Abstract
Pertuzumab, a humanized monoclonal antibody to the HER2 receptor, represents a promising new anti-HER2 agent with a novel mechanism of action targeting the inhibition of HER2 dimerization. Nonclinical and clinical data to date indicate that pertuzumab provides a broader HER2 blockade through the inhibition of HER2 heterodimerization. In preclinical experiments, pertuzumab has demonstrated superior antitumor effects when combined with other anti-HER2 treatments such as trastuzumab, compared to when used as monotherapy. Trastuzumab and pertuzumab monoclonal antibodies bind to distinct epitopes on the HER2 receptor without competing with each other, resulting in distinctive mechanisms for disrupting HER2 signaling. These mechanisms are complementary and result in augmented therapeutic efficacy when pertuzumab and trastuzumab are given in combination. Clinically, pertuzumab may have optimal therapeutic effects when given to patients with HER2-positive cancers, in combination with trastuzumab. This observation is supported by recent clinical trials in the metastatic as well as neoadjuvant setting. Intravenous pertuzumab had an acceptable tolerability profile when added to trastuzumab and chemotherapy. This overview will review recent advances in the clinical development of this HER2-targeted therapy.
Keywords:
Introduction
Overexpression of HER2 in breast cancer is correlated with high histologic grade, increased mitotic activity, p53 mutation, negative estrogen receptor (ER) status, absence of bcl2, and absence of lobular architecture. Despite associations with other known negative prognostic factors, human epidermal growth factor receptor-2 (HER2) over-expression is independently associated with poorer disease-free survival and overall survival (OS) compared with tumors that do not overexpress HER2.Citation1
Targeting the human epidermal growth factor receptor (HER) family of receptor tyrosine kinases has proven to be effective in cancer therapy. Trastuzumab, an monoclonal antibody that binds to the extracellular domain of the HER2 receptor, has become a standard treatment option for women with HER2 overexpression.Citation2 Various studies on adjuvant as well as metastatic breast cancer have proved the efficacy of this antibody.Citation3,Citation4 Yet, despite the advances that have come with the introduction of this novel therapy, there are still a number of HER2-positive breast cancers that exhibit intrinsic (primary) or de novo (secondary) resistance to HER2-targeted therapy.Citation5 As a consequence, new anti-HER2 agents are currently under development. One of the most promising is the monoclonal antibody, pertuzumab.
Pertuzumab: mechanisms of action
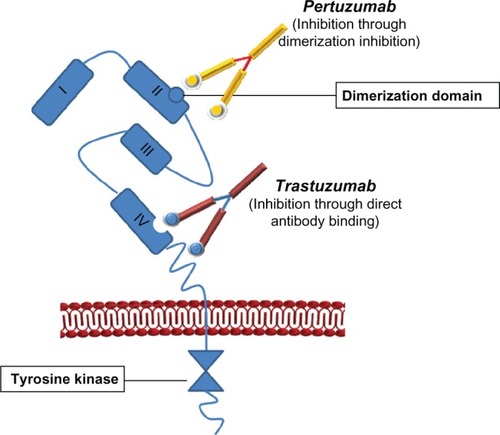
Pertuzumab (rhuMAb 2C4) is a fully humanized monoclonal antibody that is based on the human immunoglobulin (Ig) G1(κ) framework sequences. This antibody acts by blocking the association of HER2 with other HER family members, including epidermal growth factor receptor (EGFR, also known as HER1), HER3, and HER4. As a result, the formation of HER heterodimers is inhibited.Citation6,Citation7 Pertuzumab represents a new class of drugs that targets HER2 dimerization. Like trastuzumab, pertuzumab is a recombinant humanized monoclonal antibody that binds to the extracellular domain of HER2. It consists of two 449 residue heavy chains and two 214 residue light chains. Pertuzumab binds to an epitope in domain II, the dimerization domain of the HER2 extracellular domain, which is distinct from the binding site of trastuzumab in domain IV ().Citation8,Citation9 Due to their complimentary modes of action, there is strong rationale for the combination of pertuzumab with trastuzumab for the treatment of HER2 overexpressing disease.
Figure 1 Pertuzumab is a fully humanized monoclonal antibody based on the human IgG1(κ) framework sequences that consists of two heavy chains (449 residues) and two light chains (214 residues).
In vitro, pertuzumab blocks heregulin (HRG)-induced activation of the phosphatidylinositol-3-kinase (PI3 K) cell survival pathway, whereas trastuzumab does not. This suggests that pertuzumab is superior to trastuzumab in blocking ligand-activated HER2 signaling. In the breast carcinoma cell line MCF-7, pertuzumab blocks HRG-induced activation of the PI3 K cell survival pathway – as indicated by a lack of phosphorylation of a key enzyme (Akt) in this pathway – while trastuzumab does not.Citation10 Additionally, per-tuzumab activates antibody-dependent cellular cytotoxicity (ADCC) with the same potency as trastuzumab.Citation11 In human tumor xenograft models in mice, single agent pertuzumab was active against various tumors (including those without HER2 overexpression) such as lung, breast, ovarian, and prostate cancers.Citation10 Pertuzumab augmented the antitumor effect of various cytotoxic drugs representing different mechanisms of action, as well as other HER pathway inhibitors such as the HER1-targeting agent, erlotinib.Citation12 In HER2-positive xeno-graft models, pertuzumab demonstrated synergistic antitumor activity in combination with trastuzumab or trastuzumab DM-1 (T-DM1).Citation11,Citation13 The following sections will discuss the development and clinical activity of pertuzumab.
Clinical development
Due to pertuzumab’s distinct mode of action compared to trastuzumab, early clinical developments built confidence in the possibility of clinical activity in various types of cancers, even those that showed no HER2 amplification. In 2005, a Phase I trial of pertuzumab monotherapy was conducted in patients with advanced cancer.Citation14 Most of these tumors were not overexpressing the HER2 receptor. In this evaluation, pertuzumab was well tolerated at all dose levels, and a maximum tolerated dose was not reached. Toxicity was generally mild as twelve grade 3/4 events were reported, of which only six were thought to be related to the medication under examination. Symptoms included abdominal pain (14%), nausea (5%), vomiting (5%), and diarrhea (5%). Pharmacokinetic analyses of serum concentrations revealed that levels of trastuzumab rapidly declined over the first 2 to 3 days. The half-life of pertuzumab is 3 weeks and the volume of distribution approximated the serum volume. Pharmacokinetics obtained in the Phase I trial appeared to be similar to the preclinical data (see ).Citation15 Early trials used a dose ranging from 0.5 to 15 mg/kg and systemic clearance remained constant. Based on these data, a dosing interval of 3 weeks was recommended for clinical application. In the Phase II studies, a loading dose of 840 mg (followed by 420 mg q3w), was capable of attaining steady-state trough and peak concentrations by the second cycle (see ).Citation16 Clinical activity was observed in patients with HER2 low-expressing tumors who had received pertuzumab either as a single agent or in combination with cytotoxic chemotherapy. Complete responses have not been observed in any of these trials. In single agent pertuzumab studies, partial responses or stable disease lasting ≥6 months have been observed in 15% of patients with ovarian cancer and in 8% of patients with HER2 low-expressing breast cancer.Citation14,Citation17 However, due to the limited efficacy observed in these studies, generally stable diseases of relatively short duration suggested that there would be little benefit in further investigating the use of single-agent pertuzumab in unselected patients with HER2-negative diseases. Further development of this compound is focused on women with HER2-overexpressing breast cancer.
Table 1 Features and properties of pertuzumab
Pertuzumab in clinical trials for breast cancer
In a study by Baselga et al, patients with advanced HER2-positive breast cancer in whom disease progression had occurred during prior trastuzumab-based therapy, received trastuzumab weekly (4 mg/kg loading dose, then 2 mg/kg every week) or every 3 weeks (8 mg/kg loading dose, then 6 mg/kg every 3 weeks) and pertuzumab every 3 weeks (840 mg loading dose, then 420 mg every 3 weeks).Citation18 The Phase II trial (BO17929 trial) assessed the efficacy and safety profile of the combination in patients (n = 66) with HER2-positive breast cancer whose disease had progressed during prior trastuzumab-based therapy (see ). The objective response rate was 24.2%, and the clinical benefit rate was 50%. Five patients (7.6%) experienced a complete response, eleven patients (16.7%) experienced a partial response, and 17 patients (25.8%) experienced stable disease for 6 or more months. Median progression-free survival (PFS) was 5.5 months. Overall, the combination of pertuzumab and trastuzumab was well tolerated, and AEs were mild to moderate.
Table 2 Completed clinical trials examining the efficacy of pertuzumab in breast cancer
A neoadjuvant Phase II study was initiated, based on this study.Citation19 In the NEOSPHERE study, treatment-naive women with HER2-positive breast cancer were randomly assigned to four treatment groups (1:1:1:1). Depending on treatment group, patients were to receive four neoadjuvant cycles of: trastuzumab (8 mg/kg loading dose, followed by 6 mg/kg every 3 weeks) plus docetaxel (75 mg/m2), escalating, if tolerated, to 100 mg/m2 every 3 weeks (group A), or pertuzumab (loading dose 840 mg, followed by 420 mg every 3 weeks) and trastuzumab plus docetaxel (group B), or pertuzumab and trastuzumab (group C), or pertuzumab plus docetaxel (group D). The primary endpoint, examined in the intention-to-treat population, was pathological complete response (pCR) in the breast. Of the 417 eligible patients, 107 were randomly assigned to group A, 107 to group B, 107 to group C, and 96 to group D. Patients who received pertuzumab and trastuzumab plus docetaxel (group B) had a significantly improved pathological complete response rate (49 of 107 patients; 45.8% [95% CI: 36.1–55.7]) compared with those given trastuzumab plus docetaxel (group A; 31 of 107; 29.0% [95% CI: 20.6–38.5]; P = 0.0141). Twenty-three of 96 (24.0% [95% CI: 15.8–33.7]) women who received pertuzumab plus docetaxel (group D) had a pathological complete response, as did 18 of 107 (16.8% [95% CI: 10.3–25.3]) who received pertuzumab and trastuzumab (group C). Pathologic complete response was up to 63.2% with the combination of the two antibodies and docetaxel in the hormone receptor-negative subgroup.
The most common AEs of grade 3 or higher were neutropenia (61 of 107 women in group A, 48 of 107 in group B, one of 108 in group C, and 52 of 94 in group D), febrile neutropenia (eight, nine, none, and seven, respectively), and leucopenia (13, five, none, and seven, respectively). The number of serious AEs was similar in groups A, B, and D (15–20 serious AEs per group in 10%–17% of patients), but was significantly lower in group C (four serious AEs in 4% of patients). The study concluded that patients given pertuzumab and trastuzumab plus docetaxel (group B) had a significantly improved pathological complete response rate compared with those given trastuzumab plus docetaxel alone, which indicates that a chemotherapy backbone is necessary to improve the pCR rate.
The efficacy of neoadjuvant pertuzumab was confirmed by the recently presented TRYPHAENA Study.Citation20 The trial examined patients with newly diagnosed HER2-positive early breast cancer and investigated three experimental neoadjuvant regimens. The common feature of all three regimens was the combination of pertuzumab and trastu-zumab for at least three cycles. Each regimen combined these two HER2-directed antibodies with anthracycline– taxane-based or carboplatin–taxane-based standard chemotherapy backbones either concomitantly or sequentially for six cycles. Following surgery, all patients received 1 year of adjuvant trastuzumab. The primary endpoint was assessment of the safety and tolerability of neoadjuvant treatment. During neoadjuvant treatment, the left ventricular ejection fraction was monitored on a regular basis. The key secondary endpoint was the pCR rate Approximately 75 patients were randomized to each of the three neoadjuvant regimens, and all were well tolerated with no symptomatic cardiac toxicity even if both HER2-directed antibodies were given concomitantly with anthracyclines. Treatment with each regimen yielded impressive pCR rates, ranging from 45% to 66% with little difference between them. However, pCR rates were substantially higher in patients with ER-negative and progesterone receptor (PR)-negative tumors (up to 83.8%), compared to patients with ER-negative and/or PR-positive tumors. These findings, together with the previously reported results of the NEOSPHERE trial described above, clearly illustrate the potential benefits of dual HER2 inhibition using pertuzumab and trastuzumab; however, the long-term benefits of the neoadjuvant regimens investigated in the TRYPHAENA trial are still unknown. At the time of the writing, only 16% of patients had completed adjuvant therapy.
Based on the encouraging phase II data, a randomized Phase III trial has begun that enrolled women with metastatic HER2/neu positive breast cancer in first-line treatment consisting of docetaxel and trastuzumab with placebo or pertu-zumab.Citation21 In CLEOPATRA (Clinical Evaluation of Pertuzumab and Trastuzumab), an international, Phase III, double-blind, randomized registration trial, 808 patients (mean age, 54 years) received either placebo plus trastuzumab (an 8-mg/kg loading dose, followed by a 6-mg/kg maintenance dose) plus docetaxel (75 mg/m2, escalating to 100 mg/m2 if tolerated, for six cycles or more, as recommended) or pertuzumab (an 840-mg loading dose, followed by a 420-mg maintenance dose) plus trastuzumab (an 8-mg/kg loading dose, followed by a 6-mg/kg maintenance dose).Citation21 Patients were allowed to receive one previous hormonal treatment for metastatic breast cancer and/or prior systemic neoadjuvant or adjuvant therapy, including prior trastuzumab and taxanes. PFS (the primary endpoint) was reported at a median of 18.5 months with pertuzumab/trastuzumab/docetaxel and at 12.4 months with placebo/trastuzumab/docetaxel (6.1 months; hazard ratio [HR] = 0.62; 95% CI: 0.51–0.75; P < 0.0001). Calling the magnitude of effects with the pertuzumab combination “unprecedented,” the authors speculated that the new regimen could be a practice-changing discovery for the treatment of patients with HER2-positive, first-line metastatic breast cancer. Subgroup analyses revealed that the beneficial effect of pertuzumab on PFS occurred regardless of the patients’ prior exposure to adjuvant or neoadjuvant chemotherapy, their geographic region, their hormone receptor status (ie, estro-gen and/or progesterone receptor-positive or estrogen and progesterone-receptor negative), or their HER2 positivity, which was assessed by immunohistochemistry (membrane staining 3+) or by fluorescence in situ hybridization (amplificaton of the HER2 gene).
In terms of patient age, progression-free survival hazard ratios significantly favored pertuzumab in patients aged <65 or ≥65 years, and patients aged <75 years, but not in those aged ≥75 years, where the HR favored pertuzumab but the number of patients was very small (n = 19). The interim analysis of overall survival was performed after 165 events (43% of the prespecified total number for the final analysis) had occurred. More deaths occurred in the control group than in the pertuzumab group (96 [23.6%] vs 69 [17.2%]). The hazard ratio was 0.64 (95% CI: 0.47 to 0.88; P = 0.005), which did not meet the O’Brien–Fleming stopping boundary for this interim analysis of overall survival (HR ≤ 0.603; P ≤ 0.0012) and was, therefore, not significant. However, the data showed a strong trend toward a survival benefit with the pertuzumab–trastuzumab–docetaxel therapy.Citation21
Ongoing trials with pertuzumab
Several other trials are examining the efficacy of pertuzumab in patients with early breast and advanced breast cancer (see ). Both, in vitro and in vivo studies suggest that the combination of pertuzumab and trastuzumab has synergistic activity in breast cancer; therefore, clinical trials are now focusing on the combination of both antibodies.Citation22 For example, the PHEREXA study (NCT01026142) is designed to investigate the potential benefit of administering pertuzumab and trastuzmab combined with capecitabine in patients with HER2-positive metastatic breast cancer whose disease has progressed during or following trastuzumab-based therapy for first-line metastatic breast cancer.Citation23 In this multicenter, open-label, Phase II trial, patients are randomized 1:1 to receive trastuzumab in combination with capecitabine or trastuzumab, pertuzumab, and capecitabine. Enrollment began in January 2010 and 450 patients will be recruited from ∼130 sites in 19 countries. The primary endpoint is PFS (independent assessment). Secondary endpoints include overall survival, PFS (investigator assessment), and safety/tolerability. A panel of biomarkers including HER1, HER2, and HER3 receptor status and downstream markers will be analyzed.
Table 3 Ongoing or planned clinical trials investigating pertuzumab in breast cancer
The Phase III MARIANNE trial (NCT01120184) is currently underway and is comparing the efficacy of the investigational immunoconjugate trastuzumab–emtansine (trastuzumab-DM1) with or without pertuzumab with that of trastuzumab plus a taxane for the first-line treatment of patients with HER2-positive locally advanced or metastatic breast cancer.Citation24 T-DM1 is a unique antibody-drug conjugate (ADC) composed of trastuzumab, a stable thioether linker, and the highly potent cytotoxic agent, DM1 (derivative of maytansine). In a randomized Phase II trial, single-agent T-DM1 had comparable efficacy to trastuzumab plus docetaxel in the first-line treatment of HER2-positive locally advanced or metastatic breast cancer.Citation25 T-DM1 and pertu-zumab bind to different epitopes on HER2, and have distinct mechanisms of action, which suggests that the combination may result in a more complete blockade of HER2. T-DM1 plus pertuzumab showed synergistic antitumor effects in preclinical models, and the preliminary Phase Ib/II trial results showed acceptable tolerability and promising efficacy in patients with metastatic breast cancer.Citation13 This systemic chemotherapy-sparing combination has the potential to establish a new treatment paradigm by optimizing efficacy while minimizing toxicity. Patients are randomized 1:1:1 to each arm. The primary endpoint is PFS. Secondary endpoints include safety, overall response rate, overall survival, duration of response and quality of life. Target enrollment is 1092 patients across ∼310 sites globally.
Finally, the Adjuvant Pertuzumab and Herceptin IN IniTial TherapY of Breast Cancer (APHINITY) trial (NCT01358877) is examining the efficacy of pertuzumab plus trastuzumab, compared to that of trastuzumab alone in patients receiving adjuvant chemotherapy for HER2-positive, nonmetastatic breast cancer.Citation26 While the adjuvant use of trastuzumab has been shown to improve disease-free and overall survival, not all patients treated with this agent benefit from this therapy. APHINITY is a prospective, randomized, multicenter, double-blind, placebo-controlled study in patients with HER2-positive early breast cancer. Patients will be randomized to one of two arms (1:1 ratio). The investigational arm will be comprised of a course of adjuvant CT (investigators choice) consisting of either an anthracycline-taxane or a taxane-platin that contains regimens, trastuzumab, and pertuzumab for 1 year. The comparator arm will consist of the same adjuvant CT backbone with trastuzumab and placebo for 1 year.
Adverse events and cardiac safety of pertuzumab
HER2 and HER4 are crucial for mouse embryonic heart development, and studies have shown that HER2 expression is required for the development of ventricular muscles and valves in rodents, while the activation of HER2 promotes cardiomyocyte survival.Citation27–Citation30 Trastuzumab may cause an asymptomatic decline in the left ventricular ejection fraction (LVEF), known as left ventricular systolic dysfunction (LVSD).Citation21 However, symptomatic heart failure (HF) under trastuzumab is relatively uncommon. Nevertheless, due to the known effects of trastuzumab on the human heart, it is of major concern that pertuzumab may cause similar or worse declines in cardiac functions. The results of a recently published meta-analysis of 14 Phase II trials, which included 598 patients, demonstrated that pertuzumab (exposure ranging from a median of three cycles to a median of 26 cycles) was generally well tolerated.Citation31 The incidence of LVSD was low, with most events being asymptomatic and detected at scheduled evaluations. The median timing of LVSD and HF was around cycle 4 (range 1–15) with 34/39 (87%) events occurring between cycles 1 and 7. Additionally, when pertu-zumab was administered in combination with trastuzumab or nonanthracycline-containing cytotoxic chemotherapy, there was no marked increase in observed cardiac dysfunction. In this analysis of 598 unique patients exposed to pertuzumab, 35 (5.9%) cases of asymptomatic LVSD and four (0.7%) cases of symptomatic HF were reported.Citation31 Results from the completed NEOSPHERE, TRYPHAENA, and CLEOPATRA trials also indicate a low incidence of symptomatic and asymptomatic LVSD.Citation19–Citation21
Gastrointestinal toxicities (diarrhea, nausea, vomiting, and abdominal pain) and fatigue are the most frequently reported AEs associated with single-agent therapy. Diarrhea and rash are common events that increased with pertuzumab in combination with chemotherapy, compared with chemotherapy alone. The most common AEs during dual therapy with pertuzumab and trastuzumab in metastatic breast cancer (BO17929) were diarrhea, fatigue, nausea, and rash.Citation18 The majority of these AEs were NCI-CTC grade 1 or 2 in severity. The mechanisms behind diarrhea and rash are unknown, but similar side effects are observed with other agents that cause HER1 inhibition. The most frequently occurring AEs during neoadjuvant treatment of patients with locally advanced breast cancer, using pertuzumab and trastuzumab in combination with chemotherapy, were alopecia, neutropenia, diarrhea, nausea, fatigue, rash, and mucosal inflammation.Citation19,Citation20
Adding pertuzumab to the trastuzumab plus docetaxel regimen did not notably affect the overall safety profile, and the tolerability of pertuzumab plus docetaxel was also broadly comparable to the triple regimen.Citation32 Patients receiving trastuzumab and pertuzumab without docetaxel in the neoadjuvant setting reported notably fewer AEs across most body systems, compared to patients who received treatments containing chemotherapy. Serious or severe infusion-related symptoms have been rarely observed in patients (<1%) receiving pertuzumab.
Conclusion
The HER family of receptor tyrosine kinases is an important mediator in cancer development and progression, and treatment directed against individual members of this family is a successful strategy for cancer therapy. As a HER dimerization inhibitor, pertuzumab promises to be widely applicable across tumor types, particularly since it is hypothesized that HER2/neu overexpression is not required for it to be an active agent. Pertuzumab has revealed itself to be a promising agent in metastatic HER2 positive breast cancer, in which a combination with trastuzumab has shown impressive activity among women who had otherwise progressed on trastuzumab-based therapy. The antibody has also shown excellent efficacy in combination with trastuzumab and chemotherapy in a neoadjuvant setting. However, no head-to-head comparison of trastuzumab and pertuzumab exists, which is a weakness of the current data. Therefore, it is unknown if single agent activity of pertuzumab is comparable to trastuzumab. On the other hand, single-agent activity of pertuzumab without any chemotherapy backbone has proved to be very limited even in HER2 positive breast cancer. This observation has halted the further investigation of single-agent pertuzumab in breast cancer. Since HER2 overexpression serves as a predictor of trastuzumab activity, it is necessary to search for the biologic correlate that predicts the activity of pertuzumab. Nevertheless, pertuzumab remains one of the most promising agents in the treatment of HER2-positive breast cancer. Clinical trials are currently recruiting subjects to investigate pertuzumab using new regimes that may discontinue the use of chemotherapeutic agents in the future.
Disclosure
The authors report no conflicts of interest in this work.
References
- CobleighMAVogelCLTripathyDMultinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic diseaseJ Clin Oncol19991792639264810561337
- SlamonDEiermannWRobertNAdjuvant trastuzumab in HER2-positive breast cancerN Engl J Med2011365141273128321991949
- BaselgaJTreatment of HER2-overexpressing breast cancerAnn Oncol201021Suppl 7vii36vii4020943641
- SlamonDJLeyland-JonesBShakSUse of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2N Engl J Med20013441178379211248153
- HubalekMBrunnerCMatthaKMarthCResistance to HER2-targeted therapy: mechanisms of trastuzumab resistance and possible strategies to overcome unresponsiveness to treatmentWien Med Wochenschr201016019–2050651220972709
- Lee-HoeflichSTCrockerLYaoEA central role for HER3 in HER2-amplified breast cancer: implications for targeted therapyCancer Res200868145878588718632642
- Diermeier-DaucherSHasmannMBrockhoffGFlow cytometric FRET analysis of erbB receptor interaction on a cell-by-cell basisAnn N Y Acad Sci2008113028028618596360
- FranklinMCCareyKDVajdosFFLeahyDJde VosAMSliwkowskiMXInsights into ErbB signaling from the structure of the ErbB2-pertuzumab complexCancer Cell20045431732815093539
- ChoHSMasonKRamyarKXStructure of the extracellular region of HER2 alone and in complex with the Herceptin FabNature2003421692475676012610629
- AgusDBAkitaRWFoxWDTargeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growthCancer Cell20022212713712204533
- ScheuerWFriessTBurtscherHBossenmaierBEndlJHasmannMStrongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor modelsCancer Res200969249330933619934333
- FriessTScheuerWHasmannMCombination treatment with erlotinib and pertuzumab against human tumor xenografts is superior to monotherapyClin Cancer Res200511145300530916033849
- LoRussoPMWeissDGuardinoEGirishSSliwkowskiMXTrastuzumab emtansine: a unique antibody-drug conjugate in development for human epidermal growth factor receptor 2-positive cancerClin Cancer Res201117206437644722003071
- AgusDBGordonMSTaylorCPhase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancerJ Clin Oncol200523112534254315699478
- AdamsCWAllisonDEFlagellaKHumanization of a recombinant monoclonal antibody to produce a therapeutic HER dimerization inhibitor, pertuzumabCancer Immunol Immunother200655671772716151804
- NgCMLumBLGimenezVKelseySAllisonDRationale for fixed dosing of pertuzumab in cancer patients based on population pharmacokinetic analysisPharm Res20062361275128416715358
- GianniLLladoABianchiGOpen-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancerJ Clin Oncol20102871131113720124183
- BaselgaJGelmonKAVermaSPhase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapyJ Clin Oncol20102871138114420124182
- GianniLPienkowskiTImYHEfficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trialLancet Oncol2012131253222153890
- SchneeweissANeoadjuvant pertuzumab and trastuzumab concurrent or sequential with an anthracycline containing or concurrent with an anthracycline-free standard regimen: a randomized phase II study (TRYPHAENA)Paper presented at the 2011 San Antonio Breast Cancer SymposiumDecember 6–10, 2011San Antonio, TX Abstract S5–S6. Cancer Res. 2011
- BaselgaJCortesJKimSBPertuzumab plus trastuzumab plus docetaxel for metastatic breast cancerN Engl J Med2012366210911922149875
- NahtaRHungMCEstevaFJThe HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cellsCancer Res20046472343234615059883
- Munoz-MateuMUrruticoecheaASeparovicRTrastuzumab plus capecitabine with or without pertuzumab in patients with HER2-positive MBC whose disease has progressed during or following trastuzumab-based therapy for first-line metastatic disease: a multicenter, randomized, two-arm, phase II study (PHEREXA)J Clin Oncol201129Suppl Abstr TPS118
- EllisPABarriosCHImYPatreMBranleFPerezEAMARIANNE: a phase III, randomized study of trastuzumab-DM1 (T-DM1) with or without pertuzumab (P) compared with trastuzumab (H) plus taxane for first-line treatment of HER2-positive, progressive, or recurrent locally advanced or metastatic breast cancer (MBC)J Clin Oncol201129Suppl Abstr TPS102
- HurvitzSAPhase II trial on the efficacy of single-agent T-DM1 compared to trastuzumab + docetaxel in the first-line treatment of HER2-positive locally advanced or MBCAbstract 5.001: Proceedings of the European Multidisciplinary Cancer Congress (ECCO/ESMO)2011 Sep 23–27Stockholm, Sweden
- von MinckwitzGBalsegaJBradburyIAdjuvant pertuzumab and Herceptin in initial therapy of breast cancer: APHINITYCancer Res20117124 Suppl602S
- LeeKFSimonHChenHBatesBHungMCHauserCRequirement for neuregulin receptor erbB2 in neural and cardiac developmentNature199537865553943987477377
- GassmannMCasagrandaFOrioliDAberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptorNature199537865553903947477376
- CamenischTDSchroederJABradleyJKlewerSEMcDonaldJAHeart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2-ErbB3 receptorsNat Med20028885085512134143
- ZhaoYYSawyerDRBaligaRRNeuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytesJ Biol Chem19982731710261102699553078
- PerezEACardiac toxicity of ErbB2-targeted therapies: what do we know?Clin Breast Cancer20088Suppl 3S114S12018777950
- LenihanDSuterTBrammerMNeateCRossGBaselgaJPooled analysis of cardiac safety in patients with cancer treated with pertuzumabAnn Oncol201223379180021665955
- PorteraCCWalsheJMRosingDRCardiac toxicity and efficacy of trastuzumab combined with pertuzumab in patients with [corrected] human epidermal growth factor receptor 2-positive metastatic breast cancerClin Cancer Res20081492710271618451236
- BaselgaJCortesJFumoleauPPertuzumab and trastuzumab: re-responses to 2 biological agents in patients with HER2-positive breast cancer which had previously progressed during therapy with each agent given separately: a new biological and clinical observationCancer Res20096924 Suppl Abstract 5114