Abstract
The number of targeted treatments has risen exponentially over the last few years and is an important concept in the fight against cancer. This review will concentrate on some of the main treatments targeting aberrant pathways which have been tested mainly in the Phase I/II setting. These include human epidermal growth factor receptor 2 inhibitors, drug-antibody conjugates, epidermal growth factor receptor inhibitors, vascular endothelial growth factor inhibitors, reticular activating system, mammalian target of rapamycin and multi-kinase inhibitors. Further knowledge of these pathways and the predictors of response to them will enable personalized medicine to become a reality.
Introduction
With the discovery of estrogen receptors, targeted treatments became a reality. Since then, attention has turned to molecular pathways and alternative receptors as potential targets. As our knowledge of the mechanisms behind cancer cell development has improved, so too has our ability to develop therapies that can inhibit aberrant pathways. This review will examine some of the main drugs that have been investigated during the last few decades.
HER-2 receptor
The neu oncogene was first discovered in 1984 by Schechter et al.Citation1 It is a member of the epidermal growth factor receptor family (EGFR, see ) and is encoded by the proto-oncogene “v-erb-b2 erythroblastic leukemia viral oncogene homolog 2, neuro/glioblastoma derived oncogene homolog (avian).” The human equivalent is her-2, which is found on chromosome 17q21.1.Citation2 HER2 overexpression, found in approximately 22% of breast cancers,Citation3 is a marker for a more aggressive phenotype with increased growth rates, increased likelihood of early metastasis, and decreased overall survival (OS).Citation2
Figure 1 Human epidermal growth factor receptor (HER) and poly (ADP-ribose) polymerase (PARP).
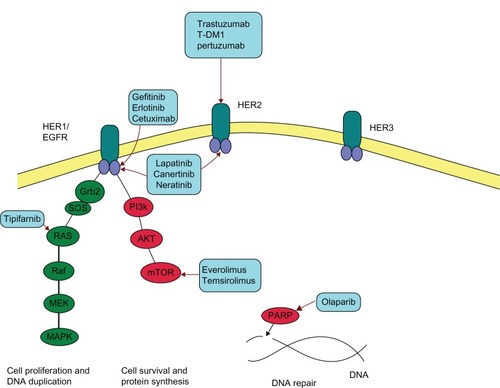
The discovery of HER2 has enabled a variety of targeted therapies to be developed. The first of these was trastuzumab (Herceptin®), a monoclonal antibody which binds to the extracellular juxtamembrane protein of HER2, therefore blocking its ability to promote cellular proliferation and survival.Citation4 Slamon et al conducted a Phase III trial in the metastatic setting which found a statistically significant improvement in overall response rate (ORR) (50% versus 32%, P < 0.001), progression free survival (PFS) (7.4 months versus 4.6 months P < 0.001) and, importantly, overall survival (OS) (25.1 months versus 20.3 months, P = 0.046) when trastuzumab was combined with different chemotherapy regimens.Citation2 As a result of this study, a new standard of care for patients with metastatic HER2 positive (HER2+) breast cancer was defined.
In the adjuvant setting, a number of large Phase III studies again showed benefit for adding trastuzumab to standard chemotherapy.Citation5 The NCCTG, N9831, and NSABP B-31 trials examined doxorubicin, cyclophosphamide, and paclitaxel with or without trastuzumab following surgery for HER2+ breast cancers.Citation5 In the combined analysis, for a total of 3969 participants at median follow up of 2.9 years (range up to 6.4 years), the 4-year disease-free survival (DFS) rate was 85.9% in the trastuzumab arm compared with 73.1% in the non-trastuzumab arm; hazard ratio (HR) 0.49 (P < 0.0001; 95% confidence interval [CI]: 0.41–0.58). The four-year OS for the combination with trastuzumab was 92.6% versus 89.4% without; HR 0.63 (P = 0.0004; 95% CI 0.49–0.81).Citation5
The multicenter HERA trial randomized 5102 women who had received locoregional therapy and a minimum of four courses of adjuvant or neoadjuvant chemotherapy to receive either observation alone (number [n] = 1698), trastuzumab for 1 year (n = 1703) or 2 years (n = 1701).Citation6 At median follow up of 23.5 months (range 0–48 months), the 1-year trastuzumab arm had a 3-year DFS of 80.6% compared with 74.3% in the observation arm; HR 0.63 (95% CI 0.53–0.75; P < 0.0001). The 3-year OS was 2.7% better with 1-year trastuzumab compared with observation; HR 0.63 (0.45–0.87; P = 0.0051).Citation6
Finally, the BCIRG 006 study compared combinations of trastuzumab with anthracycline or non-anthracycline based chemotherapy.Citation7 The investigators randomized 3222 women with early stage breast cancer HER2+ following surgery to receive either docetaxel, cyclophosphamide, and doxorubicin (AC-T) or AC-T plus trastuzumab or docetaxel, carboplatin and trastuzumab (TCH). Both trastuzumab containing regimens had significantly improved PFS and OS compared with AC-T alone. There was no significant difference in OS or PFS between the two trastuzumab regimens, but there was greater congestive cardiac failure seen in the anthracycline containing regimen compared with TCH (2.0% versus 0.4% P < 0.001).Citation7 Loss of mean left ventricular ejection fraction (defined as >10% relative loss) was 18.6% in the AC-T plus trastuzumab arm versus 9.4% in the TCH arm (P < 0.001) and this was still present in 33% of those patients at 4 years.Citation7
Pertuzumab
The monoclonal antibody pertuzumab (Perjeta®) also targets HER2 but binds to a different epitope to trastuzumab (subdomain II compared to subdomain IV) preventing dimerization of HER2.Citation8 Normally, subdomain II is responsible for dimerization of HER2 with either other HER2 receptors (homodimerization) or HER1/HER3 receptors (heterodimerization).Citation8 The pairing of receptors results in a cascade of signaling that promotes tumor growthCitation8 and may also affect tumor resistance to therapies.Citation9
A Phase II study comparing two doses of pertuzumab in 78 women with HER2 negative metastatic breast cancer (MBC) whose disease had progressed through up to two lines of previous therapy resulted in limited efficacy.Citation10 Only two patients had a partial response (PR) with 44% (18/41) of patients in the 420 mg dose arm having stable disease (SD) and 38% (14/37) of patients in the 1050 mg arm having SD lasting >12 weeks.Citation10 The authors recommended that pertuzumab should not be used as a single agent in unselected patients.Citation10 However, as pertuzumab and trastuzumab have different mechanisms of action with pertuzumab acting as a dimerization inhibitor of HER2 compared with trastuzumab which inhibits HER2 cleavage, it was hypothesized that they would work synergistically. A Phase II study performed in 66 patients with HER2+ MBC who had previously had trastuzumab in which they received pertuzumab in combination with trastuzumab demonstrated an ORR of 24.2% with 7.6% patients experiencing complete remission (CR) and 16.7% a PR, despite the documented resistance to single agent trastuzumab.Citation11
These results led to the Phase III CLEOPATRA study which randomized 808 patients with previously untreated HER2+ MBC to receive placebo, trastuzumab, and docetaxel or pertuzumab, trastuzumab, and docetaxel.Citation12 The investigators demonstrated a median PFS of 12.4 months in the control arm versus 18.5 months in the pertuzumab group (HR 0.62; 95% CI 0.51–0.75; P < 0.001).Citation12 Interim analysis at median 30 months follow up and 267 deaths (69% of planned events for the final analysis), demonstrated a median OS of 37.6 months for the placebo arm and had not yet been reached for the pertuzumab arm. The HR was significantly in favor of pertuzumab (HR = 0.66; 95% CI 0.52–0.84; P = 0.0008).Citation13 Dual targeting of a single receptor is superior, for HER2 at least, to monotherapy.
Trastuzumab-DM1
Trastuzumab-DM1 (T-DM1) or trastuzumab-emtansine as it is alternatively known, represents an interesting development in targeted chemotherapy delivery. It is an antibody-drug conjugate containing trastuzumab, which is covalently bonded to the chemotherapy agent emtansine.Citation14 Emtansine is derived from maytansine, which binds to microtubules, thereby preventing mitosis. It has previously been shown to have good cytotoxic activity, but its clinical utility was limited by its toxicity. It has however been possible to covalently bind emtansine to trastuzumab with the linker molecule (N-maleimidomethyl) cyclohexane-1-carboxylate.Citation14
In a Phase I trial Krop et al demonstrated a confirmed objective tumor response (OR) in 5/24 heavily pretreated patients with HER2+ metastatic breast cancer (MBC).Citation15 Of the 15 patients treated at the maximum tolerated dose of 3.6 mg/kg, 73% achieved either OR or SD.Citation15
Evidence of its activity was further validated in a Phase II trial of 112 patients with HER2+ MBC, whose disease had progressed through prior treatment with both trastuzumab and chemotherapy (median number of prior treatments was 8, range 2–19).Citation16 ORR in this heavily pretreated population was 25.9% (95% CI 18.4%–34.4%) by independent assessment with a follow up of greater than 12 months. Median PFS was 4.6 months (95% CI 3.9–8.6 months). It was generally well tolerated with the majority of events being grade 2 or less. The commonest grade 3 adverse events (AE) were hypokalemia (8.9%), thrombocytopenia (8.0%), and fatigue (4.5%).Citation16
EMILIA, a Phase III trial comparing T-DM1 with lapatinib combined with capecitabine in 991 HER2+ patients with MBC was recently published.Citation17 Patients had received both prior trastuzumab and a taxane. PFS was significantly better in the T-DM1 arm (9.6 months versus 6.4 months P < 0.001).Citation17 At the second interim analysis of OS at 331 deaths, T-DM1 median OS was 30.9 months versus 25.1 months with lapatinib plus capecitabine P < 0.001. ORR was also greater in the T-DM1 arm (43.6%) than in the lapatinib capecitabine arm (30.8%) P < 0.001. Frequently occurring toxicities were fatigue (45.4%), nausea (42.3%), headache (28.7%), thrombocytopenia (28.7%), and constipation (25.5%).Citation18 The main grade 3 or 4 toxicities seen with T-DM1 were thrombocytopenia (12.9%) with 2% of patients discontinuing the treatment, increased bleeding risk (29.8% for T-DM1 versus 15.8% for lapatinib plus capecitabine) and increased serum transaminases with three patients discontinuing treatment due to this.Citation17
Another Phase III trial, MARIANNE, has recently closed for recruitment and its results are awaited. It is examining T-DM1 plus placebo versus T-DM1 plus pertuzumab versus trastuzumab plus a taxane.Citation19
EGFR inhibitors
EGFR has previously been found to promote tumor cell proliferation. EGFR is also more commonly expressed in triple negative breast cancers and is associated with a poor prognosis.Citation20 A number of EGFR inhibitors have been extensively investigated as both monotherapies or in combination with other treatments.
Gefitinib
Gefitinib is a selective small molecule inhibitor of EGFR, which blocks signaling pathways involved in cell proliferation and growth.
Baselga et al performed a Phase II study examining the efficacy and pharmacodynamics of 500 mg gefitinib daily in 31 patients with MBC which had progressed through one or two previous lines of treatment, with at least 50% of patients having tumors which were EGFR receptor positive.Citation21 Despite finding that at that dose all the tumor biopsies analyzed (16 samples at day 28) had EGFR phosphorylation completely inhibited, none of the patients had CR or PR. SD was observed in 38.7% (12/31) patients and median time to progression (TTP) was 55 days (95% CI 42–88).Citation21 Other studies examining gefitinib monotherapy had a comparable low response rate.Citation22–Citation24
Gefitinib has also been investigated in combination with other agents. In a study by Ciardiello et al,Citation25 patients received gefitinib 250 mg daily with three cycles of docetaxel either at 75 mg/m2 or 100 mg/m2, increasing to six cycles if they had SD/PR/CR after three cycles. Those who had a response continued on gefitinib monotherapy until disease progression or unacceptable toxicity. They found an ORR of 54%, 22/41 patients (95% CI 45%–75%). Interestingly, the majority of responses occurred in estrogen receptor (ER) positive (ER+) tumors (70% of ER+ versus 21% ER; P = 0.01). The main toxicities were grade 3 or 4 neutropenia in 49% of patients, diarrhea in 10%, and rash in 5%. This is in contrast to a study by Engebraaten et al which terminated early due to toxicities including diarrhea and dehydration experienced with combining weekly docetaxel and gefitinib.Citation26
In the neoadjuvant setting Smith et al examined whether adding gefitinib to anastrozole would overcome development of endocrine resistance through observing changes to Ki67 during treatment.Citation27 They found no clinical/biological effect. Carlson et alCitation28 investigated adding gefitinib to fulvestrant (n = 69) or anastrozole (n = 73) as treatment for endocrine therapy naive patients with MBC. They found that the anastrozole arm had greater efficacy with an OS of 30.2 versus 23.8 months in the fulvestrant arm.Citation28
Erlotinib
Erlotinib binds intracellularly to the adenosine triphosphate binding site of the EGFR receptor, blocking downstream signaling and therefore inhibiting cell proliferation and inducing apoptosis.Citation29,Citation30 A number of Phase II studies examining erlotinib have been conducted and are summarized in .Citation29–Citation33 There are currently no Phase III trials examining the role of erlotinib in breast cancer.
Table 1 Phase II studies examining erlotinib
Cetuximab
Two Phase II trials have examined cetuximab; a monoclonal antibody against EGFR. Hobday et al enrolled 19 patients with MBC previously treated with a taxane/anthracycline to receive cetuximab 400 mg/m2 in combination with irinotecan.Citation34 The ORR was 11% (95% CI 1%–33%) with 12 patients progressing within two cycles; therefore the study terminated early.Citation34 Carey et al randomized 102 patients with triple negative MBC to receive either cetuximab alone (n = 31) or in combination with carboplatin (n = 71).Citation35 In the cetuximab monotherapy arm ORR was 6% compared with 17% in the combined arm.Citation35 Median OS was 7.5 months (95% CI 5.0–11.6) and 10.4 months (95% CI 7.7–13.1) respectively. Both arms were well tolerated with the main AEs being rash, infusion reactions, and fatigue.Citation35
Based on the evidence from Baselga,Citation21 it may be that not all tumors may be EGFR-dependent despite having EGFR receptors. To date, studies of EGFR inhibitors in breast cancer have been disappointing.
HER1/HER2 dual inhibitors
Lapatinib
Lapatinib is a dual tyrosine kinase inhibitor of both HER1 and HER2. It is thought that inhibiting both pathways overcomes tumor resistance to blockade of HER2 alone by agents such as trastuzumab.
Three Phase III studies have been conducted examining lapatinib in combination with other treatments.Citation36–Citation39 The international, multicenter, open-label NeoALTTO trial randomized women with HER2+ early stage, >2 cm disease in the neoadjuvant setting to receive either lapatinib (n = 154), trastuzumab (n = 149) or combination treatment for 6 weeks followed by 12 weeks with the addition of paclitaxel.Citation37 Patients had definitive surgery within 4 weeks of completion of treatment. The combination arm achieved significantly better pathological complete response (pCR) 51.3% (95% CI 43.1–59.5 P = 0.0002 versus trastuzumab alone) than the trastuzumab arm 29.5% (22.4–37.5) or lapatinib arm 24.7% (18.1–32.3 P = 0.74 compared with trastuzumab arm) suggesting that dual-targeting of the HER2 receptor may be a superior approach. Because the primary endpoint of the study was pCR at time of surgery, whether higher pCR rates correlate with improved survival for this population of women is uncertain. The combination was, however, well tolerated though rates of discontinuation of treatment were higher in the lapatinib containing arms and were mainly due to excess diarrhea and transaminitis.Citation37
The MA31 Phase III trial has recently published its interim analysis comparing taxane plus lapatinib to taxane plus trastuzumab as first line treatment of HER+ MBC.Citation38 At median follow up of 13.6 months, data were available for 636 patients which showed a decreased PFS with the lapatinib combination versus the trastuzumab combination median PFS 8.8 months versus 11.4 months (HR = 1.33; 95% CI 1.06–1.67; P = 0.01).Citation38
Geyer et al conducted a Phase III study randomizing women with locally advanced (LA) or metastatic HER2+ breast cancer which had progressed following a regimen which included an anthracycline, a taxane, and trastuzumab, to receive capecitabine alone (n = 163) or capecitabine and lapatinib (n = 161).Citation36 After 121 disease progression events, an interim analysis found that the combination group was superior with 49 events compared with 72 in the capecitabine monotherapy group HR 0.49 (95% CI 0.34–0.71; P < 0.001). Median TTP was 8.4 months in the combination group compared with 4.4 months for monotherapy (P < 0.001). ORR was 22% (95% CI 16%–29%) with combination therapy versus 14% (95% CI 9%–21%) with monotherapy (P = 0.09).Citation36 AEs did not lead to significantly more treatment discontinuations in the combination arm, although more diarrhea, rash, and dyspepsia were seen.Citation36 It demonstrated that targeting of the HER2 receptor beyond progression was a useful treatment option and the combination of lapatinib and capecitabine is one standard of care.
This study also demonstrated a lower risk of developing brain metastases (a major clinical problem for patients with HER2+ breast cancer) as the first site of disease progression on the combination arm (4 versus 13 months, P = 0.045).Citation39 It is thought that as it is a small molecule, lapatinib may cross the blood–brain barrier more effectively than other larger agents. A Phase II study investigated the use of lapatinib in patients with HER2+ breast cancer who had progressive brain metastases and had been previously treated with trastuzumab and whole brain radiotherapy.Citation40 The ORR was only 6% but a fifth of patients had a volumetric reduction of >20% in the volume of their brain metastases. On progression, patients were treated with lapatinib and capecitabine in combination with an ORR of 20%, though this improvement may have been due to capecitabine alone.
The CEREBEL trial examined the use of lapatinib as prophylaxis against brain metastases in patients with HER2+ MBC and no central nervous system (CNS) involvement at baseline.Citation41 Patients were randomized to receive either trastuzumab plus capecitabine (n = 218) or lapatinib plus capecitabine (n = 218). Approximately 40% of patients had not received prior trastuzumab. The primary endpoint of CNS relapse was 3% for the lapatinib arm and 4% for trastuzumab. However, both median PFS and OS were reduced in the lapatinib arm compared with trastuzumab; therefore the study was terminated early.Citation41 The efficacy of lapatinib in patients with brain metastases remains of interest, but uncertain. An ongoing study in the United Kingdom (LANTERN) is randomizing patients post radiotherapy for brain metastases to lapatinib and capecitabine or trastuzumab and capecitabine, which may provide further evidence.
Neratinib
Neratinib is an irreversible pan ErbB tyrosine kinase receptor inhibitor. A Phase II study by Burstein et alCitation42 compared patients with MBC who had received prior trastuzumab therapy (n = 66) and those who were trastuzumab naive (n = 70). They were given 240 mg neratinib and the primary endpoint of PFS at 16 weeks was evaluated. ORR was 24% in pre-treated patients (95% CI 14%–36%) and 56% in trastuzumab naive patients (95% CI 43%–69%). The treatment was generally well tolerated with diarrhea as the main grade 3/4 AE. Diarrhea improved as patients received further weeks of treatment. Further studies are ongoing.
mTOR inhibitors
The mTOR protein kinase integrates signaling from Ras and phosphatidylinositol-3-OH kinase (PI3K) resulting in further phosphorylation of downstream proteins in growth signaling pathways which, when behaving abhorrently, drive tumorigenesis.Citation43 Furthermore, mTOR signaling pathways are thought to contribute to anticancer drug resistance.Citation44
Everolimus
Everolimus inhibits mTOR through allosteric binding to mTOR complex 1 (mTORC1). Phase II studies have investigated it in combination with hormone therapy in order to exploit its potential for overcoming hormone resistance.Citation45,Citation46 The BOLERO-2 Phase III randomized study compared exemestane in combination with placebo (n = 239) or everolimus (n = 485) in women with hormone receptor positive in advanced breast cancer which had progressed while receiving prior nonsteroidal aromatase inhibitors.Citation47 The investigators found that the combination treatment had improved median PFS (based on central assessment) of 10.6 months versus 4.1 months with placebo (HR 0.36; 95% CI 0.27–0.47; P < 0.001).Citation47 Data for OS are yet to be presented, although at 12.5 months there were fewer deaths reported with combination treatment; 17.2% versus 22.7% with placebo.Citation48 The improved PFS was at the expense of more grade III toxicities for the combination arm with 19% of patients on the combination arm discontinuing treatment compared to 3% on the placebo/exemestane. The commonest grade III toxicity was stomatitis (8% versus 1%).
As previously stated, mTOR inhibitors may also be effective at overcoming drug resistance to other anti-cancer therapies. BOLERO-1 is looking at the use of everolimus in addition to trastuzumab and paclitaxel in patients with metasatic HER2+ cancer in the first line setting, with BOLERO-3 randomizing patients with metastatic HER2+ breast cancer who have received no more than three lines of cytotoxic therapy to trastuzumab and vinorelbine with or without everolimus. Results from these studies are awaited.
Temsirolimus
Although Phase II studies showed some antitumor activity and a tolerable safety profile, HORIZON, the recent Phase III study of temsirolimus versus placebo in combination with letrozole, was terminated early by the independent data monitoring committee due to lack of efficacy. Patients with hormone receptor positive MBC and no prior exposure to aromatase inhibitors were randomized to receive letrozole in combination with either temsirolimus or placebo. There was no improvement in median PFS with the addition of temsirolimus (HR 0.90 95% CI 0.76–1.07), however more grade 3/4 events were seen (37% versus 24% for placebo).Citation49 Trials of temsirolimus in combination with other drugs are ongoing.
PI3K inhibitors
The PI3K pathway plays an essential role in cell survival, differentiation and growth.Citation50 PI3Ks are activated by cell membrane receptors such as HER2 and insulin-like growth factor receptor resulting in phosphorylation of phosphatidylinositol bisphosphate to phosphatidylinositol triphosphate.Citation50 This enables Protein kinase B or AKT to bind, which activates mTOR resulting in further downstream signaling. Mutations in PI3K are found in 20%–30% of breast cancers, most frequently in hormone receptor positive cancers and less commonly in triple negative tumors.Citation51
In addition, there may be an association between PI3K mutations and hormone therapy resistance,Citation52 which is being investigated further in the Phase I/II study randomizing patients to receive letrozole in combination with XL147 or XL765.Citation50 Phase I/II trials of other PI3K inhibitors are also currently recruiting.
Ras inhibitors
Tipifarnib
Growth factor receptor pathways are integral to cellular signal transduction and growth. A component of these pathways is the attachment of Ras proteins to cell membranes, which enables interaction with membrane receptors and downstream signaling to occur. In order to attach to cellular membranes, new Ras proteins must be modified by farnesylation which involves covalent attachment of farnesyl to a COOH-terminal amino acid sequence on the Ras protein.Citation53 This process is catalyzed by farnesyl protein transferase which is inhibited by tipifarnib. In addition, tipifarnib inhibits estrogen signaling pathways and theoretically may overcome resistance to hormone therapies.Citation54 Therefore, many of the Phase II trials in the metastatic setting have combined tipifarnib with hormone therapy (see ).Citation54–Citation57 Phase III trials are yet to be conducted.
Table 2 Phase II studies of tipifarnib in MBC
PARP inhibitors
Poly(ADP-ribose) polymerase (PARP) is an enzyme responsible for repair of single stranded DNA breaks. Olaparib is a PARP inhibitor which has been investigated in two Phase II studies. Gelmon et al treated 26 patients with advanced triple-negative breast cancer with olaparib 400 mg twice daily.Citation58 There were no ORs, however, in patients who had BRCA mutated cancers; 63% (5/8) had SD for >8 weeks. For those who were BRCA wild type, 30% (7/23) had SD. The most common AEs reported were fatigue, decreased appetite, nausea, and vomiting.Citation58
In another Phase II study examining olaparib in 56 women with BRCA1 or BRCA2 positive breast cancers, an ORR of 41% (95% CI 25%–59%) was achieved in patients receiving a 400 mg twice daily dose (11/27) and 22% (95% CI 11%–41%) in those receiving 100 mg twice daily (7/27).Citation59 Similar AEs were reported and were mainly low grade. A Phase III trial has yet to be developed in breast cancer. Another inhibitor, iniparib which was initially thought to act on PARP, did not show significant improvement of PFS or OS when combined with carboplatin/gemcitabine in a Phase III study in women with pretreated MBC.Citation60 It has subsequently been demonstrated that iniparib is not an inhibitor of PARP and its development has been put on hold.
VEGF inhibitors
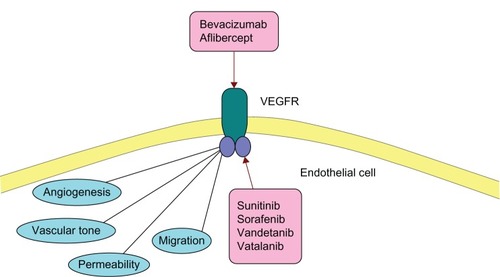
Tumor angiogenesis is thought to play a critical role in the growth and metastasis of tumors in order to supply cancerous cells with the nutrients and oxygen they need to survive. VEGF mediates formation of blood vessels and is known to be over expressed in breast cancer cells (see ).Citation61
Figure 2 Role of VEGFR (vascular endothelial growth factor receptor) in vascularization/angiogenesis and its inhibitors.
Bevacizumab
Bevacizumab is a monoclonal antibody which blocks VEGF from binding to its receptor on endothelial cells inhibiting their proliferation.Citation62 To date, unfortunately, clinical trials using the drug in breast cancer have been relatively disappointing. In the Phase III setting, four large randomized trials have been conducted.Citation63–Citation66
The AVADO trial randomized 736 patients with a local recurrence or metastatic HER2 negative breast cancer or on an intention to treat (ITT) basis to receive docetaxel with either placebo or bevacizumab at 7.5 or 15 mg/kg doses.Citation63 Bevacizumab at 15 mg/kg combined with docetaxel showed a statistically significant (but clinically small) improvement in PFS of median 10.1 months versus 8.2 months with docetaxel plus placebo (HR 0.77 95% CI 0.64–0.93 P = 0.006).Citation63 AEs of hypertension, bleeding, and proteinuria were more commonly seen in the bevacizumab arm, however there were similar numbers of AEs leading to death in both arms (2% in both bevacizumab arms, 3% placebo arm).Citation63
Another open-label trial randomized 722 women with MBC to receive either paclitaxel (n = 354) or paclitaxel plus bevacizumab (n = 368) as first line treatment (prior adjuvant chemotherapy/hormone therapy/trastuzumab was allowed).Citation66 The primary endpoint was PFS and after 624 events, the combined arm had a median PFS of 11.8 months versus 5.6 months in the paclitaxel monotherapy arm (HR 0.60, P < 0.001). ORR was 36.9% for the combination compared to 21.2%, for paclitaxel (P < 0.001).Citation66 Importantly, neither study showed an improvement in OS with the addition of bevacizumab to standard chemotherapy.
Miller et al conducted an open label study which randomized 462 patients with heavily pretreated MBC equally to receive capecitabine alone or in combination with bevacizumab.Citation64 Although they found that there were increased ORRs with the addition of bevacizumab (19.8% versus 9.1%, P = 0.001), this did not translate into improved PFS (4.9 versus 4.2 months, HR 0.98) or OS (15.1 versus 14.5 months). The only more frequent grade 3 or 4 toxicity with the combined arm was hypertension.Citation64
The RIBBON-1 study examined whether the addition of bevacizumab to chemotherapy as first line treatment in locally advanced or metastatic HER2 negative breast cancer improved efficacy.Citation65 Before 2:1 randomization, investigators allocated a patient to receive either capecitabine (n = 615), a taxane based regimen (n = 307), or an anthracycline based combination (n = 315). The addition of bevacizumab improved PFS; in the capecitabine group PFS was 6.2 months with placebo versus 9.8 months with bevacizumab (HR, 0.68; 95% CI 0.54–0.86), in the taxane/anthracycline group PFS was 8.3 months with placebo versus 10.7 months with bevacizumab (HR 0.77; 95% CI 0.60–0.99).Citation65 There was no significant difference in 1 year OS.Citation65 RIBBON-2 examined the addition of bevacizumab to chemotherapy as second line treatment in the setting of HER2 negative locally advanced or MBC; 684 patients were randomized to receive chemotherapy and placebo (n = 225) or chemotherapy and bevacizumab (n = 459).Citation67 The median PFS was improved from 5.1 months with placebo to 7.2 months with bevacizumab (HR 0.78 P = 0.0072).Citation67
There are a number of trials not yet reported, examining bevacizumab in the adjuvant setting based on the hypothesis that anti-angiogenic agents may be most effective as adjuvant treatment through preventing angiogenesis before metastases are established. E5103 is examining how the use of bevacizumab plus taxane and anthracycline chemotherapy after surgery may reduce the risk of recurrence and whether further bevacizumab maintenance adds benefit. BETH has randomized patients with HER2+ cancer to receive trastuzumab and standard chemotherapy plus bevacizumab or placebo. Preliminary results of the BEATRICE trial looking at the addition of bevacizumab to anthracycline/taxane based chemotherapy as adjuvant treatment for triple negative early breast cancer were recently presented at the San Antonio Breast cancer Symposium. At a median follow-up of 32 months, the hazard ratio for invasive disease-free survival was 0.87 (95% CI 0.72–1.07) in favor of patients assigned to chemotherapy and bevacizumab randomizes patients with triple negative breast cancer to receive standard chemotherapy plus placebo or bevacizumab as adjuvant therapy following surgery.Citation68
Aflibercept
Aflibercept is a soluble decoy protein which has high affinity for the family of VEGF which bind to VEGFR-1 and VEGFR-2.Citation20 The only Phase II study reported was terminated early due to lack of efficacy and a PFS of only 2.4 months.Citation20
Multikinase inhibitors
Sorafenib
Sorafenib is a multikinase inhibitor which targets a number of receptors including VEGF receptor-1 (VEGFR-1), VEGFR-2, VEGFR-3, Flt-3, platelet-derived growth factor receptor (PDGFR), Raf kinase, and c-Kit producing both anti-angiogenesis and anti-proliferative effects. Two Phase II studies have examined its potential efficacy in breast cancer. Moreno-Aspitia et al examined the effect on tumor response of sorafenib monotherapy.Citation69 None of the 20 patients assessable for response achieved a CR/PR although two had SD for >6 months.Citation69
Baselga et al randomized 229 patients with HER2 negative LA/MBC to receive capecitabine plus placebo or sorafenib. They found that sorafenib significantly improved PFS; 6.4 months versus 4.1 months (HR 0.58; 95% CI 0.41 to 0.81; P = 0 .001).Citation70 Toxicities of sorafenib at a dose of 400 mg twice daily were unacceptable with 20% of patients discontinuing due to AEs; therefore it was recommended that a lower dose be used in future studies.Citation70
Sunitinib
Sunitinib is also a multikinase inhibitor, blocking VEGFR, ckit, and PDGFR. Three Phase III trials have investigated its use in breast cancer. Barrios et al conducted an open label trial comparing sunitinib with capecitabine in HER2 negative advanced disease. The trial was terminated early when the independent review committee deemed that it would not reach its primary endpoint of improved PFS with sunitinib compared to capecitabine.Citation71 Nor was PFS or OS improved when used in combination with capecitabine versus capecitabine alone in another Phase III trial randomizing patients with HER2+/− advanced disease.Citation72 Finally, Bergh et al also reported negative results in their Phase III study in patients with HER2− LA/MBC comparing sunitinib in combination with paclitaxel (n = 296) versus paclitaxel alone (n = 297).Citation73 Although ORR was significantly increased with the addition of sunitinib (55% versus 42% P = 0.001), there was no significant increase in PFS or OS.Citation73 RESILIENCE, a Phase III trial examining sunitinib in combination with capecitabine is currently recruiting.
Vandetanib
Vandetanib is an inhibitor of VEGFR and EGFR as well as RET tyrosine kinase. Two trials have investigated it in the Phase II setting and further studies are ongoing. Miller et al enrolled 46 patients with previously treated MBC to receive vandetanib in a dose-finding study.Citation74 They found that it was well tolerated with the main toxicities reported as rash, prolongation of QTc interval, and diarrhea which appeared to be dose dependent. There were no ORs and one patient had SD for >24 weeks.
Boér et al randomized 64 patients with pretreated MBC on an ITT basis to receive docetaxel with vandetanib (n = 35) or docetaxel with placebo (n = 29).Citation75 They found no clinical benefit for the addition of vandetanib, however it was generally reasonably tolerated with 15 patients discontinuing due to AEs including diarrhea and neutropenia in the vandetanib arm compared to 10 with placebo.Citation75
Vatalanib
Vatalanib is a VEGFR inhibitor and at higher concentrations inhibits other tyrosine kinases such as PDGF, c-kit, and c-Fms.Citation76 The Hoosier oncology group conducted a Phase I/II study of vatalanib in combination with trastuzumab in patients with HER2+ MBC, but the trial was terminated due to low patient enrolment and toxicities.Citation77
Future aims
The evolution of targeted therapies is an important step in the creation of individualized cancer management. The possible targets for therapies multiply, at the same time as our understanding of the mechanisms underlying cancer improves. However, better understanding of which patients will gain most benefit from targeted treatments is still required. Focus is currently on developing robust biomarkers in order to aid prediction of response so that in future, patients will receive only treatments that will confer an advantage.
Disclosure
Dr Wardley has been paid honoraria from Celgene, Easai, GlaxoSmithKline, Novartis and Roche and has received travel assistance from Roche. The authors have no other conflicts of interest in this work.
References
- SchechterALSternDFVaidyanathanLThe neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigenNature198431259945135166095109
- SlamonDGodolphinWJonesLStudies of the HER-2/neu proto-oncogene in human breast and ovarian cancerScience198924449057077122470152
- RossJSSlodkowskaEASymmansWFPusztaiLRavdinPMHortobagyiGNThe HER-2 receptor and breast cancer: ten years of targeted anti–HER-2 therapy and personalized medicineThe Oncologist200914432036819346299
- HudisCATrastuzumab – mechanism of action and use in clinical practiceN Engl J Med20073571395117611206
- PerezEARomondEHSumanVJUpdated results of the combined analysis of NCCTG N9831 and NSABP B-31 adjuvant chemotherapy with/without trastuzumab in patients with HER2-positive breast cancerPaper presented at: ASCO Annual Meeting Proceedings Part I2007San Francisco
- SmithIProcterMGelberRD2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trialLancet20073699555293617208639
- SlamonDEiermannWRobertNAdjuvant trastuzumab in HER2-positive breast cancerN Engl J Med2011365141273128321991949
- AgusDBGordonMSTaylorCPhase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancerJ Clin Oncol200523112534254315699478
- ChenXYeungTKWangZEnhanced drug resistance in cells coexpressing ErbB2 with EGF receptor or ErbB3Biochem Biophys Res Commun2000277375776311062025
- GianniLLladóABianchiGOpen-label, Phase II, multicenter, randomized study of the efficacy and safety of two dose levels of pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2–negative metastatic breast cancerJ Clin Oncol20102871131113720124183
- BaselgaJGelmonKAVermaSPhase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer that progressed during prior trastuzumab therapyJ Clin Oncol20102871138114420124182
- BaselgaJCortésJKimS-BPertuzumab plus trastuzumab plus docetaxel for metastatic breast cancerN Engl J Med2012366210911922149875
- SwainSMKimSBCortesJConfirmatory overall survival (OS) analysis of CLEOPATRA: a randomized, double-blind, placebo-controlled Phase III study with pertuzumab (P), trastuzumab (T), and docetaxel (D) in patients (pts) with HER2-positive first-line (1L) metastatic breast cancer (MBC)Cancer Research20127224 Suppl 3 abstract P5-18-26
- Lewis PhillipsGDLiGDuggerDLTargeting HER2-positive breast cancer with trastuzumab-DM1, an antibody–cytotoxic drug conjugateCancer Research200868229280929019010901
- KropIEBeeramMModiSPhase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancerJ Clin Oncol201028162698270420421541
- KropIELoRussoPMillerKDA Phase II study of trastuzumab emtansine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer who were previously treated with trastuzumab, lapatinib, an anthracycline, a taxane, and capecitabineJ Clin Oncol201230263234324122649126
- VermaSMilesDGianniLTrastuzumab emtansine for HER2-positive advanced breast cancerN Engl J Med2012367191783179123020162
- DiérasVHarbeckNBuddGTTrastuzumab emtansine in HER2-positive metastatic breast cancer: pooled safety analysis from seven studiesCancer Research20127224 Suppl 3P5-18-06
- EllisPBarriosCImYPatreMBranleFPerezEMARIANNE: A phase III, randomized study of trastuzumab-DM1 (T-DM1) with or without pertuzumab (P) compared with trastuzumab (H) plus taxane for first-line treatment of HER2-positive, progressive, or recurrent locally advanced or metastatic breast cancer (MBC)J Clin Oncol201129Suppl abstr TPS102
- AlvarezRHValeroVHortobagyiGNEmerging targeted therapies for breast cancerJ Clin Oncol201028203366337920530283
- BaselgaJAlbanellJRuizAPhase II and tumor pharmacodynamic study of gefitinib in patients with advanced breast cancerJ Clin Oncol200523235323533315939921
- RobertsonJGutteridgeECheungKOwersRGefitinib (ZD1839) is active in acquired tamoxifen (TAM)-resistant oestrogen receptor (ER)-positive and ER-negative breast cancer: results from a phase II studyProc Am Soc Clin Oncol200322 abstr 23
- AlbainKElledgeRGradisharWJNataleRBOpen-label, phase II, multicenter trial of ZD 1839 (‘Iressa’) in patients with advanced breast cancerBreast Cancer Research And Treatment200276(S33)Suppl 1 abstr 20
- von MinckwitzGJonatWFaschingPA multicentre phase II study on gefitinib in taxane- and anthracycline-pretreated metastatic breast cancerBreast Cancer Res Treat200589216517215692759
- CiardielloFTroianiTCaputoFPhase II trial of gefitinib combined with docetaxel as first-line therapy in patients with metastatic breast cancerBr J Cancer200694111604160916685276
- EngebraatenOEdvardsenHLøkkevikEGefitinib in combination with weekly docetaxel in patients with metastatic breast cancer caused unexpected toxicity: results from a randomized Phase II clinical trialISRN Oncology201220128
- SmithIEWalshGSkeneAA Phase II placebo-controlled trial of neoadjuvant anastrozole alone or with gefitinib in early breast cancerJ Clin Oncol200725253816382217679728
- CarlsonRWO’NeillAVidaurreTGomezHLBadveSRandomized phase II trial of gefitinib plus anastrozole or fulvestrant in postmenopausal, metastatic breast cancerJ Clin Oncol20092715S1013
- TwelvesCTrigoJMJonesRErlotinib in combination with capecitabine and docetaxel in patients with metastatic breast cancer: a dose-escalation studyEur J Cancer200844341942618249110
- DicklerMNRugoHSEberleCAA phase ii trial of erlotinib in combination with bevacizumab in patients with metastatic breast cancerClin Cancer Res200814237878788319047117
- KaurHSilvermanPSinghDCooperBWFuPKrishnamurthiSToxicity and outcome data in a phase II study of weekly docetaxel in combination with erlotinib in recurrent and/or metastatic breast cancer (MBC)J Clin Oncol20062418S10623
- MontagnaECancelloGBagnardiVMetronomic chemotherapy combined with bevacizumab and erlotinib in patients with metastatic HER2-negative breast cancer: clinical and biological activityClin Breast Cancer201212320721422520733
- GrahamDLHillmanDWHobdayTJN0234: Phase II study of erlotinib (OSI-774) plus gemcitabine as first-or second-line therapy for metastatic breast cancer (MBC)J Clin Oncol20052316S64415659512
- HobdayTJStellaPJFitchTRN0436: a phase II trial of irinotecan plus cetuximab in patients with metastatic breast cancer and prior anthracycline and/or taxane-containing therapyJ Clin Oncol200826Suppl abstr 1081
- CareyLARugoHSMarcomPKTBCRC 001: randomized phase ii study of cetuximab in combination with carboplatin in Stage IV triple-negative breast cancerJ Clin Oncol201230212615262322665533
- GeyerCEForsterJLindquistDLapatinib plus Capecitabine for HER2-Positive Advanced Breast CancerNew England Journal of Medicine2006355262733274317192538
- BaselgaJBradburyIEidtmannHLapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trialThe Lancet3799816633640
- GelmonKABoyleFKaufmanBOpen-label phase III randomized controlled trial comparing taxane-based chemotherapy (Tax) with lapatinib (L) or trastuzumab (T) as first-line therapy for women with HER2+ metastatic breast cancer: Interim analysis (IA) of NCIC CTG MA.31/GSK EGF 108919J Clin Oncol201230Suppl 15 abstract LBA671
- CameronDCaseyMPressMA phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res TreatDec20081123533543
- LinNUDiérasVPaulDMulticenter Phase II Study of Lapatinib in Patients with Brain Metastases from HER2-Positive Breast Cancer. Clinical Cancer ResearchFebruary 15200915414521459
- PivotXSemiglazovVŻurawskiBCEREBEL (EGF111438): An open label randomized phase III study comparing the incidence of CNS metastases in patients (pts) with HER2+ Metastatic Breast Cancer (MBC), treated with Lapatinib plus Capecitabine (LC) versus Trastu-zumab plus Capecitabine (TC)Annals of Oncology201223Suppl 9 Abstract LBA11
- BursteinHJSunYDirixLYNeratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancerJ Clin Oncol20102881301130720142587
- ShawRJCantleyLCRas, PI(3)K and mTOR signalling controls tumour cell growthNature2006441709242443016724053
- JiangBHLiuLZRole of mTOR in anticancer drug resistance: perspectives for improved drug treatmentDrug Resistance Updates: Reviews and Commentaries in Antimicrobial and Anticancer Chemotherapy2008113637618440854
- BachelotTBourgierCCropetCRandomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor–positive, human epidermal growth factor receptor 2–negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO StudyJ Clin Oncol201230222718272422565002
- BaselgaJSemiglazovVvan DamPPhase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor–positive breast cancerJ Clin Oncol200927162630263719380449
- BaselgaJCamponeMPiccartMEverolimus in postmenopausal hormone-receptor–positive advanced breast cancerN Engl J Med2012366652052922149876
- Piccart-GebhartMJNoguchiSPritchardKIEverolimus for postmenopausal women with advanced breast cancer: Updated results of the BOLERO-2 phase III trialJ Clin Oncol201230Suppl abstr 559
- WolffACLazarAABondarenkoIRandomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancerJ Clin Oncol201331219520223233719
- BaselgaJTargeting the phosphoinositide-3 (PI3) kinase pathway in breast cancerOncologist201116Suppl 1121921278436
- Stemke-HaleKGonzalez-AnguloAMLluchAAn integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancerCancer Research200868156084609118676830
- TokunagaEKimuraYMashinoKActivation of PI3K/Akt signaling and hormone resistance in breast cancerBreast Cancer200613213714416755107
- EndDWSmetsGToddAVCharacterization of the antitumor effects of the selective farnesyl protein transferase inhibitor R115777 in vivo and in vitroCancer Research200161113113711196150
- DalencFDoisneau-SixouSFAllalBCTipifarnib plus tamoxifen in tamoxifen-resistant metastatic breast cancer: a negative phase II and screening of potential therapeutic markers by proteomic analysisClin Cancer Res20101641264127120145184
- LiTGuoMGradisharWA phase II trial of capecitabine in combination with the farnesyltransferase inhibitor tipifarnib in patients with anthracycline-treated and taxane-resistant metastatic breast cancer: an Eastern Cooperative Oncology Group Study (E1103)Breast Cancer Res Treat2012134134535222547107
- LiTChristosPJSparanoJAPhase II trial of the farnesyltransferase inhibitor tipifarnib plus fulvestrant in hormone receptor-positive metastatic breast cancer: New York Cancer Consortium Trial P6205Ann Oncol200920464264719153124
- JohnstonSSemiglazovVManikhasGA phase II, randomized, blinded study of the farnesyltransferase inhibitor tipifarnib combined with letrozole in the treatment of advanced breast cancer after antiestrogen therapyBreast Cancer Res Treat2008110232733517851757
- GelmonKATischkowitzMMackayHOlaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised studyLancet Oncol201112985286121862407
- TuttARobsonMGarberJEOral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trialLancet376973723524420609467
- O’ShaughnessyJSchwartzbergLSDansoMAA randomized phase III study of iniparib (BSI-201) in combination with gemcitabine/carboplatin (G/C) in metastatic triple-negative breast cancer (TNBC)J Clin Oncol201129Suppl 15 abstr 1007
- RelfMLeJeuneSScottPAEExpression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor β-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesisCancer Res19975759639699041202
- ShihTLindleyCBevacizumab: an angiogenesis inhibitor for the treatment of solid malignanciesClin Ther200628111779180217212999
- MilesDWChanADirixLYPhase III Study of Bevacizumab Plus Docetaxel Compared With Placebo Plus Docetaxel for the First-Line Treatment of Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast CancerJ Clin Oncol201028203239324720498403
- MillerKDChapLIHolmesFARandomized Phase III Trial of Capecitabine Compared With Bevacizumab Plus Capecitabine in Patients With Previously Treated Metastatic Breast CancerJ Clin Oncol200523479279915681523
- RobertNJDiérasVGlaspyJRIBBON-1: randomized, double-blind, placebo-controlled, phase iii trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2–negative, locally recurrent or metastatic breast cancerJ Clin Oncol201129101252126021383283
- MillerKWangMGralowJPaclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancerN Engl J Med2007357262666267618160686
- BrufskyAHurvitzSAPerezEAFinal overall survival (OS) and safety analyses of RIBBON-2, a randomised phase III trial of bevacizumab (BEV) versus placebo (PL) combined with second line chemotherapy (CT) for HER2-negative-BEV-naive metastatic breast cancer (MBC)J Clin Oncol201230Suppl 27 abstr 100
- CameronDBrownJDentRPrimary results of BEATRICE, a randomized phase III trial evaluating adjuvant bevacizumab-containing therapy in triple-negative breast cancerCancer Research20127224, Suppl 3 Available from: http://cancerres.aacrjournals.org/cgi/content/short/72/24_MeetingAbstracts/S6-5?rss=1Accessed May 20, 2013
- Moreno-AspitiaAMortonRFHillmanDWPhase II trial of sorafenib in patients with metastatic breast cancer previously exposed to anthracyclines or taxanes: North Central Cancer Treatment Group and Mayo Clinic Trial N0336J Clin Oncol2009271111519047293
- BaselgaJSegallaJGMRochéHSorafenib in combination with capecitabine: an oral regimen for patients with HER2-negative locally advanced or metastatic breast cancerJ Clin Oncol201230131484149122412143
- BarriosCHLiuMCLeeSCPhase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2-negative advanced breast cancerBreast Cancer Res Treat2010121112113120339913
- CrownJDierasVStaroslawskaEPhase III trial of sunitinib (SU) in combination with capecitabine (C) versus C in previously treated advanced breast cancer (ABC)J Clin Oncol201028Suppl abstr 18
- BerghJBondarenkoIMLichinitserMRFirst-line treatment of advanced breast cancer with sunitinib in combination with docetaxel versus docetaxel alone: results of a prospective, randomized phase III studyJ Clin Oncol201230992192922331954
- MillerKDTrigoJMWheelerCA multicenter phase II trial of ZD6474, a vascular endothelial growth factor receptor-2 and epidermal growth factor receptor tyrosine kinase inhibitor, in patients with previously treated metastatic breast cancerClin Cancer Res20051193369337615867237
- BoérKLángILlombart-CussacAVandetanib with docetaxel as second-line treatment for advanced breast cancer: a double-blind, placebo-controlled, randomized phase II studyInvest New Drugs201230268168720830502
- WoodJMBoldGBuchdungerEPTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administrationCancer Res20006082178218910786682
- US National Institutes of HealthPTK787 + Trastuzumab for HER2 Overexpressing Metastatic Breast Cancer Available from: http://clinicaltrials.gov/ct2/show/results/NCT00216047Accessed June 23rd, 2013