Abstract
Since its first documentation, breast cancer (BC) has been a conundrum that ails millions of women every year. This cancer has been well studied by researchers all over the world, which has improved the patient outcome significantly. There are many diagnostic markers to identify the disease, but early detection and then subclassification of this cancer remain dubious. Even after the correct diagnosis, more than half the patients come back with a more aggressive and metastatic tumor. The underpinning mechanism that governs the resistance includes over-amplification of receptors, mutations in key gene targets, and activation of different signaling. A plethora of drugs have been devised that have shown promising results in clinical settings. However, in recent times, the role played by cancer stem cells in disease progression and their interaction in mediating the resistance to cellular insults have come into the limelight. As breast cancer stem cells (BCSCs) are dormant in nature, it is highly likely that they fail to directly respond to the cytotoxic drugs which are meant for ablating rapidly proliferating cells. Furthermore, the absence of well-characterized, drug-able surface markers to date, has limited the application of targeted therapies in complete eradication of the disease. In this review, our intent is to discuss versatile therapeutics in practice followed by discussing the upcoming therapy strategies in the pipeline for BC. Furthermore, we focus on the roles played by BCSCs in mediating the resistance, and therefore, the aspects of new therapeutics against BCSCs under development that may ease the burden in future has also been discussed.
Introduction
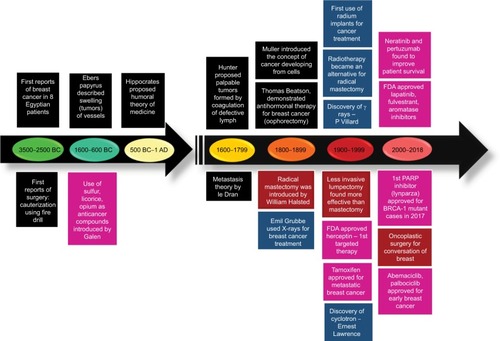
The first reported case of any kind of cancer was that of a breast cancer (BC) in around 1600 BC in Egypt, which is not surprising as the organ allowed easier identification. BC, since its first documentation, has been well-studied but is still a leading cause of deaths in women worldwide.Citation1 Extensive research has shown that this is due to the heterogeneous nature of the disease itself, which predicts the therapeutic response. Based on the presence or absence of the well-established biomarkers on the surface of BC cells, they are classified into luminal A, luminal B, HER2, and triple negative breast cancer (TNBC).Citation2 Each subclass has diverse risk factors for incidence, disease progression, therapeutic response, and favored organ sites of metastases. Out of these subtypes, TNBC is the most aggressive subtype and shows high metastatic potential. This can be attributed to the high number of cancer stem cell (CSC) population present within the tumor. There is a plethora of therapeutic modalities administered to BC patients on the basis of their initial diagnosis. Landmark discoveries like radical mastectomy, lumpectomy, radiation implants, tamoxifen, trastuzumab, and so on have remarkably helped in improving disease outcome, for a wide number of patients (). However, >50% of treated patients come back with a more aggressive disease. This aggressive behavior and resistance to the predicted therapies have marked a major challenge for BC clinics. Reports have shed a light onto the various mechanisms that might be responsible for these resistance mechanisms. Knowledge of the signaling cascades has led to the development of numerous molecules with potential to reduce the burden of the resistance encountered in BC. Even though chemotherapy and radiotherapy have undergone substantial improvements and refinements in efficacy and administration in the past decade, orthodox cancer treatments remain futile for many patients, predominantly whose cancer has been diagnosed at a later stage.Citation3 Delay in cancer diagnosis reduces the overall treatment efficacy mainly due to the increased likelihood of the manifestation of metastatic disease, but also partly because more advanced disease requires more intensive treatment, which may, itself, cause treatment intolerance. In this review, first we discuss the conventional chemotherapeutics in practice followed by the novel therapeutics that are being developed against drug-resistant BC. Further, we focus on the role of BCSCs in mediating the resistance. Lastly, aspects of currently developing treatment strategies against BCSCs are discussed.
BC therapy: conventional approaches
In the genome era, rapid advances in molecular understanding have subdivided BC into ten interclasts.Citation2 However, based on the current clinical practices, BC is known to have four primary subtypes. Luminal BCs are positive for steroid hormone receptors (estrogen receptor [ER] and progesterone receptor [PR]), which is further classified into two groups (A and B). The luminal A (ER+/PR+/HER2−) type tumors are less aggressive than other subtypes and take much longer to grow as well. These cancer cells also respond better to hormonal interventions and have a better prognosis.Citation4 Luminal B subgroup (ER+/PR+/HER2+) typically shows high Ki67 proliferative index marker as well as HER2 expression. BC cells belonging to luminal B subgroup usually show poorer prognosis than luminal A, but respond better to standard chemotherapy. Since patients of this subgroup also show high HER2 expression, targeted therapy for HER2 might also be employed in some cases.Citation4 In HER2+, BCs, which have amplification or overexpression of the HER2/ERBB2 oncogene, are generally treated with anti-HER2 therapies including the antibody drug trastuzumab and small molecule inhibitor lapatinib. Basal-like BC lacks the hormonal receptors as well as HER2 receptor and therefore is often known as triple negative breast cancer (TNBC). Standard chemotherapeutic regimens involving platinum-based drugs are majorly administered for treating TNBCs.
Majority of BC patients (~77%) have hormonal receptor-positive diseases, which comprise 23.7% from ER+/PR+/HER2− (luminal A) and ~53% from ER+/PR+/HER2+ (luminal B). Approximately, 23%–30% of BC patients show HER2 amplification. TNBC represents about 10%–12% of the total BC population.Citation4 Endocrine therapy is currently the gold standard treatment regimen to treat the hormone receptor+ BCs. This therapy works either by making the hormone effect ineffective or by lowering the hormone level itself. Therapeutic drugs prescribed to the patients include 1) tamoxifen, which acts by blocking the estrogen uptake by ER; 2) exemestane, anastrozole, and letrozole that belong to aromatase inhibitor class of drugs, which inhibits the conversion of androgens to estrogens thereby depleting estrogen in the body; 3) leuprolide and goserelin (luteinizing hormone-releasing hormone analogs), these drugs suppress the synthesis of hormone from the ovary; and 4) fulvestrant (a specific ER inhibitor), which makes it suitable for refractory BC patients. Administration of the above drugs for treating hormone receptor+ BC is recommended until there is clinical resistance or metastasis, where chemotherapy is employed.Citation5 As different endocrine drugs work by distinct mechanism, a combinatorial approach can show improved efficacy. However, the effectiveness of this combination treatment has not been proved well in the patient scenario.Citation5 Therefore, the current consensus is that both endocrine therapy-naïve advanced BC and high endocrine-sensitive patients can benefit from the combination endocrine therapy.Citation6
The patient group having HER2 gene amplification or protein overexpression is generally administered molecular targeted therapy; a range of targeted drugs have been approved as single agent or in combination with standard chemo regimen. The receptor-targeted therapeutic agents include 1) trastuzumab (specific anti-HER2 monoclonal antibody [mAb]); 2) ado-trastuzumab emtansine, which is trastuzumab conjugated with emtansine (microtubule inhibitor); 3) pertuzumab (specific anti-HER2 mAb with distinct binding site on HER2 extracellular region compared to trastuzumab); 4) lapatinib, a small molecule inhibitor (TKI) capable of inhibiting both HER2 and epidermal growth factor receptor (EGFR) signaling. The standard regimen for early stage HER2+ cases includes neoadjuvant therapy with a combination of HER2 targeted therapy and chemotherapy.Citation7 Subsequently, this treatment is followed by surgery, radiotherapy, and 1 year of HER2-targeted therapy. Endocrine adjuvant can be added based on the specific receptor status in patient. The successful advent of molecular targeted therapy against HER2+ BC can be seen by the substantial increase in overall survival (OS) of patients from ~1.5–5 years.Citation7
TNBC is aggressive by nature and defiant to treat as well when compared to hormone-positive and HER2+ BC. TNBC can be further subdivided into six subtypes based on transcriptomic heterogeneity and response to chemotherapy. These subtypes are mesenchymal (M), a mesenchymal stem-like (MSL), basal-like (BL1 and BL2), a luminal androgen receptor (LAR), and an immunomodulatory (IM) type.Citation8 Both M and MSL subtypes have enhanced expression of factors regulating epithelial–mesenchymal transition (EMT), but intriguingly only the MSL subtype has diminished expression of genes involved in proliferation. The BL1 subtype is categorized by augmented expression of cell cycle and DNA damage repair genes, while the BL2 subtype shows higher expression of growth factor receptors and myoepithelial markers. The LAR subtype is regulated by the androgen receptor (AR) and characterized by luminal gene expression. The IM subtype comprises of BC cells encoding immune checkpoint regulatory genes such as programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1), antigens, and immunomodulatory cytokines. Detailed analysis shows activation of immune signal transduction pathways in this subtype, which is likely from both the tumor cells and infiltrating lymphocytes.Citation8
Until now, standard chemotherapy remains the mainstay of treatment in TNBCs. The absence of the receptors precludes the application of targeted therapies against advanced stage disease. The only US Food and Drug Administration (FDA)-approved therapy is chemotherapy drugs such as anthracycline, taxane, and platinum drugs with or without bevacizumab.Citation9 The median OS of patients with metastatic disease ranges between 9 months and 1 year.Citation9 Given the suboptimal treatment outcome with standard therapeutic agents, identification of novel targets and therapy is the need of the hour.
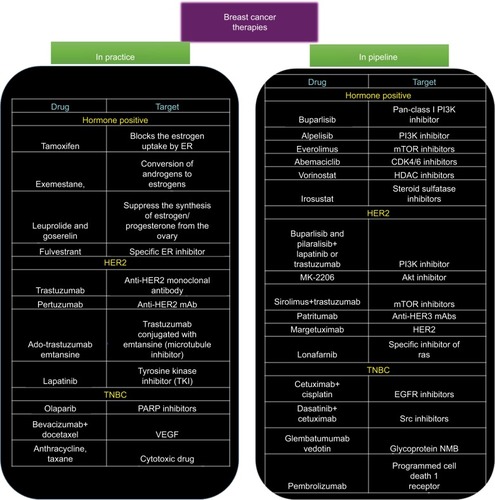
Even with the development of so many different agents, the BC patient scenario is still very disappointing. This can be attributed to the innate biology of the cancer cells to outsmart the current therapies. Over the past decade, it has been identified that cancer cells employ various strategies to overcome the cytotoxic effects such as activation of other signaling pathways, altered metabolism, change in the cell cycle machinery, and epigenetic changes to name a few. This knowledge has led to the development of agents with potential to overcome the resistance of BC cells (). In the following sections, we discuss some of the promising therapeutic strategies that are being investigated to treat drug-resistant BC.
Figure 2 Snapshot of various chemotherapeutic modalities that are being prescribed in clinics and the novel therapeutic drugs that are being developed.
BC therapy: developments in the challenging dogma
Hormonal therapy-resistant BC
Development of resistance in hormone receptor-positive BC against their targeted treatment agents is now a well-established phenomenon. Resistant cancer is often metastatic in nature and the underpinning genomic alterations occur majorly in ER cascade. However, other signaling pathways might also get activated and are involved. Mammalian target of phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/rapamycin (mTOR) (PI3K–Akt–mTOR) signaling circuit is considered as one of the prime contributing factors to the resistance in a variety of cancers including BC with hormonal drug resistance.Citation10 This signaling cascade is reported to be overactivated in almost 70% of BC, with PIK3CA (PI3K catalytic subunit p110α) being the frequently mutated and/or amplified genes.Citation11 In addition, activation of escape pathways like HER2 signaling as well as altered cell cycle kinetics has been observed to mediate resistance against ER therapies Thus, to tackle ER+ metastatic BC, there is a need to develop novel therapeutic approach with a potential to either minimize or reverse drug resistance. To address the conundrum of resistance, spectrum of different chemotherapeutic agents has been developed.
PI3K inhibitors
Combinatorial therapies targeting both hormonal receptors and PI3K/AKT/mTOR pathways have been appraised to reverse the resistance to hormonal therapies. Combinatorial treatment with PI3K inhibitors and aromatase inhibitors has been employed as a second-line of treatment for advanced luminal A cases. Buparlisib (a pan-class I PI3K inhibitor) was reported to considerably improve progression-free survival (PFS) in patients, specifically in those having PIK3CA mutation. However, PI3K inhibitors such as pilaralisib,Citation12 voxtalisib,Citation12 and buparlisibCitation13 cannot be employed in treating patients due to their high toxicity. Recently, taselisib and alpelisib are also under Phase III trials (NCT02340221 and NCT02437318, respectively) and are reported to be efficacious primarily due to their high selectivity and lesser toxicity. These α-specific PI3K inhibitors showed promising results in patients harboring PIK3CA mutations.Citation14 Irrespective of PIK3CA status, both taselisibCitation15 and pictillisibCitation16 in combination with letrozole or anastrozole were found to augment antitumor effects in early luminal A patients when employed as neoadjuvant treatment. Buparlisib and alpelisib are currently under Phase II efficacy investigation (NCT01923168).
mTOR inhibitors
Everolimus (derived from sirolimus) has been approved by the US FDA for treating ER/PR + advanced BC in combination with exemestane. Everolimus has also been employed in combination with letrozole, but its clinical efficacy was a failure as it could not reverse the resistant BC.Citation17 Another derivative of sirolimus, that is, temsirolimus, was a complete defeat as it could hardly show any clinical benefits either as first-line therapy in combination with letrozole or as a single agent in second-line therapy in advanced ER/PR + BCs.Citation18
Cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors
It is known that cancer cells have aberrant cell cycle machinery that aids them to divide and proliferate infinitely. Thus somehow, inhibiting the cell cycle in these cancer cells might provide a way to overcome the resistance in cancer cells. The proteins involved in regulating cell cycle belong to cyclin-dependent kinase (CDK) family. In addition, CDK4/6 has been shown to regulate the cell cycle progression by its reversible interaction with cyclin D1. Thus, among the emerging therapies against CDKs, CDK4/6 inhibitors such as abemaciclib, ribociclib, and palbociclib are the most promising candidates. These CDK4/6 inhibitors block the phosphorylation of retinoblastoma protein, resulting in the downregulation of E2F-response genes to mediate cell cycle arrest at the G1-S stage. These small molecule inhibitors have also been reported to dephosphorylate the forkhead box protein M1 (transcription factor), causing inhibition in cellular proliferation.Citation19 Interestingly, it was observed that hormone-resistant tumors are still dependent on CDK4/6-cyclin D1 for their growth and proliferation.Citation20
Promising results have led to the FDA approval of combination treatment using ribociclib and palbociclib along with aromatase inhibitor as the first-line treatment for ER+/PR+/HER2− advanced BC. They have been reported to significantly improve the PFS in advanced BC patients by 10 months and the PFS rate by 20% after 18 months, respectively, compared to letrozole alone.Citation21 Abemaciclib has been shown to prolong the median PFS by 7 months,Citation22 when employed as second-line treatment in combination with fulvestrant in ER+/PR+/HER2− advanced BC. To assess the efficacy of ribociclib and abemaciclib alone, they are currently in Phase III trials (NCT02422615 and NCT02246621). Although the mechanism of action of these CDK4/6 inhibitors is alike, abemaciclib showed greater monotherapy response and induced lesser neutropenia as compared to other inhibitors, primarily due to its more specific CDK4 inhibition.Citation21 In addition to the conventional/direct approach of targeting the signaling cascade involved in mediating resistance, there has been development of inhibitors which have the potential to reverse the resistance.
Histone deacetylase (HDAC) inhibitors
Resistance to conventional hormonal therapy has also been attributed to the histone deacetylation-mediated loss of ER expression in ER+ patients.Citation1 In line with this observation, application of HDAC inhibitors has been shown to upregulate the expression of ERα and aromatase, thereby aiding in suppression of the signaling governed by ER.Citation23 Entinostat when combined with exemestane and vorinostat in combination with tamoxifen has shown promising antitumor activity when employed as second-line treatment for ER+/PR+ advanced BC in combination with, compared to, their monotherapy counterparts.Citation23
Steroid sulfatase inhibitors
Steroid sulfatase also known as arylsulfatase C is a sulfatase enzyme involved in the metabolism of steroids. Interestingly, the enzymatic activity of steroid sulfatase was reported to be substantially increased in ERα-positive BC cells.Citation24 Thus, inhibiting the enzymatic activity reduces the estrogenic steroids and suppresses tumor growth. Encouraging results were reported from the Phase II trial of the combinatorial treatment using irosustat along with conventional aromatase inhibitor.Citation25 SR16157 (dual-acting steroid sulfatase inhibitor) which is a direct inhibitor of steroid sulfatase and releases ERα modulator has also been assessed for its effects in hormone-dependent BC.Citation26
Resistant BC against HER2 targeted therapy
The growing reports of primary and acquired resistance to lapatinib or trastuzumab are alarming and severely hampering its clinical significance as HER2+ BC therapeutics. Thus, identifying the resistance mechanisms and discovery of potential therapeutic agents to tackle the resistance is the need of the hour. Various groups have identified and verified numerous therapeutic agents that might play an important role in the fight against metastatic HER2+ BC.
PI3K/Akt/mTOR inhibitors
The aberrant activation of PI3K/Akt/mTOR pathway is also considered to be mediator of resistance in HER2+ BC, thus combining the inhibitors of this pathway with HER2 targeted candidates and studying the efficacy of the drugs is an area of active research. Buparlisib and pilaralisib (pan-class I PI3K inhibitors), when administered with lapatinib,Citation27 trastuzumab,Citation28 or trastuzumab and paclitaxel,Citation29 was proven to be efficacious and safe in patients having HER2+ advanced disease. In addition, MK-2206 (an Akt inhibitor) also showed promising antitumor activity when combined with trastuzumab and paclitaxelCitation28 or trastuzumabCitation30 alone in patients with HER2+ advanced BC. To directly target the mTOR, everolimus was combined with trastuzumab and vinorelbine; however, the clinical outcome of advanced HER2+ BC patients did not improve.Citation31 Surprisingly, this combination demonstrated better anticancer activity than trastuzumab alone in HER2+ patients who are hormone receptor negative.Citation31 Recent drugs such as sirolimusCitation32 and ridaforolimusCitation33 when administered in combination with trastuzumab have demonstrated promising results in refractory HER2+ BC.
Inhibitors targeting HER-family receptors
Receptor ligands switching between HER-family members (HER1 [EGFR], HER3, or HER4) can activate the signaling cascade, this phenomenon is reported to make trastuzumab redundant.Citation34 In addition, HER2/HER3 heterodimers have also been associated with trastuzumab resistance.Citation35 Thus suppression of the HER-family members may be promising to deal with the conundrum of resistance in HER2+ advanced BC. In view of this, an irreversible TKI, neratinib was developed that has the ability to inhibit HER1/HER2/HER4. It has been reported that administration of neratinib after trastuzumab adjuvant therapy has significantly improved the 2-year invasive disease-free survival in HER2+ patients.Citation36 Preclinical study has also shown encouraging antitumor activity of an anti-HER3 mAb (patritumab) by inhibiting HER2/HER3 heterodimers. Further, it was shown that it is efficacious and has lower toxicity in patients with advanced HER2+ disease.Citation37 A novel mAb margetuximab (HER2 targeting) was also assessed in a first Phase I trial for its antitumor activity and was found to be well tolerated and had promising activity even as a single therapeutic agent.Citation38 Further, this antibody inhibitor is currently undergoing trials to test its efficacy as a single agent (NCT02492711) and/or in combination with pembrolizumab (targets PD-1 receptor of lymphocytes) (NCT02689284).Citation38 Among other ongoing efforts, trastuzumab is conjugated with emtansine (microtubule inhibitor) which utilizes the specificity of trastuzumab for targeting HER2+ BC cells and microtubule cytotoxicity for killing the cells.Citation39 It has been approved as a second-line treatment for lapatinib/trastuzumab-relapsed/refractory HER2+ BC patients.Citation40
Immunotherapy
The use of immune system against tumor cells serves as an area of extensive research with the aim to develop a vaccine against cancer. One of the first devised immunotherapeutic agents was nelipepimut-S, derived from the extracellular region of HER2. It has been extensively analyzed as a potential vaccine to prevent relapse in high-risk BC patients.Citation41 The combinatorial application of nelipepimut-S and trastuzumab in HER2+ early BC is studied in Phase IIb clinical trial (NCT02297698). Recombinant HER2 protein (dHER2) was also studied for the potential vaccine and exhibited immunogenicity to augment T-cell-mediated response against HER2+ BC.Citation42 Follow-up studies are being carried out to elucidate its role as monotherapy in HER2+ advanced BC as well as advanced BC refractory to trastuzumab or lapatinib.Citation43
TNBC
Among the BC subtypes, TNBC has fewer choice of therapeutic drugs, primarily due to the lack of well-characterized molecular targets. Therefore, the need of the hour is to identify novel targets and develop effective agents against these targets to achieve improved clinical benefits. Till now the agents developed against TNBCs are primarily based on drug repurposing.
Anti-angiogenic agents
Vascular endothelial growth factor (VEGF), which is a key angiogenic factor implicated in various cancers, has been reported to be higher in TNBC as compared to non-TNBC BC.Citation44 A well-known anti-VEGF mAb, bevacizumab, demonstrates suppression of tumor neovasculature growth and inhibits metastasis. It was also reported in a Phase III trial that supplementation of bevacizumab to docetaxel (first-line chemotherapy) resulted in improved response rate.Citation45 It was also observed that the combination of bevacizumab and docetaxel had minimal or no side effects when compared to docetaxel alone.
Poly(ADP-ribose) polymerase (PARP) inhibitors
A major breakthrough toward the understanding of the heterogeneity of the TNBCs came in the form of detection of a subclass of sporadic TNBC that has deficiency in the homologous-repair pathway, which is a characteristic of BRCA1/2-mutated BC. In line with this observation, the therapeutic drugs administered to these patients incorporate PARP inhibitors or platinum drugs (DNA targeting) like carboplatinCitation46 along with conventional chemotherapy.Citation47 BRCA1/2 genes are responsible for encoding tumor-suppressor genes that are involved in repairing DNA double-stranded breaks via homologous recombination. Whereas, PARP enzymes repair the single-stranded breaks. Patients harboring germline BRCA1/BRCA2 mutation (gBRCA+) benefit the most after administration of PARP inhibitors, probably due to synthetic lethality.Citation48
When considering PARP inhibitors, olaparib seems to be a success story. In addition to olaparib, other inhibitors targeting PARP such as talazoparib is currently in Phase III trial (NCT01945775). Talazoparib has shown promising preclinical results, which can be attributed to its strong affinity to DNA by trapping PARP–DNA complexes.Citation49 It has also demonstrated strong anticancer activity as a monotherapeutic drug in advanced gBRCA+ BC.Citation50 Rucaparib (Phase II, NCT02505048) and niraparib (Phase III, NCT01905592) are being explored in case of gBRCA+ advanced BC patients as a single agent as well as in combination with standard chemotherapy (niraparib: Phase I/II, NCT02657889; rucaparib: Phase II, NCT01074970).
The decision of using either PARP inhibitors or carboplatin in TNBC is generally determined by three DNA-based homologous recombination deficiency scores, which highly correlates with the germline genetic defects in BRCA1/2.Citation51 However, none of the abovementioned therapeutic agents is beneficial against all TNBC because of the heterogeneous nature of TNBC. Thereby, an urgent need for the identification and characterization of novel clinically important molecular biomarkers for further refinement of the in-practice treatment approaches.
EGFR inhibitors
The EGFR has been reported to be overexpressed in TNBC. Therefore, numerous clinical trials are underway to evaluate the antitumor activity of cetuximab in combination with platinum-based drugs like cisplatin in metastatic TNBC patients.Citation52,Citation53 Identification of a subpopulation of TNBC patients that might respond well to EGFR inhibitors is an area of active research efforts.Citation54 Low expression of α-crystalline B chain, lack of KRAS expression, and higher expression of PTEN in tumors might be correlated with favorable response.Citation54
SRC inhibitors
SRC is a non-receptor signaling kinase which is a downstream molecule of several growth factor receptors such as PDGFR, EGFR, HGFR, and IGF-1R which have been reported to be deregulated in TNBC. Dasatinib, when tested as monotherapy for TNBC in Phase II trial (CA180059), showed substandard result.Citation55 However, when tested in cell lines, dasatinib in combination with anti-EGFR mAb: cetuximab and cisplatin showed synergistic antitumor activity in different TNBC cell lines.Citation56 The combination of three drugs resulted in more prominent induction of apoptosis and inhibition of MAPK and EGFR phosphorylation than all the other combinations.Citation56 In addition, cancer cell migration and invasiveness were also substantially suppressed by dasatinib as well as combination treatment with dasatinib, cetuximab, and cisplatin in TNBC cell lines.Citation56 Thus, clinical investigations are required to further access the use of dasatinib-containing amalgamations in TNBC patients that have tumors expressing both EGFR and c-Src.
Monoclonal antibodies
Glembatumumab vedotin is a mAb conjugated with a cytotoxic drug aimed at targeting glycoprotein NMB-overexpressing (gpNMB+) TNBC.Citation57 gpNMB is a transmembrane protein that has been linked with tumor invasion and promote metastasis and is overexpressed in about 40% of TNBC.Citation58 Phase II trial done on gpNMB+ advanced TNBC patients showed significant improvement in PFS and OS in glembatumumab vedotin-treated patients as compared to conventional therapy.Citation59
Breast cancer stem cells (BCSCs): the troublemakers
BC is widely understood as a heterogeneous disease which in turn contributes to therapy failure and disease progression.Citation60 There is not only intratumoral heterogeneity, that is, diversity within a tumor in context to phenotypic, functional, and genetic variations, but also intertumoral diversity, that is, the diversity between primary and metastasized tumor. To explain the intratumoral heterogeneity, two theories have been put forward. The first one was clonal evolution theory/stochastic theory, introduced by Peter Nowell, according to which cancer is an evolutionary process in which most neoplasms arise from single cell and progression of tumor results from stepwise accumulation of mutations within original clones following selection of more aggressive subclones. Accordingly, each dominant subclone possess similar tumorigenic potential.Citation61
The second theory proposed is CSC theory. According to this hypothesis, only a small population of cells, called CSCs, are capable of self-renewal and have the potential to initiate tumor. In CSC model, cancers originates from the malignant transformation of a stem or progenitor cells through the deregulation of self-renewal program or from transformation of committed cells through dedifferentiation of mature cells that gain a self-renewal potential.Citation62
The first CSCs from solid tumors were identified in breast tumors,Citation63 subsequently CSCs were isolated from other organs. Al-Hajj et al were the first to identify a subpopulation of BC which had the potential to form tumors in immune-deficient Nonobese Diabetic (NOD)/Severe Combined Immunodeficiency (SCID) mice.Citation63 They used a set of cell surface markers to isolate cells with increased tumorigenic capacity. In particular, cells that were CD44+CD24lowEpCAM+ and lineage negative (cells lacking markers CD2, CD3, CD10, CD16, CD18, CD31, CD64, and CD140b), isolated from one primary breast tumor and eight metastases, were able to form heterogeneous tumors eight out of nine times and were termed as BCSCs. Surprisingly, as few as 200 CD44+CD24lowEpCAM+lin- cells transplanted into NOD/SCID mice could form tumors with 100% efficiency, while CD44−CD24+EpCAM− cells could not form tumors. Different subtypes of BC constitute different proportion of BCSCs contributing to different disease outcome. Among the BC subtypes, the highest amount of CSCs was observed in patients with TNBC (basal) subtype and has been correlated with its aggressiveness.Citation64
With momentous discovery of CSCs, their pivotal role in driving key processes during cancer development such as tumor growth, metastasis, recurrence, as well as treatment resistance was established. However, the signaling pathway that regulates CSCs and that might be involved in promoting the resistance toward the conventional therapies remains largely elusive. Hedgehog, Notch, and Wnt pathways have been shown to play crucial role in promoting resistance to therapy. These pathways are generally involved in the development of embryo and adult tissue homeostasis. Deregulation of the Notch and Hedgehog pathways, which normally regulates stem cell self-renewal and differentiation, results in BCSC phenotype.Citation65 The Wnt pathway plays an important role in maintaining and preserving undifferentiated state of stem cells.Citation66 Hedgehog pathway, which is an embryonic development organizer pathway, is also deregulated in BC, thereby activating Gli1 and Ptch1 genes (positive modulators of the hedgehog pathway) and thus leading to BCSC proliferation.Citation67 The Notch pathway is involved in cell differentiation during both embryogenesis and adulthood. Notch pathway deregulation activates genes important for regulating proliferation and apoptosis inhibition in cancer cells.Citation68 The transcription factors targeted by Notch signaling include CDKN1A, cyclinD1, c-myc, and HES-related repressor protein. These pathways have been reported to be activated in BCSCs.Citation69 In addition, other transcriptional factors involved in maintaining the potency of BCSCs have also been identified. The transcriptional factors such as Sox2, Oct4, and Nanog act as master regulators of pluripotency and maintain the undifferentiated state of BC cells.Citation70 Of the basal-like breast carcinomas, 43% exhibit higher Sox2 expression, indicating a less differentiated phenotype.Citation71 Another member of the Sox family, Sox4, induces changes associated with the EMT process that is responsible for increased invasiveness and mobility of cancer cells in vivo.Citation72 Recently, the ability of BCSCs to undergo EMT has been scrutinized, leading to the identification of partial EMT. Reports suggest that the circulating tumor cells (CTCs) survive in blood by exhibiting both epithelial and mesenchymal (E/M) phenotypes. The CTCs employ the collective cell migration properties of the epithelial cells and enhance their attachment to the extracellular matrix by achieving mesenchymal properties.Citation73 This significantly enhances the chance of survival and promote distant metastasis. Several evidences suggest that the expressions of Oct3/4, Nanog, and Sox2 are strongly associated with different CSCs, including BCSCs.Citation74
Apart from the genes that maintain the potency of stem cells, the BCSCs can be distinguished based on the following unique features:
Presence of classical cell surface marker such as CD44+CD24−, in addition CD133, CD44+ CD49 fhi CD133/2hi. CD49f and CD61 have also been introduced as BCSC marker.Citation75 These markers can be detected by flow cytometer, via employing specific mAbs.
High expression of BC resistance protein 1, also known as ATP-binding cassette (ABC) transporter G family ABCG2 or CD338.Citation75 This can be tested by using orthodox side population assay.
Ability to form mammospheres in suspension culture and the overexpression of aldehyde dehydrogenase-1 (ALDH1).Citation75
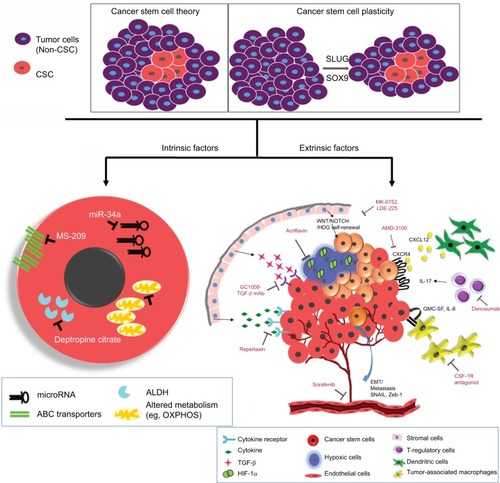
Chemoresistance to the conventional therapies can be divided into two main groups, namely intrinsic resistance due to genetic alterations and extrinsic resistance including microenvironment influences ().Citation76 Intrinsic resistance includes overexpression of ABC transporter, overexpression of ALDH1, enhanced DNA repair mechanism, an altered cell cycle, and resistance to apoptosis. The extrinsic cause of resistance includes all micro-environment influences such as hypoxia or EMT.
Figure 3 Schematic diagram showing intrinsic and extrinsic factors that aid the breast cancer stem cells to evade and survive against therapeutic insults.
Notes: Two widely accepted theories regarding the origins of CSCs are highlighted. According to “Cancer stem cell theory,” an inherent subpopulation of dormant cancer cells that are pluripotent in nature with the ability to repopulate the depleted pool of cancer cells following therapy. The “cancer stem cell plasticity theory” describes that therapeutic insults and EMT triggers a few breast cancer cells to undergo a switch, converting epithelial cells to pluripotent CSCs. The interplay of microenvironment and intrinsic cascades of CSCs aids them to elude the conventional therapy. Intrinsic factors depict microRNAs, drug transporters, ALDH, and altered cellular metabolism as pivotal processes that can be targeted. Further, extrinsic factors include a plethora of various components from the tumor microenvironment. Some of the important players being hypoxia, angiogenesis, EMT, immune cells, and stromal cells promoting proliferative signaling like cytokines, TGF-β, and self-renewal signals like Wnt/Notch/Hedgehog. Some of the inhibitors/mAbs against these resistance promoting factors have also been depicted.
Intrinsic factors of resistance
The small pool of cells, that is, BCSCs that evade chemotherapy is possibly because of the presence of ABC transporters. Increased level of ABCG2 in BCSCs was shown to enable rapid expulsion of cytotoxic drugs, conferring cellular resistance to antitumor drugs.Citation77 Increased levels of P-glycoprotein which belongs to ABC transport family also confer resistance to antineoplastic drugs by manipulating several cellular processes like the p53 network which plays a role in mediating chemoresistance. New tumors arising from BCSCs show a chemoresistant phenotype and are often accompanied by activating mutations.Citation75 Hu et alCitation78 observed that Akt signaling altered the subcellular localization of BCRP, thereby regulating drug efflux activity in CSCs. Inhibitors of PI3K, blocked Akt signaling, resulted in the suppression of cancer cell proliferation, but also enhanced the sensitivity of chemoresistant cells.Citation78
Aldefluor assays indicated that highly tumorigenic BC cells were ALDH positive. These BCSCs had the similar differentiation and self-renewal properties when compared to CSC.Citation79 ALDH1A1 and ALDH3A1 are important in the protection and the differentiation of CSCs via the conversion of retinol to retinoic acid.Citation80 ALDH1 has the ability of metabolizing toxic chemotherapeutic agents into nontoxic molecules, particularly cyclophosphamide class, by converting aldophosphamide to carboxyphosphamide and thus eliminating the lethal effects of the acrolein and phosphoramide mustard (metabolite of cyclophosphamide).Citation80,Citation81 It has been observed that metastatic breast tumors overexpress ALDH.Citation82
Alteration of cell cycle kinetics is another alternative intrinsic mechanism of resistance reported in BCSC.Citation83,Citation84 This feature aids the BCSC to evade death due to chemotherapeutic agents targeting rapidly dividing cells.Citation76,Citation81 This quiescent state of BCSC is also responsible for relapsed disease after a long-periods of time. A dexterous DNA repair mechanism in the BCSCs is another example of intrinsic resistance mechanism.Citation84 BCSC uses the augmented activity of ChK1 and ChK2 allowing them to escape from mitotic catastrophe and to repair their damaged DNA proficiently.Citation84,Citation85 This state of dormancy and robust DNA repair mechanism contributes to the resistance of BCSC against standard chemotherapeutic regimes.
In recent times, miRNAs are also shown to govern and regulate the BC resistance against the standard therapies. miRNAs are short, non-coding RNAs that regulate crucial biological processes and are frequently deregulated in cancer. Suppression of miR200c has been shown to promote tumorigenicity of BCSCs and normal mammary stem cells. In addition, it was shown that suppression of miR-200c triggers migration and invasion of cancer cells in the neighboring tissues.Citation86 Loss of miR-205 in BCSC populations has been shown to result in drug resistance properties.Citation87 Another report demonstrated that miR-141 is inhibited in BCSCs, contributing to the dedifferentiation of BC cells into stem-like cells which in turn enhances the stem population.Citation88 Alternatively, reduced expression of miR-34a in human BC resulted in inhibition of stem cell properties. A report has shown that miR-34a regulates Notch-1 pathway in sustaining stem cell properties of BCSC populations, thereby suggesting that the miR-34a/Notch-1 pathway might be a potential therapeutic target for treating BC.Citation89 Further, enhanced expression of let-7 miRNA has been shown to be involved in tumorigenesis of BCSCs. Other report has revealed that isoform let-7c along with Wnt signaling cascade regulates BCSC renewal in vivo.Citation90 In addition, miR-1 has been shown to be associated with Wnt signaling pathway which is critical for the aggressiveness of BC.Citation91 Most of the knowledge gained in recent times has provided a foundation for the future development of miRNA-based therapy against BCSCs.
Extrinsic cause of resistance
The interaction between the microenvironment and CSC is a dynamic process resulting in continuous remodeling of both.Citation84 EMT plays a crucial role in chemoresistance and aids in cancer metastasis.Citation92 Augmented drug efflux, suppressed apoptotic signaling pathways, and slow cellular proliferation are associated with EMT, and this contributes to the resistance of BC cells against anticancer drugs.Citation93 Gefitinib or erlotinib, prescribed to BC patients with high EGFR, often relapses. EMT-associated transcription factor Snail is reported to enhance the expression of AXL receptor tyrosine kinase. Signals transduced by AXL allow the BC cells to override the cytostatic effects of EGFR inhibitors. EMT also triggers other processes that enable the BC cells to elude the lethal effect of cytotoxic T cells. Elevated expression of PD-L1 is one such major evasion mechanism employed by BC cells.Citation75 PD-1, an inhibitory immune-checkpoint receptor, expressed by cytotoxic T cells recognizes the PD-L1 on the cancer cells and diminishes their function. Further, enhanced secretion of thrombospondin-1 by mesenchymal cells induces the development of regulatory T cells within the tumor micro-environment that suppresses the cytotoxic T cells.
In addition to chemoresistance, EMT process also equips the BC cells to evade cytotoxic effects of radiation. Radio-resistant BC cells that acquire mesenchymal properties have been reported to be more invasive, attributed to enhanced traction forces and membrane ruffling.Citation94 The intricate program of EMT is not only responsible for the development of BCSC, but deeper understanding in the process would help us to develop novel approaches to target cells that evade conventional therapeutic regimens. Paracrine signals from the Notch/Wnt/Hedgehog pathway influence EMT by cytoskeleton rearrangements which results in mesenchymal-like phenotype.Citation95
BCs have the tendency to recruit mesenchymal cells from the normal breast stromaCitation96 or from the bone marrow.Citation97 For instance, mesenchymal stem cells (MSCs) expressing ALDH1 are selectively recruited to areas of actively dividing tumor, where they interact with BCSCs via cytokine loops of CXCL7 and IL-6.Citation97 These cytokine signaling augments the self-renewal of BCSCs.Citation97 In addition, MSCs have also been shown to protect the BCSCs via recruitment of regulatory T cells.Citation98 Immunohistochemical analysis has established the presence of such interacting MSC/BCSC in tumor biopsies of BC patients.Citation97 High ALDH1 expression in BC cells has been shown to be an independent predictor of poor outcome in patients with BC.Citation79 Further, MSCs have the capability to differentiate into adipocytes and tumor-associated fibroblasts, which might also interact with tumor cells and can influence disease progression.Citation99
Gabbiani and Majno were the first who reported the morphological alterations in the stimulated myofibroblasts of dormant tumor- and wound-associated fibroblasts.Citation100 Confirming the observation, in an experimental mouse model, it was demonstrated that acute wounding of the mammary gland by dermal incision augmented BC growth and metastasis.Citation101 While the exact underpinning mechanisms remain elusive, it is believed that paracrine signals from the developing tumors induce epigenetic changes in the neighboring stromal fibroblasts.Citation102 Certainly, the expression profile of cancer-associated fibroblasts (CAFs) is similar to that of wound-associated fibroblasts; this profile has been linked with poor outcome of patients.Citation103,Citation104 A report suggests that transforming growth factor beta (TGF-β; growth factor) may be involved in regulating the epigenetic changes, leading to fibroblast activation.Citation105 In addition, cytokines like CXCL12 (also known as SDF-1) released by BC-associated fibroblasts might help in the proliferation of cancer cells, which expresses CXCR4 (SDF-1 receptor).Citation106 The high levels of free SDF-1 in serum has been correlated with poor outcome in BC patients.Citation107,Citation108
Interleukin-6 (IL-6) and IL-8 have been associated with both chronic inflammation and tumor growth.Citation109,Citation110 Many cell types present in the tumor microenvironment including macrophages, immune cells, and mesenchymal cells have been reported to secrete both IL-6 and IL-8.Citation110 In addition, the high levels of both of these cytokines in serum have been related to poor BC patient outcome.Citation111,Citation112 IL-6 has been shown to promote angiogenesis, tumorigenicity, and metastasis.Citation113 In clinics, correlation of high IL-6 serum levels and poor outcome in BC patients justifies the studies aimed to elucidate the role that these cytokines play in tumorigenesis. A report demonstrated that IL-6 is directly involved in BCSC self-renewal, that was mediated by the IL-6 receptor/GP130 complex via STAT3 activation.Citation114 IL-6 has been shown to be a vital element of positive feedback loop that regulates these MSCs and BCSCs.Citation97 Utilizing relative gene expression profiling, it was identified that CXCR1 (IL-8 receptor) was overexpressed on BCSCs and also IL-8 was able to induce the self-renewal of the BCSCs.Citation115 Further, blocking the receptor activity in mouse xenografts significantly reduced the population of BCSCs, resulting in decreased tumorigenicity and metastasis. The production of inflammatory cytokines such as IL-6 and IL-8 is controlled by the NF-κB signaling pathway.Citation116
Hepatocyte growth factor (HGF), released by mammary stromal cells, might also play an important role in developing mammary tumors.Citation117 HGF serves as a co-stimulatory signal to activate the Wnt pathway during colon carcinogenesis;Citation118 however, involvement of similar pathways in breast carcinogenesis is still unknown. Another vital growth factor released by activated fibroblasts includes fibroblast growth factors (FGFs). It was recently reported that estrogen regulates the BCSC population via paracrine signaling cascade involving FGF9.Citation119 Additional factors such as PDGF, IGF, Wnt, Hedgehog ligands, Notch ligands, and matrix metalloproteinases (MMPs) are released in the tumor microenvironment that controls tumor proliferation, invasion, and metastasis.Citation120–Citation125
Endothelial cells are involved in blood vessel formation and might play an important role in developing the tumor microenvironment via direct interaction with tumor cells. Endothelial cells have been reported to be a significant constituent of normal neuronal and hematopoietic stem cell niches.Citation126,Citation127 It has been observed that cytokines produced by endothelial cells regulate CSCs.Citation128,Citation129 Remarkably, the tumor vasculature is substantially different from the normal vasculature, as exemplified by the differential expression of almost 1,000 genes between them, including JAK3, MMPs, and FGF receptors.Citation128 Even though several pro-angiogenic factors have been recognized, VEGF is the principal facilitator of this process,Citation130 and because of this, it has become the primary target of many anti-angiogenic therapeutics. Bevacizumab and two small molecule multi-kinase VEGF inhibitors, sunitinib and sorafenib, are currently approved for clinical application. Bevacizumab was approved against metastatic BC as it can prolong the time taken to tumor progression.Citation131 However, more recent studies have suggested discouraging the result that the effect is severely limited and that the combination of bevacizumab and cytotoxic chemotherapy failed to increase the OS of patient.Citation132 These results are corroborated with reports in mouse models that application of anti-angiogenic agents might accelerate BC invasion and metastasis.Citation133,Citation134 By studying mouse model of human BC, a report also suggests that these anti-angiogenic agents increase the CSC pool through tissue hypoxia.Citation135 Anti-angiogenesis drugs might also augment tumor growth by stimulating HGF production from tumor-associated stromal cells.Citation136
New approaches to develop therapeutics against BCSCs
As stated above resistance to conventional therapeutic regime such as radiation and cytotoxic chemotherapy has been the motivation behind the development of specific agents capable of targeting the CSC population. BCSCs show enhanced expression of CD44 and ABC transporters promoting survival of these stem cells. These surviving cells again give rise to tumors that have enhanced chemo-tolerance and metastatic ability resulting in relapse. Here we would discuss some of the current approaches used for targeting BCSC. The promising therapies employed against BCSCs have been summarized in .
Table 1 Upcoming therapeutic modalities against breast cancer stem cells
Targeting signaling cascades
There are significant reports of dysregulation of Notch pathway in a substantial fraction of human BCs.Citation137,Citation138 Of the various approaches, one of the most clinically promising candidates is γ-secretase inhibitor. Activation of Notch signaling is regulated by this proteolytic enzyme (γ-secretase), which cleaves Notch receptors and releases the intracellular domain, which in turn acts as a transcription factor and regulates important oncogenic gene functions.Citation139 Therefore, γ-secretase inhibitors were designed for treating BC patients in an early-phase clinical trial. The most severe effect observed has been the gastrointestinal toxicity due to goblet cell hyperplasia, which is an on-target effect of Notch inhibition.Citation140 Although, a moderate dosage along with the administration of high dose of corticosteroids was able to lower the toxicity.Citation141 Combination of γ-secretase inhibitor with taxane chemotherapy has also been clinically investigated in a Phase I trial.Citation141 Other pathway regulating BCSC is the Hedgehog pathway. This pathway has been reported to be active in tumor cells as well as in the tumor stroma.Citation142 Oral Hedgehog inhibitors were clinically tested, and they appear to be fairly nontoxic.Citation143 Phase II clinical studies employing this drug compounds in combination with conventional cytotoxic agents are underway.
Despite the outstanding clinical efficiency of HER2-targeted therapy, almost one third of HER2-positive BC patients do not respond to these agents, and chemoresistance may develop in these patients with chronic exposure. Increasing evidence indicates that resistance may be associated with the activation of other receptor kinases, gain of function mutations of PI3K, loss of PTEN tumor suppressor gene, or truncation of the extracellular domain of HER2.Citation144 These mutations cause aberrant activation of the downstream PI3K/Akt/mTOR pathway and are generally correlated with poor prognosis after conventional trastuzumab therapy.Citation144 Confirmatory evidence has recently shown that the PI3K/Akt/mTOR pathway plays a significant role in regulating BCSC pool. This ensues via Akt activation of the Wnt pathway through phosphorylation of GSK/3β and direct phosphorylation of β-catenin on serine552 amino acid which results in its nuclear transport.Citation145 This observation suggests that suppressing Akt that is downstream of HER2 signaling might efficiently target BCSCs in HER2-resistant tumors. Indeed, perifosine (Akt inhibitor) has demonstrated promising prospect by effectively targeting the BCSC pool in breast tumor xenografts.Citation145 Encouraged by the abovementioned observations, a spectrum of PI3K and Akt selective inhibitors are being clinically investigated, providing us the direct assessment of the effects of these agents in controlling the stem cell population.
Targeting tumor microenvironment
The role of cytokine signaling in maintaining and promoting CSCs is well-documented. Among the cytokines, IL-6 and IL-8 play an important role in the maintenance of BCSC population. These two cytokines promote the inflammatory cascade via NF-κB pathway leading to chronic inflammation and augmentation of tumorigenesis. Interestingly, anti-inflammatory agents such as statins show a decrease in BC risk.Citation146 Statins lower the levels of pro-inflammatory cytokines, as revealed by lowered CRP levels.Citation147 A recent report has demonstrated that antibodies against the CXCR1 (IL-8 receptor) or repertaxin (small molecule CXCR1/CXCR2 inhibitor) has the potential to target BCSC in mouse xenograft models impeding tumor growth and metastasis.Citation145 Repertaxin was initially developed to avert graft rejection and has shown to have promising effect in Phase I trials. Repertaxin was reported to mediate stem cell death in bulk cellular population through bystander effect involving release of Fas cell surface death receptor (FAS) ligand. CXCR1 inhibits FOXO3A localization and FAS ligand expression through AKT signaling. Treatment with repertaxin suppressed AKT, resulting in nuclear FOXO3A and FAS ligand expression. Conventional chemotherapeutics are also known to cause cell death via a bystander effect through FAS ligand, but that in turn induces IL-8 which protects BCSC from FAS ligand. This suggested that repertaxin might cause blockade of this effect and efficiently kill the BCSC population. Repertaxin also suppressed the BCSC population in vitro as well as in tumor xenografts. As a monotherapy, repertaxin had a nonsignificant effect on tumor growth, but drastically reduced tumor volume when employed in combination with docetaxel. Further, repertaxin was able to reduce metastatic lesions and secondary tumor formation. These promising results show that repertaxin can sensitize BCSC to bystander effect via FAS ligand and that CXCR1 blocking might represent a novel approach to targeting and eliminating breast CSCs. Further, mAbs targeting IL-6 or its receptor are currently being assessed in clinical trials for multiple myeloma.Citation148
The downregulation of caveolin-1 (CAV1) in CAFs is a well-studied biomarker which is associated with oncogenic transformation. It has been observed that inhibition of CAV1 in CAFs resulted in hyper-proliferative phenotype of BC cells. The replacement of CAV1 with CAV1 mimetic eliminated the proliferative behavior of the cells.Citation149 It was also reported that CAFs and MSCs sensitized MCF7 cells to the RAD001 (an mTOR inhibitor) and augmented the cytotoxic effect of RAF265 (an RAF inhibitor) on MDA-MB-231 cells through the inhibition of ERK1/2 phosphorylation.Citation150 However, both CAFs and MSCs had no significant effect on the response to TKI258 (a PDGFR/FGFR/VEGFR inhibitor) in BC cell lines.Citation150 This demonstrates that CAFs may not be involved in all the mechanisms of drug resistance, but heterogeneity of CAFs should be taken into account during drug response.
Tumor and adjoining stromal cells are known to secrete CCL2, which is an essential chemoattractant for macrophages. CCL2 and its receptor, CCR2, are involved in monocyte recruitment onto the tumor periphery. Lu et al observed that overexpression of CCL2 promotes both bone and lung metastases in BC. Targeting the tumor-derived CCL2 via a neutralizing mAb reduced metastasis to bone and lung.Citation151 Adverse side effects of anti-CCL2 therapy have raised serious concern as it has shown to aggravate metastasis via increasing macrophage recruitment within weeks of treatment termination.
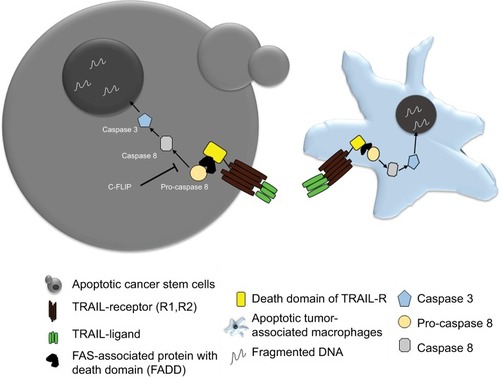
Adipocytes are one of the main components of the breast and have been shown to play a role in tumor development. In line with this observation, a few chemopreventive agents have been tested for their efficacy against BC cells. Sulforaphane, which is a compound present in broccoli, has been extensively studied and shown promising results.Citation152 However, the limited understanding about the role of adipocytes in promoting tumorigenesis is limiting the scope of available options to target these adipocytes. Tumor necrosis factor-related apoptosis inducing ligand (TRAIL) is a well-known death receptor that can mediate ligand (TRAIL-R1, TRAIL-R2)-induced apoptosis, in tumor cells. TRAIL-mediated therapies are in Phase I clinical trials for TNBC.Citation153 It has been reported that TRAIL can be a valuable tool for targeting patients who have limited treatment options.Citation153 However, there are some tumors that show resistance to TRAIL therapy, with CSC being major contributors in therapy resistance. The overexpression of anti-apoptosis proteins like c-FLIP can lead to the resistance of anti-TRAIL therapy. Thus combining the c-FLIP inhibition with anti-TRAIL antibodies can lead to an effective eradiation of CSC and thus overcoming therapy resistance.Citation154
Also, the macrophages and monocytes express a large amount of TRAIL receptors, that is, TRAIL-1R and TRAIL-2R. Thus, recombinant TRAIL therapy can help in selectively inducing apoptosis of the tumor-associated macrophages (TAM), which form a primary signaling arm for tumor microenvironment. Therefore, TRAIL therapy can provide dual benefits all together, where it can selectively eliminate tumor cells and also control the pro-tumor signals coming from TAM present in the microenvironment ().Citation155
Figure 4 Schematic showing TRAIL therapy against CSC and TAM.
Abbreviations: BCSC, breast cancer stem cell; CSC, cancer stem cell; FAS, Fas cell surface death receptor; TAM, tumor-associated macrophage; TRAIL, tumor necrosis factor-related apoptosis inducing ligand.
Targeting CSC metabolism
CSCs show a distinct dependency on glucose and mitochondrial metabolism. It is shown that the multipotent cells rely majorly on glycolysis. The stem cell pool of basal-like BC, which is CD44+/EPCAM+, is dependent on aerobic glycolysis. Overexpression of FBP1, which promotes gluconeogenesis and inhibits glycolysis, reduces the number of spheroids in basal-like BC.Citation156 A well-known regulator for mitochondrial metabolism is BCL-2 protein, which forms a complex with Bcl-2-associated death promoter and glucokinase. Inhibition of BCL-2 activation can lead to the inhibition of oxidative phosphorylation (OXPHOS) leading to the reduction of CSC depending on OXPHOS.Citation157 Increase in mitochondrial activity can promote metastasis and confer resistance to DNA damage in BC.Citation158 A transcription factor peroxisome proliferator-activated receptor gamma, co-activator 1 alpha (PPARGC1A, also known as PGC-1α), is an important target for cancer cell metabolism as it couples with OXPHOS and supports migration and invasion of cells. This factor has been reported to be highly expressed in BCSCs and its inhibition lead to decreased stemness.Citation159 Fatty acid oxidation is another major arm of supporting tumor cell growth and proliferation. It has been reported that various stem cell pools rely on fatty acid oxidation.Citation160 NANOG is known to repress OXPHOS and activate fatty acid oxidation. Thus using etomoxir, a carnitine palmitoyltransferase-1 inhibitor, could reduce the spheroid formation ability of BC in vitro and also reduced in vivo tumorigenic potential.Citation161 A mitochondria inhibitor VLX600 has been reported to target the quiescent cell pool within the tumor, in vivo.Citation162 Salinomycin, which is an antibiotic extracted from Streptomyces albus has been shown to reduce the stemness by targeting the Wnt pathway, which is crucial for main taining stem cell proliferation.Citation163 Wnt signaling is a known regulator of cell metabolism, where a study has shown that using a therapeutic approach of administering Wnt antagonist frizzled-related protein 4 caused metabolic reprogramming, which led to apoptosis of CSC under variable glucose conditions.Citation164 Recent reports suggest that targeting iron metabolism can be fruitful in targeting CSC since altered iron metabolism can cause increase in ROS and oxidative stress.Citation165
Nano-therapeutics against CSC
Nanoparticle (NP)-mediated therapy is an effective strategy of drug delivery for cancer therapeutics. NPs are also being employed for targeting stem cell subpopulations within tumor bulk, where CSC marker-targeted NPs offer an advantage of specificity and precision. Thus using biocompatible polymers like liposome, PLGA, and so on, which are coated with antibodies/aptamers against BCSC-specific markers, can help in specific delivery of chemotherapeutic drug, RNAi, or antibodies to the stem cell population. BCSCs generally show enhanced expression of CD44, and studies have shown that paclitaxel- and salinomycin-loaded liposomal NP coated with CD44 antibody can target the CD44+ CSC population of MDA-MB-231 cells.Citation166 Iron oxide magnetic NPs coated with CD44 antibody and loaded with gemcitabine have been used for targeting the stem cell population in BC.Citation167 These particles have been shown to have an added advantage of hyperthermia. NPs containing a combination of chemotherapeutic agents along with autophagy inhibitor chloroquine (CQ) are an upcoming line of therapy which can target the tumor bulk as well as CSC pool within a tumor.Citation168 Doxorubicin and CQ NP have shown to reduce the ALDH high population of MDA-MB-231 cells.
Administration of decitabine, a DNA hypermethylation inhibitor encapsulated in NP made with polyethylene glycol, could sensitize the tumor bulk and CSC population to chemotherapy. Also, when these NPs were combined with doxorubicin, they could reduce the ALDH+ population in mammospheres of MDA-MB-231 cells.Citation169 Anticancer drugs are frequently being incorporated into liposomes, for efficient drug delivery. An anticancer compound ESC8 was used along with dexamethasone (Dex)-associated liposome (DX), to form ESC8-entrapped liposome named DXE, showed promising results in reducing the drug-resistant cell population.Citation170 Drug targets against Notch, TGF-β, and Wnt/β-catenin pathways are also being used in combination with NPs.
NPs are also used to deliver siRNA to tumor. A cationic lipid-based polymer was developed along with an siRNA and TGF-βR-I receptor inhibitor LY364947.Citation171 Similarly, a cationic liposomal delivery of miR-34a could reduce the expression of CSC markers ALDH and CD44, thereby delaying tumor growth.Citation172 An interesting observation made by a group showed that graphene oxide (carbon nanomaterial) itself has the potential to induce differentiation of stem cells and reducing their in vitro sphere forming ability, thereby it can be a good source to target tumor bulk as well as the CSC population.Citation173 Carbon nanotubes are capable of mediating a thermal effect, and they have been studied in BC cells where the BCSCs were found to be sensitive to these carbon nanotube thermal therapy.Citation174 Further, conjugation of these carbon-based nanomaterials with stem cell receptor targeting can help achieve specificity. Also, novel methods of gene delivery using NPs are being effectively used to reduce tumor burden. An interesting study showed that specific gene delivery targeting the glucocorticoid receptor using a cationic liposome has the potential to reduce tumor growth in vivo.Citation175
Conclusion
BC is a complex and heterogeneous disease, a culmination of a variety of cells that exert influence on one another, thereby making the disease management complex. Cellular/clonal heterogeneity within tumors and disease relapse are the major threats from the clinical point of view. We have now started to understand the quiescent, self-renewable pool of cells within a tumor population, the so-called BCSCs, which can govern the therapy resistance, metastasis, and disease relapse. Also the stem cells have unique mechanisms to withstand drug/radiation insults, for example, presence of large number of drug efflux pumps, and enhanced DNA repair machinery and thus posing big in terms of future developments of cancer therapeutics.
Tumor microenvironment is another key domain helping in maintenance of CSCs. Various elements like cytokine flux, tumor-associated immune cells, stromal cells, and CAFs impact chemokine receptor signaling, cytoskeletal rearrangements, hypoxia, angiogenesis, as well as cell metabolism. Altered cancer cell metabolism is a consequence of cancer condition where the BCSCs depend particularly on glycolysis. Mitochondrial OXPHOS is an alternative backup for stem cell survival. A large number of preclinical and clinical studies are being conducted on today’s date, targeting eradication of stem cell pool at the tumor site. Studies have also begun to explain the concept of CSC plasticity, where the non-CSCs can revert back to CSCs, and therefore, greater attention is needed as it will be indispensable for CSC management. The advancement in nanotherapeutics and nanomedicine is also greatly changing the face of treatment options by providing novel approaches of combining multimode treatment options. Together, all these dimensions added to BC research is surely going to give us an edge in reducing the impact of therapy resistance and improve disease outcomes in future days.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2017CA Cancer J Clin201767173028055103
- CurtisCShahSPChinS-FThe genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroupsNature2012486740334635222522925
- Centers for Disease Control and PreventionCancers diagnosed at late-stages despite available screening tests [Press release] Available from: https://www.cdc.gov/media/pressrel/2010/r101124.htmlAccessed February 18, 2019
- HaqueRAhmedSAInzhakovaGImpact of breast cancer subtypes and treatment on survival: an analysis spanning two decadesCancer Epidemiol Biomarkers Prev201221101848185522989461
- ReinertTBarriosCHOptimal management of hormone receptor positive metastatic breast cancer in 2016Ther Adv Med Oncol20157630432026557899
- MehtaRSBarlowWEAlbainKSCombination anastrozole and fulvestrant in metastatic breast cancerN Engl J Med2012367543544422853014
- WuerstleinRHarbeckNNeoadjuvant therapy for HER2-positive breast cancerRev Recent Clin Trials2017122819228164759
- LehmannBDJovanovićBChenXRefinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selectionPLoS One2016116e015736827310713
- BerradaNDelalogeSAndréFTreatment of triple-negative metastatic breast cancer: toward individualized targeted treatments or chemosensitization?Ann Oncol201021Suppl 7vii30vii3520943632
- MillerTWHennessyBTGonzález-AnguloAMHyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancerJ Clin Invest201012072406241320530877
- MillerTWRexerBNGarrettJTArteagaCLMutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancerBreast Cancer Res201113622422114931
- BlackwellKBurrisHGomezPPhase I/II dose-escalation study of PI3K inhibitors pilaralisib or voxtalisib in combination with letrozole in patients with hormone-receptor-positive and HER2-negative metastatic breast cancer refractory to a non-steroidal aromatase inhibitorBreast Cancer Res Treat2015154228729726497877
- di LeoASeok LeeKCiruelosEAbstract S4-07: BELLE-3: a phase III study of buparlisib + fulvestrant in postmenopausal women with HR+, HER2–, aromatase inhibitor-treated, locally advanced or metastatic breast cancer, who progressed on or after mTOR inhibitor-based treatmentCancer Res2017774 SupplementS407
- BaselgaJCortésJDelaurentiisMSANDPIPER: Phase III study of the PI3-kinase (PI3K) inhibitor taselisib (GDC-0032) plus fulvestrant in patients (pts) with estrogen receptor (ER)-positive, HER2-negative locally advanced or metastatic breast cancer (BC) enriched for pts with PIK3CA- mutant tumorsJ Clin Oncol20173515_supplTPS1119
- SauraCde AzambujaEHlauschekDLBA10_PRPrimary results of LORELEI: a phase II randomized, double-blind study of neoadjuvant letrozole (let) plus taselisib versus let plus placebo (Pla) in postmenopausal patients (PTS) with ER+/HER2-negative early breast cancer (EBC)Ann Oncol201728suppl_5mdx440.001
- SchmidPPinderSEWheatleyDPhase II randomized preoperative Window-of-Opportunity study of the PI3K inhibitor Pictilisib plus anastrozole compared with anastrozole alone in patients with estrogen receptor-positive breast cancerJ Clin Oncol201634171987199426976426
- BaselgaJCamponeMPiccartMEverolimus in postmenopausal hormone-receptor-positive advanced breast cancerN Engl J Med2012366652052922149876
- FlemingGFMaCXHuoDPhase II trial of temsirolimus in patients with metastatic breast cancerBreast Cancer Res Treat2012136235536322245973
- XuHYuSLiuQRecent advances of highly selective CDK4/6 inhibitors in breast cancerJ Hematol Oncol20171019728438180
- ShahANCristofanilliMThe growing role of Cdk4/6 inhibitors in treating hormone receptor-positive advanced breast cancerCurr Treat Options Oncol2017181628197838
- Barroso-SousaRShapiroGITolaneySMClinical development of the CDK4/6 inhibitors Ribociclib and Abemaciclib in breast cancerBreast Care201611316717327493615
- SledgeGWToiMNevenPMONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapyJ Clin Oncol201735252875288428580882
- AcharyaMRSparreboomAVenitzJFiggWDRational development of histone deacetylase inhibitors as anticancer agents: a reviewMol Pharmacol200568491793215955865
- StanwaySJDelavaultPPurohitASteroid sulfatase: a new target for the endocrine therapy of breast cancerOncologist200712437037417470679
- PalmieriCSteinRCLiuXIris study: a phase II study of the steroid sulfatase inhibitor irosustat when added to an aromatase inhibitor in ER-positive breast cancer patientsBreast Cancer Res Treat2017165234335328612226
- RasmussenLMZaveriNTStenvangJPetersRHLykkesfeldtAEA novel dual-target steroid sulfatase inhibitor and antiestrogen: SR 16157, a promising agent for the therapy of breast cancerBreast Cancer Res Treat2007106219120317268816
- GuerinMRezaiKIsambertNPIKHER2: a phase Ib study evaluating buparlisib in combination with lapatinib in trastuzumab-resistant HER2-positive advanced breast cancerEur J Cancer201786283628950146
- SauraCBendellJJerusalemGPhase Ib study of Buparlisib plus trastuzumab in patients with HER2-positive advanced or metastatic breast cancer that has progressed on trastuzumab-based therapyClin Cancer Res20142071935194524470511
- TolaneySBurrisHGartnerEPhase I/II study of pilaralisib (SAR245408) in combination with trastuzumab or trastuzumab plus paclitaxel in trastuzumab-refractory HER2-positive metastatic breast cancerBreast Cancer Res Treat2015149115116125537644
- HudisCSwantonCJanjigianYYA phase 1 study evaluating the combination of an allosteric Akt inhibitor (MK-2206) and trastuzumab in patients with HER2-positive solid tumorsBreast Cancer Res2013156R11024252402
- HurvitzSAAndreFJiangZCombination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): a phase 3, randomised, double-blind, multicentre trialLancet Oncol201516781682926092818
- Acevedo-GadeaCHatzisCChungGSirolimus and trastuzumab combination therapy for HER2-positive metastatic breast cancer after progression on prior trastuzumab therapyBreast Cancer Res Treat2015150115716725687356
- SeilerMRay-CoquardIMelicharBOral ridaforolimus plus trastuzumab for patients with HER2+ trastuzumab-refractory metastatic breast cancerClin Breast Cancer2015151606525239224
- NahtaREstevaFJHER2 therapy: molecular mechanisms of trastuzumab resistanceBreast Cancer Res20068621517096862
- HettmanTSchneiderMBlumSAbstract B161: U3-1287 (AMG 888), a fully human anti-HER3 mAb, demonstrates preclinical efficacy in HER2+ and HER2− breast cancer modelsMol Cancer Ther20098Supplement 1B161
- ChanADelalogeSHolmesFANeratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trialLancet Oncol201617336737726874901
- MukaiHSaekiTAogiKPatritumab plus trastuzumab and paclitaxel in human epidermal growth factor receptor 2-overexpressing metastatic breast cancerCancer Sci2016107101465147027452985
- BangYJGiacconeGImSAFirst-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumorsAnn Oncol20172885586128119295
- DiérasVBachelotTThe success story of trastuzumab emtansine, a targeted therapy in HER2-positive breast cancerTarget Oncol20149211112223852665
- LianosGDVlachosKZorasORoukosDHPotential of antibody-drug conjugates and novel therapeutics in breast cancer managementOnco Targets Ther2014749124711706
- MittendorfEACliftonGTHolmesJPFinal report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patientsAnn Oncol20142591735174224907636
- LimentaniSACamponeMDorvalTA non-randomized dose-escalation phase I trial of a protein-based immunotherapeutic for the treatment of breast cancer patients with HER2-overexpressing tumorsBreast Cancer Res Treat2016156231933026993131
- HamiltonEBlackwellKHobeikaACPhase 1 clinical trial of HER2-specific immunotherapy with concomitant HER2 kinase inhibition [corrected]J Transl Med2012102822325452
- LinderholmBKHellborgHJohanssonUSignificantly higher levels of vascular endothelial growth factor (VEGF) and shorter survival times for patients with primary operable triple-negative breast cancerAnn Oncol200920101639164619549711
- RobertNJDiérasVGlaspyJRIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancerJ Clin Oncol201129101252126021383283
- CastrellonABPidhoreckyIValeroVRaezLEThe role of carboplatin in the neoadjuvant chemotherapy treatment of triple negative breast cancerOncol Rev2017111
- RobsonMImSASenkusEOlaparib for Metastatic Breast Cancer in Patients with a Germline BRCA MutationN Engl J Med2017377652353328578601
- BryantHESchultzNThomasHDSpecific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymeraseNature2005434703591391715829966
- BrownJSKayeSBYapTAPARP inhibitors: the race is onBr J Cancer2016114771371527022824
- TurnerNCTelliMLRugoHSFinal results of a phase 2 study of talazoparib (TALA) following platinum or multiple cytotoxic regimens in advanced breast cancer patients (pts) with germline BRCA1/2 mutations (ABRAZO)J Clin Oncol20173515_suppl1007
- TimmsKMAbkevichVHughesEAssociation of BRCA1/2 defects with genomic scores predictive of DNA damage repair deficiency among breast cancer subtypesBreast Cancer Res201416647525475740
- CareyLARugoHSMarcomPKTBCRC 001: randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancerJ Clin Oncol201230212615262322665533
- BaselgaJGómezPGreilRRandomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancerJ Clin Oncol201331202586259223733761
- TomaoFPapaAZaccarelliETriple-negative breast cancer: new perspectives for targeted therapiesOnco Targets Ther2015817725653541
- FinnRSBengalaCIbrahimNDasatinib as a single agent in triple-negative breast cancer: results of an open-label phase 2 studyClin Cancer Res201117216905691322028489
- KimEMMuellerKGartnerEBoernerJDasatinib is synergistic with cetuximab and cisplatin in triple-negative breast cancer cellsJ Surg Res2013185123123923899511
- RoseAANBiondiniMCurielRSiegelPMTargeting GPNMB with glembatumumab vedotin: current developments and future opportunities for the treatment of cancerPharmacol Ther201717912714128546082
- RoseAAGrossetAADongZGlycoprotein nonmetastatic B is an independent prognostic indicator of recurrence and a novel therapeutic target in breast cancerClin Cancer Res20101672147215620215530
- YardleyDAWeaverRMeliskoMEEmerge: a randomized phase II study of the antibody-drug conjugate Glembatumumab Vedotin in advanced glycoprotein NMB-Expressing breast cancerJ Clin Oncol201533141609161925847941
- HanahanDWeinbergRAHallmarks of cancer: the next generationCell2011144564667421376230
- NowellPCThe clonal evolution of tumor cell populationsScience197619442602328959840
- KresoADickJEEvolution of the cancer stem cell modelCell Stem Cell201414327529124607403
- Al-HajjMWichaMSBenito-HernandezAMorrisonSJClarkeMFProspective identification of tumorigenic breast cancer cellsProc Natl Acad Sci USA200310073983398812629218
- ChekhunSVZadvornyTVTymovskaYOCD44+/CD24- markers of cancer stem cells in patients with breast cancer of different molecular subtypesExp Oncol2015371586325804234
- MuñozPIliouMSEstellerMEpigenetic alterations involved in cancer stem cell reprogrammingMol Oncol20126662063623141800
- LingLNurcombeVCoolSMWnt signaling controls the fate of mesenchymal stem cellsGene20094331–21719135507
- LiuSDontuGMantleIDHedgehog signaling and bmi-1 regulate self-renewal of normal and malignant human mammary stem cellsCancer Res200666126063607116778178
- RizzoPOsipoCForemanKRational targeting of Notch signaling in cancerOncogene200827385124513118758481
- FarnieGClarkeRBMammary stem cells and breast cancer--role of Notch signallingStem Cell Rev20073216917517873349
- YamanakaSInduction of pluripotent stem cells from mouse fibroblasts by four transcription factorsCell Prolif200841Suppl 1515618181945
- Rodriguez-PinillaSMSarrioDMoreno-BuenoGSox2: a possible driver of the basal-like phenotype in sporadic breast cancerMod Pathol200720447448117334350
- ZhangJLiangQLeiYSOX4 induces epithelial-mesenchymal transition and contributes to breast cancer progressionCancer Res201272174597460822787120
- SaitohMInvolvement of partial EMT in cancer progressionJ Biochem2018164425726429726955
- O’BrienCAPollettAGallingerSDickJEA human colon cancer cell capable of initiating tumour growth in immunodeficient miceNature2007445712310611017122772
- ChuthapisithSEreminJEl-SheemeyMEreminOBreast cancer chemoresistance: emerging importance of cancer stem cellsSurg Oncol2010191273219251410
- RebucciMMichielsCMolecular aspects of cancer cell resistance to chemotherapyBiochem Pharmacol20138591219122623435357
- Hirschmann-JaxCFosterAEWulfGGA distinct “side population” of cells with high drug efflux capacity in human tumor cellsProc Natl Acad Sci USA200410139142281423315381773
- HuCLiHLiJAnalysis of ABCG2 expression and side population identifies intrinsic drug efflux in the HCC cell line MHCC-97L and its modulation by Akt signalingCarcinogenesis200829122289229718820285
- GinestierCHurMHCharafe-JauffretEALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcomeCell Stem Cell20071555556718371393
- CrokerAKAllanALInhibition of aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and radiation resistance of stem-like ALDHhiCD44+ human breast cancer cellsBreast Cancer Res Treat20121331758721818590
- EconomopoulouPKaklamaniVGSiziopikouKThe role of cancer stem cells in breast cancer initiation and progression: potential cancer stem cell-directed therapiesOncologist201217111394140122941971
- Charafe-JauffretEGinestierCIovinoFAldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancerClin Cancer Res2010161455520028757
- CleversHThe cancer stem cell: premises, promises and challengesNat Med201117331331921386835
- Maugeri-SaccàMVigneriPde MariaRCancer stem cells and chemosensitivityClin Cancer Res201117154942494721622723
- EylerCERichJNSurvival of the fittest: cancer stem cells in therapeutic resistance and angiogenesisJ Clin Oncol200826172839284518539962
- LiHLiuLGuoLHERG K+ channel expression in CD34+/CD38-/CD123(high) cells and primary leukemia cells and analysis of its regulation in leukemia cellsInt J Hematol200887438739218415658
- LapidotTSirardCVormoorJA cell initiating human acute myeloid leukaemia after transplantation into SCID miceNature199436764646456487509044
- BonnetDDickJEHuman acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cellNat Med1997377307379212098
- CollinsATBerryPAHydeCStowerMJMaitlandNJProspective identification of tumorigenic prostate cancer stem cellsCancer Res20056523109461095116322242
- BapatSAMaliAMKoppikarCBKurreyNKStem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancerCancer Res20056583025302915833827
- SinghSKHawkinsCClarkeIDIdentification of human brain tumour initiating cellsNature2004432701539640115549107
- CreightonCJLiXLandisMResidual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating featuresProc Natl Acad Sci USA200910633138201382519666588
- ShibueTWeinbergRAEMT, CSCs, and drug resistance: the mechanistic link and clinical implicationsNat Rev Clin Oncol2017141061162928397828
- DesaiSBaraiABukhariABDeASenSα-Actinin-4 confers radio-resistance coupled invasiveness in breast cancer cells through AKT pathwayBiochim Biophys Acta Mol Cell Res20181865119620829055790
- GuptaSIljinKSaraHFZD4 as a mediator of ERG oncogene-induced Wnt signaling and epithelial-to-mesenchymal transition in human prostate cancer cellsCancer Res201070176735674520713528
- KarnoubAEDashABVoAPMesenchymal stem cells within tumour stroma promote breast cancer metastasisNature2007449716255756317914389
- LiuSGinestierCOuSJBreast cancer stem cells are regulated by mesenchymal stem cells through cytokine networksCancer Res201171261462421224357
- LimPKBlissSAPatelSAGap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cellsCancer Res20117151550156021343399
- PittengerMFMackayAMBeckSCMultilineage potential of adult human mesenchymal stem cellsScience1999284541114314710102814
- GabbianiGMajnoGDupuytren’s contracture: fibroblast contraction? An ultrastructural studyAm J Pathol19726611311465009249
- StueltenCHBarbulABuschJIAcute wounds accelerate tumorigenesis by a T cell-dependent mechanismCancer Res200868187278728218794114
- HuMYaoJCaiLDistinct epigenetic changes in the stromal cells of breast cancersNat Genet200537889990516007089
- FarmerPBonnefoiHAnderlePA stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancerNat Med2009151687419122658
- FinakGBertosNPepinFStromal gene expression predicts clinical outcome in breast cancerNat Med200814551852718438415
- BierieBMosesHLTumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancerNat Rev Cancer20066750652016794634
- OrimoAGuptaPBSgroiDCStromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretionCell2005121333534815882617
- BurgerJAKippsTJCXCR4: a key receptor in the crosstalk between tumor cells and their microenvironmentBlood200610751761176716269611
- ChuQDPanuLHolmNTHigh chemokine receptor CXCR4 level in triple negative breast cancer specimens predicts poor clinical outcomeJ Surg Res2010159268969519500800
- SchellerJRose-JohnSInterleukin-6 and its receptor: from bench to bedsideMed Microbiol Immunol2006195417318316741736
- WaughDJWilsonCThe interleukin-8 pathway in cancerClin Cancer Res200814216735674118980965
- ChaoMPAlizadehAATangCTherapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemiaCancer Res20117141374138421177380
- BenoyIHSalgadoRvan DamPIncreased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survivalClin Cancer Res200410217157716215534087
- AraTDeclerckYAInterleukin-6 in bone metastasis and cancer progressionEur J Cancer20104671223123120335016
- IliopoulosDHirschHAStruhlKAn epigenetic switch involving NF-kappaB, Lin28, let-7 microRNA, and IL6 links inflammation to cell transformationCell2009139469370619878981
- GinestierCLiuSDiebelMECXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenograftsJ Clin Invest2010120248549720051626
- BarnesPJKarinMNuclear factor-κB – a pivotal transcription factor in chronic inflammatory diseasesN Engl J Med199733615106610719091804
- MichieliPMazzoneMBasilicoCTargeting the tumor and its microenvironment by a dual-function decoy Met receptorCancer Cell200461617315261142
- VermeulenLde SousaEMeloFvan der HeijdenMWnt activity defines colon cancer stem cells and is regulated by the microenvironmentNat Cell Biol201012546847620418870
- FillmoreCMGuptaPBRudnickJAEstrogen expands breast cancer stem-like cells through paracrine FGF/Tbx3 signalingProc Natl Acad Sci USA201010750217372174221098263
- DufraineJFunahashiYKitajewskiJNotch signaling regulates tumor angiogenesis by diverse mechanismsOncogene200827385132513718758482
- HiscoxSParrCNakamuraTInhibition of HGF/SF-induced breast cancer cell motility and invasion by the HGF/SF variant, NK4Breast Cancer Res Treat200059324525410832594
- LebedisCChenKFallavollitaLBoutrosTBrodtPPeripheral lymph node stromal cells can promote growth and tumorigenicity of breast carcinoma cells through the release of IGF-I and EGFInt J Cancer200210012812115579
- PietrasKRubinKSjöblomTInhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapyCancer Res200262195476548412359756
- SingerCFKronsteinerNMartonEMMP-2 and MMP-9 expression in breast cancer-derived human fibroblasts is differentially regulated by stromal-epithelial interactionsBreast Cancer Res Treat2002721697712000221
- DaleTCWeber-HallSJSmithKCompartment switching of Wnt-2 expression in human breast tumorsCancer Res19965619432043238813115
- SugiyamaTKoharaHNodaMNagasawaTMaintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemo-kine signaling in bone marrow stromal cell nichesImmunity200625697798817174120
- CalabreseCPoppletonHKocakMA perivascular niche for brain tumor stem cellsCancer Cell2007111698217222791
- BhatiRPattersonCLivasyCAMolecular characterization of human breast tumor vascular cellsAm J Pathol200817251381139018403594
- KeithBSimonMCHypoxia-inducible factors, stem cells, and cancerCell2007129346547217482542
- HolashJWiegandSJYancopoulosGDNew model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGFOncogene199918385356536210498889
- MillerKWangMGralowJPaclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancerN Engl J Med Overseas Ed20073572626662676
- PetrelliFBarniSBevacizumab in advanced breast cancer: an opportunity as second-line therapy?Med Oncol20122911421161443
- Pàez-RibesMAllenEHudockJAntiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasisCancer Cell200915322023119249680
- EbosJMLeeCRCruz-MunozWAccelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesisCancer Cell200915323223919249681
- SantosSJKakaralaPHeathAClouthierSWichaMSAbstract 3336: anti-angiogenic agents increase breast cancer stem cells via generation of tumor hypoxiaCancer Res2011718 Supplement3336
- ShojaeiFLeeJHSimmonsBHHGF/c-Met acts as an alternative angiogenic pathway in sunitinib-resistant tumorsCancer Res20107024100901010020952508
- SorianoJVUyttendaeleHKitajewskiJMontesanoRExpression of an activated Notch4(int-3) oncoprotein disrupts morphogenesis and induces an invasive phenotype in mammary epithelial cells in vitroInt J Cancer200086565265910797286
- UyttendaeleHSorianoJVMontesanoRKitajewskiJNotch4 and Wnt-1 proteins function to regulate branching morphogenesis of mammary epithelial cells in an opposing fashionDev Biol199819622042179576833
- Coleman-VaughanCMalADeAMcCarthyJVThe $γ$-Secretase Protease Complexes in Neurodegeneration, Cancer and Immunity. In Chakraborti S, Dhalla NS, editorsPathophysiological Aspects of ProteasesSingaporeSpringer20174787
- MilanoJMckayJDagenaisCModulation of Notch processing by gamma-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiationToxicol Sci200482134135815319485
- RealPJFerrandoAANotch inhibition and glucocorticoid therapy in T-cell acute lymphoblastic leukemiaLeukemia20092381374137719357700
- TianHCallahanCADupreeKJHedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesisProc Natl Acad Sci USA2009106114254425919246386
- von HoffDDLorussoPMRudinCMInhibition of the hedgehog pathway in advanced basal-cell carcinomaN Engl J Med2009361121164117219726763
- NagataYLanKHZhouXPTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patientsCancer Cell20046211712715324695
- KorkayaHPaulsonACharafe-JauffretERegulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signalingPLoS Biol200976e100012119492080
- KochharRKhuranaVBejjankiHCalditoGFortCStatins to reduce breast cancer risk: a case control study in U.S. female veteransJ Clin Oncol20052316_suppl514514
- RidkerPMCannonCPMorrowDC-reactive protein levels and outcomes after statin therapyN Engl J Med20053521202815635109
- KastritisECharidimouAVarkarisADimopoulosMATargeted therapies in multiple myelomaTarg Oncol2009412336
- MercierICasimiroMCWangCHuman breast cancer-associated fibroblasts (CAFs) show caveolin-1 down-regulation and Rb tumor suppressor functional inactivation: implications for the response to hormonal therapyCancer Biol Ther2008781212122518458534
- DittmerAFuchsAOerleckeIMesenchymal stem cells and carcinoma-associated fibroblasts sensitize breast cancer cells in 3D cultures to kinase inhibitorsInt J Oncol201139368969621667024
- LuXKangYChemokine (C-C motif) ligand 2 engages CCR2+ stromal cells of monocytic origin to promote breast cancer metastasis to lung and boneJ Biol Chem200928442290872909619720836
- LiQXiaJYaoYSulforaphane inhibits mammary adipogenesis by targeting adipose mesenchymal stem cellsBreast Cancer Res Treat2013141231732424002734
- RahmanMPumphreyJGLipkowitzSThe TRAIL to targeted therapy of breast cancerAdv Cancer Res2009103437319854352
- PiggottLOmidvarNPérezSMEberlMClarksonRWESuppression of apoptosis inhibitor c-FLIP selectively eliminates breast cancer stem cell activity in response to the anti-cancer agent, TRAILBreast Cancer Res2011135R8821914219
- LiguoriMBuracchiCPasqualiniFFunctional TRAIL receptors in monocytes and tumor-associated macrophages: a possible targeting pathway in the tumor microenvironmentOncotarget2016727416624167627191500
- de FrancescoEMSotgiaFLisantiMPCancer stem cells (CSCs): metabolic strategies for their identification and eradicationBiochem J201847591611163429743249
- DeshmukhADeshpandeKArfusoFNewsholmePDharmarajanACancer stem cell metabolism: a potential target for cancer therapyMol Cancer20161516927825361
- SnyderVReed-NewmanTCArnoldLThomasSMAnantSCancer stem cell metabolism and potential therapeutic targetsFront Oncol2018820329922594
- SanchoPBurgos-RamosETaveraAMYC/PGC-1α balance determines the metabolic phenotype and plasticity of pancreatic cancer stem cellsCell Metab201522459060526365176
- ManciniRNotoAPisanuMEMetabolic features of cancer stem cells: the emerging role of lipid metabolismOncogene201837182367237829445137
- de FrancescoEMMaggioliniMTanowitzHBSotgiaFLisantiMPTargeting hypoxic cancer stem cells (CSCs) with doxycycline: implications for optimizing anti-angiogenic therapyOncotarget2017834561265614228915578
- ZhangXFryknäsMHernlundEInduction of mitochondrial dysfunction as a strategy for targeting tumour cells in metabolically compromised microenvironmentsNat Commun201451329524548894
- JangamreddyJRGhavamiSGrabarekJSalinomycin induces activation of autophagy, mitophagy and affects mitochondrial polarity: differences between primary and cancer cellsBiochimica et Biophysica Acta (BBA) - Molecular Cell Research2013183392057206923639289
- DeshmukhAArfusoFNewsholmePDharmarajanARegulation of cancer stem cell metabolism by secreted frizzled-related protein 4 (sFRP4)Cancers201810240
- El HoutMdos SantosLHamaïAMehrpourMA promising new approach to cancer therapy: targeting iron metabolism in cancer stem cellsSemin Cancer Biol20185312513830071257
- WuDSiMXueHYWongHLNanomedicine applications in the treatment of breast cancer: current state of the artInt J Nanomedicine2017125879589228860754
- HongISJangGBLeeHYNamJSTargeting cancer stem cells by using the nanoparticlesInt J Nanomedicine201510Spec Iss25126026425092
- SunRShenSZhangYJNanoparticle-facilitated autophagy inhibition promotes the efficacy of chemotherapeutics against breast cancer stem cellsBiomaterials2016103445527376558
- LiSYSunRWangHXCombination therapy with epigenetic-targeted and chemotherapeutic drugs delivered by nanoparticles to enhance the chemotherapy response and overcome resistance by breast cancer stem cellsJ Control Release201520571425445694
- AhmadAMondalSKMukhopadhyayDBanerjeeRAlkharfyKMDevelopment of liposomal formulation for delivering anticancer drug to breast cancer Stem-Cell-Like cells and its pharmacokinetics in an animal modelMol Pharm20161331081108826832839
- ZuoZQChenKGYuXYPromoting tumor penetration of nanoparticles for cancer stem cell therapy by TGF-β signaling pathway inhibitionBiomaterials201682485926751819
- PramanikDCampbellNRKarikariCRestitution of tumor suppressor microRNAs using a systemic nanovector inhibits pancreatic cancer growth in miceMol Cancer Ther20111081470148021622730
- FiorilloMVerreAFIliutMGraphene oxide selectively targets cancer stem cells, across multiple tumor types: implications for non-toxic cancer treatment, via “differentiation-based nano-therapy”Oncotarget2015663553356225708684
- BurkeARSinghRNCarrollDLThe resistance of breast cancer stem cells to conventional hyperthermia and their sensitivity to nanoparticle-mediated photothermal therapyBiomaterials201233102961297022245557
- MukherjeeANarayanKPPalKSelective cancer targeting via aberrant behavior of cancer cell-associated glucocorticoid receptorMol Ther200917462363119223869