Abstract
Purpose
Oral tamoxifen, a triphenylethylene (TPE), is useful for breast cancer prevention, but its adverse effects limit acceptance by women. Tamoxifen efficacy is related to its major metabolites 4-hydroxytamoxifen (4-OHT) and N-desmethyl-4-hydroxytamoxifen (endoxifen [ENX]). Transdermal delivery of these to the breast may avert the toxicity of oral tamoxifen while maintaining efficacy. We evaluated the relative effciency of skin permeation of 4-OHT and ENX in vitro, and tested oleic acid (OA) as a permeation-enhancer.
Methods
4-OHT, ENX, and estradiol (E2) (0.2 mg/mL of 0.5 μCi 3H/mg) were dissolved in 60% ethanol-phosphate buffer, ±OA (0.1%–5%). Permeation through EpiDerm™ (Matek Corp, Ashland, MA) and split-thickness human skin was calculated based on the amount of the agents recovered from the receiver fluid and skin using liquid scintillation counting over 24 hours.
Results
In the EpiDerm model, the absorption of 4-OHT and ENX was 10%–11%; total penetration (TP) was 26%–29% at 24 hours and was decreased by OA. In normal human skin, the absorption of 4-OHT and ENX was 0.3%; TP was 2%–4% at 24 hours. The addition of 1% OA improved the permeation of ENX significantly more than that of 4-OHT (P < 0.004); further titration of OA at 0.25%–0.5% further improved the permeation of ENX to a level similar to that of estradiol.
Conclusion
The addition of OA to ENX results in a favorable rapid delivery equivalent to that of estradiol, a widely used transdermal hormone. The transdermal delivery of ENX to the breast should be further developed in preclinical and clinical studies.
Introduction
For more than three decades, oral administration of tamoxifen (TAM), a triphenylethylene (TPE), has been a standard component of the treatment of estrogen receptor-α (ERα)–positive breast cancer,Citation1 and, more recently, has been used for prevention, for both pre- and postmenopausal women.Citation2 However, TAM is a prodrug that requires conversion by the phase I drug metabolizing enzymes cytochrome P450 (CYP2D6 and CYP3A4/5) to its major antiestrogenic metabolites, 4-hydroxytamoxifen (4-OHT), and N-desmethyl-4-hydroxytamoxifen (endoxifen [ENX]), which have equivalent affinity for ERα that is 100× > TAM. Recent reports suggest that ENX is the dominant metabolite responsible for the therapeutic effect of TAM because of its greater abundance (10× higher than 4-OHT in serum) and its ability to cause proteosomic degradation of ERα.Citation3–Citation5
The effectiveness of TAM may be compromised in about 33% of women because of enzyme polymorphisms, which result in decreased availability of ENX. In addition, long-term systemic exposure to TAM is associated with hot flashes, night sweats, and menstrual irregularity, as well as the more serious risks of thromboembolism and endometrial cancer.Citation6 Thus, the systemic delivery of TAM is problematic both from the perspective of efficacy through inefficient metabolism and toxicity through high systemic exposure. However, in women with ductal carcinoma in situ (DCIS) and those at high risk for breast cancer, effective concentrations are required only in breast tissue; systemic exposure is redundant, and side effects related to it may be largely avoided by transdermal delivery of active TAM metabolites through breast skin, as suggested by Mauvais-Jarvis and others.Citation7–Citation9 Promising results have been reported from a presurgical study of postmenopausal women with estrogen receptor (ER)–positive breast cancer. The topical application of 4-OHT gel to the breast skin resulted in inhibition of tumor cell proliferation to the same degree as that seen with the standard dose of oral TAM (20 mg/day) but with much lower plasma levels, 2%–11% of those achieved with oral TAM.Citation9 In the present study, the in vitro skin permeation of 4-OHT and ENX was evaluated, first using a reconstituted human epidermal skin model, and then normal human skin. Oleic acid (OA) was investigated as a permeation enhancer with the objective of increasing uptake of ENX to that of estradiol (E2), a well-established transdermal agent.
Materials and methods
Reagents
TAM, 4-OHT, E2, absolute ethanol, polyoxyethylene 20-oleyl ether (POE[20]), and OA were purchased from Sigma-Aldrich Corp (St Louis, MO), and ENX was obtained from Toronto Research Chemicals Inc (Toronto, Canada). [N-methyl-3H]tamoxifen (3H-tamoxifen) and Z-4-hydroxy[N-methyl-3H]tamoxifen (3H-4-OHT), (72 Ci/mmol), were obtained from Amersham Biosciences (Piscataway, NJ). CYP2D6 was purchased from BD Biosciences (San Jose, CA). The in vitro human-reconstituted epidermis skin model (EpiDerm™ skin model EPI-200) and permeation devices (EPI-100-FIX) were obtained from MatTek Corp (Ashland, MA). Franz diffusion cells (#4G-01-00-07-05) were purchased from PermeGear Inc (Hellertown, PA).
Preparation of 3H-endoxifen
Twenty microcuries of 3H-4-OHT were incubated in 0.5 mL of 70 mM phosphate buffer (PB) containing 10 μM Mg2+, pH 7.4, with 0.5 nmol CYP2D6 for 30 minutes at 23°C. The product was extracted into ethyl ether and was chromatographed on a silica gel thin layer. TAM, 4-OHT, N-desmethyltamoxifen, and ENX in this system had RF values of 0.60, 0.29, 0.25, and 0.06, respectively. Rechromatography in a second solvent system (benzene:methanol, 1:1) gave RF values of 0.51 and 0.25 for 3H-4-OHT and 3H-ENX, respectively. The products were eluted into methanol and stored at 4°C. The yield of 3H-ENX was >50%. The specific activity of the product was 36 Ci/mmol.
Skin preparations
The EpiDerm was incubated for 1 hour at 37°C with 5% CO2 prior to dosing. Each of the EpiDerm batches was used within 3 days of delivery. The acquisition of anonymous human skin samples from the operating room was approved by the Institutional Review Board of Northwestern University. The subcutaneous fat from fresh mastectomy and abdominoplasty specimens was removed and the full-thickness skin was immobilized to obtain split-thickness skin (STS) using a surgical blade (George Tiemann and Co, Hauppauge, NY) or an electric dermatome (Robins instrument Inc, Chatham, NJ). The thickness of STS samples was measured with an electronic digital micrometer (Tresna Instruments Co, Guilin, China) as 367 ± 0.039 microns.
Skin imaging
The EpiDerm and STS samples were cut into 3 mm × 10 mm pieces, placed horizontally in a Cryomold® (Tissue-Tek®; Sakura Finetek USA, Torrance, CA), embedded in a tissue freezing medium (OCT™ Compound; Tissue-Tek), frozen in the microtome/cryostat chamber, sectioned at 7 microns, and stained with Mayer’s hematoxylin and eosin. Images were taken with a 20× objective using a Nikon Eclipse (Tokyo, Japan) optical microscope with a 10 micron bar superimposed on the skin images.
Skin cell viability
Ethanol toxicity to the skin was determined using the 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) toxicology kit (MTT-100, MatTek) by measuring epidermal cell viability of the EpiDerm. A PB (2 mM KH2PO4 4 mM Na2HPO4, pH 7.0) was used as a negative control and 70% (v/v) ethanol-PB was the test material. 0.4 mL of each solution was loaded in the donor chamber of the MatTek permeation device (MPD) (EPI-100-FIX) and 5 mL of Dulbecco’s modified Eagle’s medium (DMEM)-based assay medium (EPI-100-ASY) was added in each receiver well. Skin samples were incubated at 37°C with 5% CO2. After 6 and 24 hours, skin samples were processed by the MTT assay, following the manufacturer’s protocol.
Diffusion studies
Permeation of TPEs using the MPD
TPE solutions (0.2 mg/mL of 0.5 μCi 3H-TPEs/mg) were prepared in the control vehicle, 60% (v/v) ethanol-PB. To evaluate OA effect on permeation of TPEs, the control vehicle was supplemented with 1%–5% (v/v) OA. The receiver chamber contained 5 mL of phosphate-buffered saline (PBS) with stirring at 37°C. Skin samples were placed in the MPD with a skin exposure area of 0.256 cm2. A drug solution of 0.2 mL was loaded into the donor chamber (final dose was 312.6 μg/cm2 for EpiDerm and 156.3 μg/cm2 for human STS). General procedures for the permeation study followed the manufacturer’s protocol. After 6 and 24 hours, receiver fluid was collected the solution remaining in the donor chamber was removed and the exposed skin and donor chamber were washed twice with 0.4 mL of PBS and cleaned with a cotton swab and removed from the permeation device. The amount of 3H-TPEs from the washes of the donor chamber, the donor and receiver fluids, and the skin samples was determined by liquid scintillation counting (Beckman Coulter LS6500, Fullerton, CA). For each receiver fluid 1 mL aliquots in triplicate were placed in 20 mL liquid scintillation glass vials, and 10 mL of Ecolite (+)™ (MP Biomedicals, Solon, OH) liquid scintillation fluid was added. Skin samples were cut into small pieces with surgical scissors and homogenized using an ultrasonic processor (Cole Parmer, Vernon Hills, IL); the 3H-TPEs were extracted three times into methanol; the solvent was evaporated and resuspended in 0.5 mL methanol and 10 mL of Ecolite (+) liquid scintillation fluid. 3H-TPEs from the donor chamber were measured as follows: 3H-TPEs from the cotton swabs were extracted into 1 mL of methanol three times, then this was added together with the remaining donor solution and washes; the solvent was evaporated and the residue was dissolved in 0.5 mL methanol and 10 mL of Ecolite (+) liquid scintillation fluid. Total recovery of 3H-TPEs was greater than 95%.
Permeation of ENX using Franz diffusion cells
ENX and estradiol (E2) (0.2 mg/mL of 0.5 μCi 3H/mg), were prepared in the control vehicle, 60% (v/v) ethanol-PB. OA (0.1%–1%) was added to the control vehicle, to test its permeation-enhancing effect on ENX. Static Franz diffusion cells (skin exposure area of 0.38 cm2 and receiver chamber volume of 5 mL) were used for the permeation experiments. PBS was supplemented with 4% (w/v) POE(20) to overcome the possible artificial dermal retention of lipophilic compounds.Citation10 0.1 mL of drug solution was loaded into the donor chamber (final dose was 78.9 μg/cm2). Samples of 0.25 mL were collected at the predetermined intervals over 24 hours. After 24 hours of sampling, the exposed skin area was washed as described, and the epidermis was separated from the dermis by forceps. All other procedures were the same as described previously.
Data and statistical analysis
Permeation parameters of TPEs using the MPD were expressed as the mean and standard error of the mean (SEM) of the percent of the applied dose from replicate experiments. Absorption is defined as the amount (μg/cm2) of TPEs reaching the receiver fluid. The total penetration (TP) was defined as the sum of the absorption and skin contents of TPEs at the predetermined time points. For Franz diffusion cell experiments, the permeation profiles of ENX and E2 were analyzed by plotting the absorption (μg/cm2) of the compound as function of time (h). The permeation rate (or flux) at steady state (μg/cm2/h) was calculated from the slope of the linear portion of the permeation curve over 24 hours. The lag time was determined by extrapolating the linear portion of the curve to the x-axis. Permeation parameters of ENX and E2 were expressed as the mean and SEM. The Kruskal–Wallis and Wilcoxon rank sum tests were used for comparisons across the compounds and between OA conditions, with Bonferroni corrections for significance testing as detailed in the Results. Statistical analyses were done using the SAS statistical software (SAS OnlineDoc® 9.2, SAS Institute Inc, Cary, NC).
Results
Stratum corneum thickness and ethanol skin toxicity
The stratum corneum (SC) of EpiDerm was thinner than normal human skin (). The skin toxicity of 70% (v/v) ethanol-PB was evaluated in the EpiDerm by the MTT assay. Epidermal cells of EpiDerm treated with PB remained highly viable (100%), whereas 37% and 44% of epidermal cells died when treated with 70% ethanol-PB for 6 and 24 hours, respectively ().
Figure 1 Skin images of EpiDerm™ and human STS. Top panel depicts the composition of skin layers. in the lower panel skin images of the EpiDerm (left) and the epidermis of human STS (right) are compared at the same magnification (20×) with a 10 micron bar, showing that SC of human skin is twice as thick as the SC of the EpiDerm.
Figure 2 Assessment of ethanol skin toxicity. Toxicity of 70% (v/v) ethanol-PB was evaluated in the EpiDerm™ by the MTT assay. 0.1 mL of PB and 70% ethanol-PB were loaded on the EpiDerm and MTT assay was performed after 6 and 24 h incubation at 37°C. The fraction of epidermal cells remaining highly viable after 24 h at 37°C was measured as the Abs of formazan at 570 nm, subtracting out a background reading for all samples at 650 nm with UV-visible spectrometer. Percent cell viability was determined at each of the dose concentrations using the following formula: % viability = 100× ([Abs570 − Abs650] of sample)/([Abs570 − Abs650] of a negative control) Negative control was PB and all data were normalized using data of PB at 6 h, and reported in mean ± SD.
![Figure 2 Assessment of ethanol skin toxicity. Toxicity of 70% (v/v) ethanol-PB was evaluated in the EpiDerm™ by the MTT assay. 0.1 mL of PB and 70% ethanol-PB were loaded on the EpiDerm and MTT assay was performed after 6 and 24 h incubation at 37°C. The fraction of epidermal cells remaining highly viable after 24 h at 37°C was measured as the Abs of formazan at 570 nm, subtracting out a background reading for all samples at 650 nm with UV-visible spectrometer. Percent cell viability was determined at each of the dose concentrations using the following formula: % viability = 100× ([Abs570 − Abs650] of sample)/([Abs570 − Abs650] of a negative control) Negative control was PB and all data were normalized using data of PB at 6 h, and reported in mean ± SD.](/cms/asset/529ef4ae-f114-40ca-92bf-b65cb705e5ad/dbct_a_24367176_f0002_b.jpg)
Permeation of TPEs across EpiDerm
The permeation parameters of the TPEs (TAM, 4-OHT, and ENX) across the EpiDerm were investigated with the addition of 1% OA and are summarized in . In the control vehicle the absorption of TPEs (approximately 1% of each TPE) was essentially equivalent at 6 hours (P = 0.21) but increased at 24 hours, in the order of 4-OHT > ENX > TAM (Kruskal–Wallis P = 0.005, among all TPEs). For the total penetration (TP) of TPEs we found that the TP of TAM and 4-OHT was similar, but the TP of ENX was significantly lower than that of TAM and 4-OHT (P = 0.006 for both) at 6 hours. At 24 hours, the total permeation of all TPEs was similar, ranging from 25%–29%.
Table 1 Detrimental effect of OA on permeation of TPEs across EpiDerm™
On addition of 1% OA into the control vehicle, a uniform and significant decrease in the absorption of TAM, 4-OHT and of ENX over 24 hours was found. The largest reduction was in the TP of TAM (approximate 80% reduction, P = 0.0008). The exception to this adverse trend was a transient increase in the absorption of ENX at 6 hours, in contrast to TAM and 4-OHT, which decreased significantly at 6 hours.
Permeation of TPEs across human skin samples
Next, we examined the absorption and TP of 4-OHT and ENX across normal human STS, comparing three concentrations of OA (1, 2.5, and 5%) to the control vehicle over 24 hours (). In the control vehicle, the absorption of 4-OHT and ENX were equivalent, but the TP of ENX was approximately half that of 4-OHT (P = 0.002). On addition of 1%–5% OA to the control vehicle, both the absorption and the TP of 4-OHT and ENX increased (Kruskal–Wallis P < 0.0001, for all comparisons). The addition of 1% OA enhanced the absorption of 4-OHT and ENX by approximately 3× and 18×, and the TP of these agents by 3× and 18×, respectively, compared with vehicle controls. The addition of 1% OA improved the TP of ENX 1.4× higher than that of 4-OHT (P = 0.004). At higher concentrations of OA (2.5%–5%) the absorption of 4-OHT and ENX did not further improve; rather, the absorption of ENX decreased when compared with 1% OA (1% vs 2.5% OA: P = 0.002, 1% vs 5% OA: P = 0.0013).
Table 2 OA enhances permeation of 4-OHT and ENX across human STS at 24 h
Permeation of ENX at lower concentrations of OA
Having established that 1% OA enhanced permeation of ENX to a greater extent than 4-OHT in human skin, we explored the effect of lower concentrations of OA (0.1%–1%) on permeation of ENX using Franz diffusion cells over 24 hours (). The permeation parameters (lag time, flux, absorption, skin content, and TP) of ENX were compared with that of E2, since the transdermal delivery of this hormonal agent is well established ( and ).
Figure 3 Permeation parameters of ENX compared with E2. The applied dose of the compounds was 78.9 (μg/cm2). The skin samples from three subjects were used and each treatment condition was tested in duplicate on the skin samples from a subject in each experiment. (A) permeation profile, (B) lag time, (C) flux (μg/cm2/h), and (D) absorption at 24 h (μg/cm were expressed as the mean ± SEM, n = 5–6. The P-values were determined using the Wilcoxon rank sum test.
Abbreviations: ENX, endoxifen; E2, estradiol; OA, oleic acid; SEM, standard error of the mean.
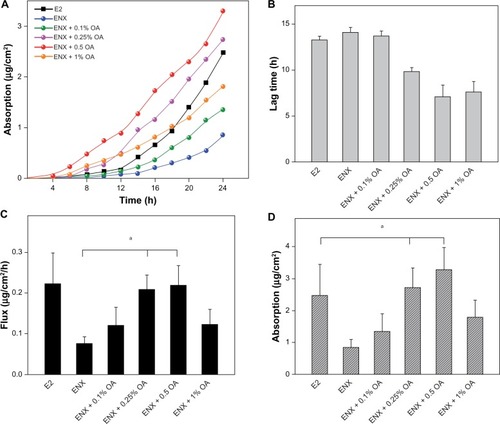
Figure 4 Skin content and total penetration of ENX compared with E2. The applied dose of the compounds was 78.9 (μg/cm2). The skin samples from three subjects were used and each treatment condition was tested in duplicate on the skin samples from each subject in each experiment. (A) Skin content (μg/cm2) of the compounds was measured separately from epidermis and dermis and was combined as total skin content after 24 h. (B) TP of the compounds was determined as the sum of absorption and total skin contents after 24 h. All measurements were expressed as the mean ± SEM, n = 5–6. The P-values were determined using the Wilcoxon rank sum test.
Abbreviations: ENX, endoxifen; E2, estradiol; OA, oleic acid; SEM, standard error of the mean; TP, total penetration.
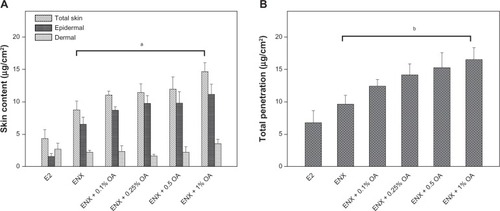
In the control vehicle, the lag time of ENX and E2 was similar, the flux of ENX was <E2 ( and ), and the absorption of ENX was 50% than that of E2 (P = 0.045) (). In contrast, the total skin content of ENX was 1.8× higher than that of E2 (P = 0.045). This increase was attributable to the epidermal content of ENX, which was 4× higher than that of E2 (P = 0.006), while the dermal content of two compounds was similar (). Finally, the TP of ENX was slightly higher (1.5×) than that of E2, but the difference was not significant (P = 0.17) ().
Next, we evaluated the effect of lower concentrations of OA (0.1%–1%) on the permeation parameters of ENX. The addition of 0.1% OA to the control vehicle showed nonsignificant permeation-enhancing effects on the lag time and the flux of ENX in comparison with that in the control vehicle. On addition of 0.25% and 0.5% OA, lag time of ENX was shorter (P = 0.0062 for both), the flux was significantly enhanced by 2.6–2.8× (P < 0.05 for both), and the absorption was increased by 3.2× and 3.9×, respectively, in comparison with that of ENX in the control vehicle. At the concentration of 1% OA, we observed that there was no further improvement in the permeation parameters of ENX (). Thus, the flux and the absorption of ENX was lower than that of E2 in the control vehicle, but the addition of 0.25%–0.5% OA enhanced the flux of ENX equivalent to that of E2 and increased the absorption of ENX superior to that of E2 (P < 0.05) ( and ).
For the skin content, the partitioning of ENX into skin layers was improved by 0.1%–1% OA. These improvements were proportional to the concentration of OA added to the control vehicle, and mainly driven by epidermal rather than dermal content of ENX (). At a concentration of 1% OA the total skin content of ENX was improved by 1.7× in comparison with that in the control vehicle (P = 0.045) (). This was 3.4× higher than that of E2 in the control vehicle (P = 0.01).
Finally, we observed that the TP of ENX was in the range of 15.7%–20.9% (12.4–16.5 μg/cm2) in the presence of 0.1%–1% OA (), so that, with a 1% concentration of OA, the TP of ENX was 1.7× higher than in the control vehicle (P = 0.029), and 2.4× higher than that of E2 in the control vehicle ().
Discussion
Transdermal delivery has long been recognized as an effective form of systemic therapy, with distinct pharmacokinetic and related advantages, but, due to the effectiveness of the barrier function of the stratum corneum, only a small number of drugs have been successfully formulated for this purpose. Existing data on transdermal permeation of 4-OHT suggest that the relatively small and lipophilic nature of this molecule renders it suitable for transdermal formulation.78 A study using an alcoholic gel formulation of 4-OHT suggests that, when applied to the skin of the breast of postmenopausal women with ER-positive breast cancer, sufficient breast tissue concentrations are achieved for an antiproliferative effect on tumor cells of equal magnitude to that seen with standard doses of oral TAM.Citation9 Other studies using the same gel for the treatment of mastalgia show a benefit 4-OHT gel at a concentration of 4 mg/dayCitation11 The authors are using the same formulation of 4-OHT in a multicenter presurgical study in DCIS patients (NCT00952731), with the primary endpoint of decreased cell proliferation.
The authors have investigated the relative permeation of 4-OHT and ENX because the binding affinities of 4-OHT and ENX are both 25× greater for ERα and 56× greater for ERβ than that of TAM.Citation12,Citation13 ENX has been reported to have an advantage over 4-OHT in that it causes proteosomic degradation of ERα and may have more selective antiestrogenic effects.Citation5 Therefore, ENX is expected to give better therapeutic efficacy than 4-OHT, but its specific toxicity profile is currently unknown. If ENX shares even some of the toxicity of the parent drug and its percutaneous absorption in humans is equivalent to (or better) than that of 4-OHT, it would be an excellent candidate for transdermal delivery. Additionally, the chemical structure of ENX would render it more promising for transdermal application because it is more amenable to conjugation to nanoparticles for controlled release. For this reason, the authors have investigated the relative in vitro percutaneous absorption of ENX in comparison with those of 4-OHT and TAM.
It was found that the total penetration of ENX into human skin was not as efficient as that of 4-OHT in the control vehicle, but the addition of 1% OA greatly improved both absorption and TP of ENX over 24 hours. Although significant increases in these parameters were also seen for 4-OHT, the increase in ENX permeation was larger and brings ENX permeation into a range that is very compatible with transdermal therapy. OA is a well-known permeation enhancer that has been employed to increase absorption of TPEs in the 60% (v/v) ethanol-PB vehicle.Citation14 Several researchers have observed a permeation-enhancing effect with OA in ethanol-water systems across hairless rodent skin.Citation15–Citation17 In the present study, an ethanol-based vehicle was necessary to solubilize TPEs and ethanol also has the advantage of being a widely used skin permeation enhancer used in topical drug-delivery systems for estradiol, progesterone, fentanyl, and other drugs. It is not clear why ENX benefited more from the addition of OA than 4-OHT, but this may be related to a difference in their structure. ENX is smaller and more polar than 4-OHT because one methyl group at a tertiary amine is replaced with a hydrogen, resulting in a secondary amine that is more hydrophilic than the tertiary amine of 4-OHT. Because OA appears to make the stratum corneum fluidicCitation18,Citation19 and ethanol gives a continuous driving force,Citation14,Citation20 ENX may move faster through the skin than 4-OHT. The amine group of ENX may have a favorable balance of hydrophilic and hydrophobic properties to deal with the stratum corneum, which would allow ENX to traverse the stratum corneum more easily. The results here agree with previous findings using hairless rat skin, which showed that the co-solvent system of OA-ethanol-water efficiently increased skin permeation of both lipophilic and hydrophilic drugs.Citation15
The effect of OA as a permeation enhancer was markedly divergent between the EpiDerm and human STS; the general permeation-enhancing effect of OA was not only absent in the EpiDerm model, but also the permeation was significantly reduced. The EpiDerm model does not have the papillary dermal layer of normal human STS, and the thickness of the stratum corneum was almost 50% < human STS used in our experiments. Thus, the reason for the inhibition of permeation by OA in the EpiDerm may be related to its lipid characteristics and thin, imperfectly developed stratum corneum, derived from cultured human keratinocytes, so that the favorable effects of OA on the partitioning of compounds through the skin are not observed. This suggests that the reconstituted epidermis is not a suitable model for the testing of permeation enhancers, such as OA, that depend on partitioning with lipids in the stratum corneum.
The permeation enhancing effect of OA was assessed at lower concentrations (0.1%–1%) to find an optimal concentration of OA for ENX in 60% ethanolic solution, with E2 as a reference transdermal compound, to determine whether the permeation of ENX with OA can be improved to a level consistent with effective transdermal delivery. The results show that the addition of 0.25%–0.5% OA maximizes the flux of ENX over 24 hours and higher concentrations of OA do not result in further improvement. Overall, 0.25%–0.5% OA seems to be the optimal concentration for 60% ethanolic vehicle system as a fast and efficient transdermal delivery of ENX. Furthermore, although ENX alone permeates human skin slower than E2, the addition of OA not only improves the absorption of ENX to a level similar to that of E2, but also significantly increases skin deposition of ENX. Together, these results suggest that ENX is an excellent candidate for transdermal delivery.
The direct delivery of active metabolites to the breast through its skin envelope averts first-pass metabolism in the liver, potentially avoiding changes in the clotting cascade that lead to the prothrombotic effects of TAM and raloxifene.Citation2,Citation21,Citation22 Since risk of thromboembolism is a major concern not only with TAM use, but also with all selective estrogen–receptor modulators (SERMs) tested clinically to date, its avoidance would be a significant advantage for women considering SERM therapy for breast cancer prevention and for treatment of DCIS. Additionally, the very low plasma concentrations of following transdermal application of 4-OHT observed in the studies conducted so farCitation7,Citation8,Citation11 suggest that uterine toxicity and hot flashes would be reduced by the transdermal delivery of active TAM metabolites to the breast. Furthermore, limitations on the bioavailability of active metabolites that are caused by polymorphisms in TAM metabolizing genesCitation3,Citation4 would be overcome by this approach.
Finally, the issue of whether transdermal delivery to the breast is a local or a systemic treatment deserves consideration. The preliminary studies conducted by Mauvais-Jarvis and colleagues in the 1980s and 1990s showed that 4-OHT applied through the skin of the breast concentrates in the breast at 10× higher levels than when it is applied to the arm or shoulder.78 The investigators attributed this accumulation to the binding of 4-OHT to ER present in breast tumors and breast epithelium. In fact, ERα expression in nonmalignant breast tissue is very low but ERβ is high and may account for at least some of the localization in the breast. However, receptor binding alone is insufficient to explain 4-OHT retention in the breast.Citation23,Citation24 A more plausible explanation relates to the embryological origin of the breast as a skin appendage (ie, a modified sweat or apocrine gland). Studies of the embryology of the breast suggest that the breast gland (parenchyma) and its skin envelope are a single unit with a well-developed internal lymphatic (and venous) circulation.Citation25 These embryological studies are supported by the fact that the skin and parenchyma of the breast drain to the same sentinel nodes.Citation26,Citation27 Therefore, other drugs applied to the skin of the breast should concentrate in the parenchyma to a greater degree than can be expected based on systemic absorption and delivery through the circulation, and the model of transdermal local pharmacotherapy for breast cancer prevention and for DCIS therapy should be extendable to a variety of agents as long as they show sufficient dermal permeation.
It is too early to draw any conclusion for potential clinical use since it remains uncertain whether a level of ENX in the breast by transdermal delivery is equivalent to the clinical efficacy of oral TAM. To address this, the authors have initiated an in vivo preclinical study to assess mammary-gland and systemic distribution of ENX by transdermal delivery, and to evaluate the in vivo therapeutic efficacy of ENX, compared with standard doses of oral TAM in the hairless rat model.Citation28 These studies will guide the development of a clinical study to evaluate the efficacy of this approach for prevention of ER positive breast cancer in the near future.
Conclusion
These results demonstrate that the addition of OA improves absorption of ENX through human skin in vitro to the same range as that seen for E2, providing strong justification for the development of ENX for local transdermal delivery to the breast. The data raise questions about the suitability of the EpiDerm model for the evaluation of skin permeation enhancement and suggest that the permeation dynamics of human skin differ substantially from those of reconstituted human epidermis.
Disclosure
The authors report no conflicts of interest in relation to this work.
Acknowledgments
The authors thank Dr Russell O Potts, Russ Potts Consulting, LLC, San Francisco, CA, for valuable discussions regarding setup of in vitro human skin permeation experiment and permeation enhancers. The present study was supported by grants from Lynn Sage Breast Cancer Foundation and the Susan G Komen Foundation.
References
- JordanVCChemoprevention of breast cancer with selective oestrogen-receptor modulatorsNat Rev Cancer200771465317186017
- CuzickJPowlesTVeronesiUOverview of the main outcomes in breast-cancer prevention trialsLancet2003361935429630012559863
- GoetzMPRaeJMSumanVJPharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashesJ Clin Oncol200523369312931816361630
- GoetzMPKnoxSKSumanVJThe impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifenBreast Cancer Res Treat2007101111312117115111
- WuXHawseJRSubramaniamMGoetzMPIngleJNSpelsbergTCThe tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor alpha for degradation in breast cancer cellsCancer Res20096951722172719244106
- FisherBCostantinoJPWickerhamDLTamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 StudyJ Natl Cancer Inst19989018137113889747868
- Mauvais-JavisPBaudotNCastaigneDBanzetPKuttennFtrans-4-Hydroxytamoxifen concentration and metabolism after local percutaneous administration to human breastCancer Res1986463152115253943109
- PujolHGiraultJRouanetPPhase I study of percutaneous 4-hydroxy-tamoxifen with analyses of 4-hydroxy-tamoxifen concentrations in breast cancer and normal breast tissueCancer Chemother Pharmacol19953664934987554041
- RouanetPLinares-CruzGDravetFNeoadjuvant percutaneous 4-hydroxytamoxifen decreases breast tumoral cell proliferation: a prospective controlled randomized study comparing three doses of 4-hydroxytamoxifen gel to oral tamoxifenJ Clin Oncol200523132980298715860853
- BronaughRLStewartRFMethods for in vitro percutaneous absorption studies. VI: preparation of the barrier layerJ Pharm Sci19867554874913735088
- ManselRGoyalANestourELMasini-EteveVO’ConnellKA phase II trial of Afimoxifene (4-hydroxytamoxifen gel) for cyclical mastalgia in premenopausal womenBreast Cancer Res Treat2007106338939717351746
- LimYCDestaZFlockhartDASkaarTCEndoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifenCancer Chemother Pharmacol200555547147815685451
- LimYCLiLDestaZEndoxifen, a secondary metabolite of tamoxifen, and 4-OH-tamoxifen induce similar changes in global gene expression patterns in MCF-7 breast cancer cellsJ Pharmacol Exp Ther2006318250351216690721
- WilliamsACBarryBWPenetration enhancersAdv Drug Deliv Rev200456560361815019749
- KimDDKimJLChienYWMutual hairless rat skin permeation-enhancing effect of ethanol/water system and oleic acidJ Pharm Sci19968511119111958923324
- KimMJDohHJChoiMKSkin permeation enhancement of diclofenac by fatty acidsDrug Deliv200815637337918686081
- GoodmanMBarryBWAction of penetration enhancers on human skin as assessed by the permeation of model drugs 5-fluorouracil and estradiol. I. Infinite dose techniqueJ Invest Dermatol19889143233273171212
- FangJYHwangTLLeuYLEffect of enhancers and retarders on percutaneous absorption of flurbiprofen from hydrogelsInt J Pharm2003250231332512527159
- YuBDongCYSoPTBlankschteinDLangerRIn vitro visualization and quantification of oleic acid induced changes in transdermal transport using two-photon fluorescence microscopyJ Invest Dermatol20011171162511442745
- OgisoTPakuTIwakiMTaninoTPercutaneous penetration of fluorescein isothiocyanate-dextrans and the mechanism for enhancement effect of enhancers on the intercellular penetrationBiol Pharm Bull19951811156615718593481
- CosmanFBaz-HechtMCushmanMShort-term effects of estrogen, tamoxifen and raloxifene on hemostasis: a randomized-controlled study and review of the literatureThromb Res2005116111315850603
- CuzickJForbesJEdwardsRFirst results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trialLancet2002360933681782412243915
- KhanSARogersMAKhuranaKKMeguidMMNumannPJEstrogen receptor expression in benign breast epithelium and breast cancer risk [see comments]J Natl Cancer Inst199890137429428781
- RickettsDTurnbullLRyallGEstrogen and progesterone receptors in the normal female breastCancer Res199151181718222004366
- AckermanABKesslerGGyorfTTsouHCGottliebGJContrary view: the breast is not an organ per se, but a distinctive region of skin and subcutaneous tissueAm J Dermatopathol200729221121817414452
- PovoskiSPOlsenJOYoungDCProspective randomized trial comparing intradermal, intraparenchymal, and subareolar injection routes for sentinel lymph node mapping and biopsy in breast cancerAnn Surg Oncol2006132101116372150
- KlimbergVSRubioITHenryRCowanCColvertMKorourianSSubareolar versus peritumoral injection for location of the sentinel lymph nodeAnn Surg1999229686086410363900
- LienEASolheimEUelandPMDistribution of tamoxifen and its metabolites in rat and human tissues during steady-state treatmentCancer Res19915118483748441893376