BRCA1 that have been discovered in these studies contribute valuable insight and prompt further research necessary to understand the relationship between the two proteins as well as the effects of herbal supplements on these proteins.
The concentration-dependent decrease in ER-α expression due to treatment with BC is consistent with other findings that show a change in the expression of ER-α after BC treatment. Furthermore, these studies also suggest that the antiproliferative effects of T-47D cells may be due to BC’s antiestrogen effects.Citation20 The hormone studies, which examined cell viability in both the T-47D and MCF-7 cell lines, show that the proliferative effects of E2 were diminished when E2 treatments were combined with BC. This is consistent with other research, which showed a decrease in MCF-7 cell proliferation with BC treatment.Citation15 There is a corresponding decrease in ER-α expression when BC, in combination with E2, is used. ESR1 gene expression levels were reduced with the treatment of 10 nM E2 and with 400 μM BC in both cell lines. Combination treatment with E2 and BC also decreases the expression of ESR1 compared to the controls in both cell lines. Consistent with our findings, BC appears to decrease the expression of proteins that are involved in translation. Based on the observations of BC effects on cell viability, ER-α and PR protein expression along with concentration-dependent alterations in the sensitivity of both estrogens and antiestrogens in our results and the results from previous studies performed in our lab, there is a possibility that a dual mechanism for BC is both ER-dependent and independent. This is further indicative of the possible estrogenic properties of BC, as supported by other findings.Citation20 However, some studies have shown that BC does not act by mimicking the effects of estrogen; rather, it induces apoptosis (cell death) and prevents proliferation through other biochemical pathways.Citation26 Other studies suggest that the growth-inhibitory effects of BC on breast cancer cells are related to its chemical structure of triterpene glycosides.Citation15 Further research is needed for any definitive conclusion on whether or not BC acts through estrogen-like activity.
Our findings contribute to a better understanding of the effects of BC not only on ER-α expression but also on PR and BRCA1 expression in breast cancer cells. It is clear from our studies that this herbal compound substantially alters hormone-dependent breast cancer cells, but more extensive research is required to elucidate its exact mechanism of action.
Acknowledgments
We would like to thank Molly McDonald for her technical assistance. We would also like to thank NatureX for their donation of black cohosh. Funding was provided by the Center of Biomedical Research and Prevention Research Center, and by the Honors College at Oakland University, Rochester, MI, USA. An abstract of this paper was presented at the 98th Annual Meeting of the Endocrine Society in Boston April 1–4, 2016, as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in the meeting proceedings. Presentation Number: FRI-125: http://press.endocrine.org/doi/abs/10.1210/endo-meetings.2016. ED.1.FRI-125.
Disclosure
The authors report no conflicts of interest in this work.
References
- HarbeckNGnantMBreast cancerLancet201638910074P1134P1150
- WoolstonCBreast cancerNature20155277578S10126580154
- SzmydMThe effects of black cohosh on the regulation of estrogen receptor (ERα) and progesterone receptor (PR) in breast cancer cellsBreast Cancer20181011129403307
- SiebertAESanchezALDindaSMoudgilVKEffects of estrogen metabolite 2-methoxyestradiol on tumor suppressor protein p53 and proliferation of breast cancer cellsSyst Biol Reprod Med201157627928722077725
- DindaSSanchezAMoudgilVKEffects of LY117018 (a SERM analog of raloxifene) on tumor suppressor proteins and proliferation of breast cancer cellsHorm Mol Biol Clin Investig201021211217
- PowellCESotoAMSonnenscheinCIdentification and characterization of membrane estrogen receptor from MCF7 estrogen-target cellsJ Steroid Biochem Mol Biol2001772–39710811377974
- TungNWangYCollinsLCEstrogen receptor positive breast cancers in BRCA1 mutation carriers: clinical risk factors and pathologic featuresBreast Cancer Res2010121R1220149218
- JiaMDahlman-WrightKGustafssonJÅEstrogen receptor alpha and beta in health and diseaseBest Pract Res Clin Endocrinol Metab201529455756826303083
- YipCHRhodesAEstrogen and progesterone receptors in breast cancerFuture Oncol201410142293230125471040
- RosenEMFanSPestellRGGoldbergIDBRCA1 gene in breast cancerJ Cell Physiol20031961194112767038
- ElstrodtFHollestelleANagelJHBRCA1 mutation analysis of 41 human breast cancer cell lines reveals three new deleterious mutantsCancer Res2006661414516397213
- VaidyaYVaidyaPVaidyaTDuctal carcinoma in situ of the breastIndian J Surg201577214114626139969
- WiechmannLKuererHMThe molecular journey from ductal carcinoma in situ to invasive breast cancerCancer2008112102130214218383519
- SotoAMSonnenscheinCThe role of estrogens on the proliferation of human breast tumor cells (MCF-7)J Steroid Biochem198523187944021494
- GaubeFWolflSPuschLKrollTCHamburgerMGene expression profiling reveals effects of Cimicifuga racemosa (L.) NUTT. (black cohosh) on the estrogen receptor positive human breast cancer cell line MCF-7BMC Pharmacol2007711117880733
- MerchantSStebbingJBlack cohosh, hot flushes, and breast cancerLancet Oncol201516213713825638678
- BorrelliFErnstEAlternative and complementary therapies for the menopauseMaturitas201066433334320580501
- BorrelliFErnstEBlack cohosh (Cimicifuga racemosa) for menopausal symptoms: a systematic review of its efficacyPharmacol Res200858181418585461
- BorrelliFErnstEBlack cohosh (Cimicifuga racemosa): a systematic review of adverse eventsAm J Obstet Gynecol2008199545546618984078
- BorrelliFIzzoAAErnstEPharmacological effects of Cimicifuga racemosaLife Sci200373101215122912850238
- EinbondLSWen-CaiYHeKGrowth inhibitory activity of extracts and compounds from Cimicifuga species on human breast cancer cellsPhytomedicine2008156–750451117980565
- HowellAOsborneCKMorrisCWakelingAEICI 182,780 (Faslodex(TM)): development of a novel, “pure” antiestrogenCancer200089481782510951345
- AnK-CSelective estrogen receptor modulatorsAsian Spine J201610478779127559463
- SaluzzoJHallmanKMAleckKThe regulation of tumor suppressor protein, p53, and estrogen receptor (ERα) by resveratrol in breast cancer cellsGenes Cancer2016711–1241442528191286
- WangLDiLJBRCA1 and estrogen/estrogen receptor in breast cancer: where they interact?Int J Biol Sci105566575
- ViereckVEmonsGWuttkeWBlack cohosh: just another phytoestrogen?Trends Endocrinol Metab200516521422115927480