Abstract
Mounting evidence indicates that anomalies in the inflammatory and immune response pathways are essential to tumorigenesis. However, tumor-based innate immunity initiated by transformed breast epithelia tissues has received much less attention. This review summarizes published reports on the role of the toll-like receptor signaling pathway on breast cancer risk, disease progression, survival, and disease recurrence. Specifically, we discuss the underlying biological mechanisms that contribute to the tumorigenic and/or anti-tumorigenic properties of toll-like receptors and their associated agonists in relation to breast tumorigenesis and cancer treatment. Further, we use results from preclinical, clinical, and population-based studies as prompts for the exploration of new and more effective breast cancer therapies. As the knowledge base of innate immunity’s involvement in breast cancer progression increases, current and new immune-modifying strategies will be refined to effectively treat breast cancer.
Introduction
Breast cancer, a heterogeneous disease, is a major public health issue for women worldwide. Moreover, it is the leading cause of cancer-related death among women in the USA. In 2012, the American Cancer Society estimated that 226,800 women would receive a breast cancer diagnosis and 39,500 would die from the disease.Citation1 Although there have been improvements in the early detection of breast cancer, a 23% 5-year survival rate plagues women with distant stage disease.Citation2 While breast cancer incidence rates are higher among women of European rather than African descent in the USA, African-American women are more likely to die from this disease. Major breast cancer risk factors include increasing age, overweight/obesity, weight gain after age 18, hormone replacement therapy, alcohol consumption, high breast tissue density, high bone mineral density, exposure to high-dose radiation to the chest, null parity, long menstrual history, having one’s first child after age 30, family history of breast cancer, and inheritance of susceptibilities in high (BRAC1, BRAC2) and low penetrance genes. Recently, studies have suggested that imbalances in inflammatory and immune-associated proteins may also contribute to breast cancer and disease progression.Citation3–Citation6
The immune system consists of both innate and adaptive immune response pathways.Citation7,Citation8 Adaptive immunity is a finely tuned, highly selective response mechanism mediated by antigen-specific T and B lymphocytes in response to infection. In contrast, the evolutionary conserved innate immune system undergoes activation in response to nonspecific exogenous and endogenous insults that possess pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). Of the two inflammatory divisions, innate immune responses serve as the first line of defense.
Several cellular mechanisms, including the toll-like receptor (TLR) pathway, play a crucial role in the innate immune system.Citation9,Citation10 In particular, TLR signaling pathways promote survival, proliferation and apoptosis, as well as interferon (IFN), cytokine, and chemokine production. However, there is strong evidence showing that infectious agents activate TLRs and promote tumor progression in gastric cancer, Kaposi’s sarcoma, hematological malignancies, and prostate cancer.Citation11–Citation13 Although pathogenic-induced breast cancer remains inconclusive,Citation14–Citation20 TLR ligand stimulation can either enhance or inhibit tumor growth, as summarized in .
Table 1 Impact of compounds that affect toll-like receptor TLR2 and TLR4 activity or expression in breast cancer
Recently, single nucleotide polymorphisms (SNPs) within the TLR signaling pathway have garnered a great deal of attention as candidate cancer detection, prognostication, and clinical management tools. It is speculated that genetic susceptibilities in TLR targets may alter the sensitivity of TLRs to their ligands, which may modify downstream signaling, including release of inflammatory and immune responses that favor tumor development. While it is feasible that TLR sequence variants are related to breast cancer outcomes, this research focus remains understudied.
In this review, we evaluate the TLR signaling pathway in relation to TLR breast cancer risk, disease progression, and tumor behavior. In particular, we highlight the clinical impact of synthetic TLR ligands alone or in combination with other therapeutic regimens on breast tumorigenesis. Lastly, we propose future research avenues to explore the contribution of innate immune mechanisms that support tumor progression and new treatment strategies that target these pathways.
TLR signaling pathway
TLRs are essential type I transmembrane glycoproteins that regulate inflammatory responses against harmful pathogens, as shown in .Citation21–Citation24 In humans, at least ten members have been identified and categorized as cell surface or intracellular TLRs based on their substrate specificity. TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are cell surface receptors, while TLR3, TLR7, TLR8, and TLR9 are embedded in lysosomal, endosomal, as well as endoplasmic reticulum membranes.Citation15 Cell surface receptors primarily recognize lipid or protein PAMPs and DAMPs in response to infection and tissue damage, respectively.Citation25 In contrast, secretory pathway receptors are triggered by DAMPs and nucleic acids, which are presumably released during cell lysis and sequestered in endolysosomes for degradation.Citation26–Citation28 Accessory molecules for TLR ligand recognition, subcellular localization and/or function have been identified for TLR2, TLR3, TLR4, TLR6, TLR7 and TLR9 (reviewed in Lee et al, 2012).Citation29
Figure 1 The toll-like receptor (TLR) signaling pathway is initiated via activation of TLRs, followed by adaptor complex formation, interleukin-1R-associated kinase (IRAK) and/or tumor necrosis factor receptor-associated factor 6 (TRAF6) activation to induce subsequent mitogen-activated protein kinase, nuclear factor-kappa B (NF-κB), and interferon-regulatory factor (IRF) activation, nuclear translocation, and regulation of pro- or anti-inflammatory gene expression.
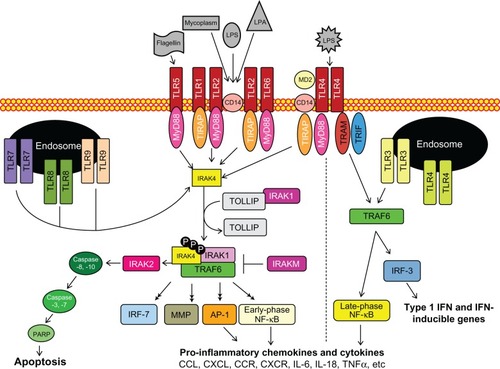
With the exception of TLR3, which uses the MyD88-independent pathway and TLR4, which uses both the MyD88-dependent and -independent pathways, activated TLRs signal via the MyD88-dependent pathway. Following stimulation, TLRs bind to the sorting adaptor complex toll/interleukin receptor homology domain adaptor protein/myeloid differentiation factor 88 adapter-like protein (TIRAP/MAL), which recruits MyD88 and subsequently activates regulatory serine/threonine interleukin-1Receptor-associated kinases (IRAKs). Four IRAKs participate in TLR signaling, namely IRAK1, IRAK2, IRAKM (IRAK3), and IRAK4. IRAK4 is the central regulatory, kinase, signaling its own autophosphorylation as well as phosphorylation of IRAK1, IRAK2, and tumor necrosis factor receptor-associated factor 6 (TRAF6), as depicted in .Citation30–Citation32 After IRAK1 dissociates from toll-interacting protein (TOLLIP) it binds and activates phospho-TRAF6, which in turn activates mitogen-activated protein (MAP) kinases and nuclear factor-kappa B (NF-κB). As a result, several transcription factors, including NF-κB and activator protein-1, undergo nuclear translocation, resulting in the secretion of proinflammatory chemokines and cytokines (ie, tumor necrosis factor-α, interleukin [IL]-6, and IL-8)Citation33–Citation36 as well as the induction of interferon-regulatory factors (IRFs, IRF1, IRF3, IRF5, IRF7, and IRF8) and matrix metalloproteinases (MMPs). Besides chemokine and cytokine production, IRAK activation stimulates the Fas-associated protein with death domain (FADD) expression and thereby promotes programmed cell death (apoptosis) via activation of caspase-8 and -10.Citation37,Citation38
The MyD88-independent signaling pathway via activated TLR3 and TLR4 recruits the sorting adaptor toll/interleukin receptor domain-containing adaptor inducing interferon (TRIF) and the signaling adaptor toll/interleukin receptor domain-containing adaptor inducing interferon-related adaptor molecule (TRAM). Association of the TRIF/TRAM complex coupled with ligand-bound TLR3 or TLR4 upregulates IRF3/7 via NF-κB activation but intermediates in the MyD88-independent signaling pathway have not been well-characterized. The targeted activation/inhibition of either MyD88-dependent or -independent pathways should prove a useful approach to altering the innate immune system’s complex autocrine and paracrine induction of growth and immune cell recruitment and tumor cell biology.
The TLR signaling pathway and breast tumorigenesis
Several TLRs, expressed in breast cancer cell lines and/or archival tissue, have been evaluated in relation to tumor behavior. Xie et al observed modest mRNA levels of TLR1, TLR2, TLR3, TLR5, TLR6, and TLR8 but not of TLR4, 7, 9, and 10 in MCF-7 and MDA-MB-231 cells.Citation39 Moreover, higher levels of TLR2 or its gene product were present in invasive MDA-MB-231 breast cancer cell lines when compared with MCF-7 cells, based on quantitative reverse transcriptase polymerase chain reaction, (RT-PCR) protein analysis, and flow cytometry. In MDA-MBA-231 cells only, TLR2 inactivation positively influenced cell invasion through a variety of mechanisms including: NF-κB activation, which plays a central role in breast cancer progression;Citation40,Citation41 induction of phosphorylation of nuclear hormone receptor NR2C2 and I kappa B α in the TLR2/NF-κB signaling pathway; and secretion of IL-6, VEGF and, MMP-9. TLR3 activation inhibited tumor growth by inducing pro-apoptotic and inhibiting cell proliferation in in vitro and in vivo studies.Citation42,Citation43 Unlike Xie et al analysis of all TLRs in MDA-MB-231 cells, Yang et al confirmed the presence of only four TLR mRNAs and proteins (TLR4 > TLR6 > TLR5 > TLR7), in this cell line.Citation44 Yang et al also demonstrated that TLR4 silencing inhibited MDA-MB-231 cell proliferation and inflammatory IL-6 and IL-8 secretion.Citation44 Commensurate with Yang’s findings, González-Reyes et al revealed that high expression of TLR4 was associated with disease recurrence, large tumor size, and distant metastatic disease in a case-only study of 74 women.Citation45 In this same study, TLR3 and TLR9 were also linked to tumor size and tumor stage, respectively. In two independent studies, expression and/or ligand stimulation of TLR9 by CpG oligonucleotides promoted: cell migration in an estrogen-receptor negative BT-20 cell line,Citation46 cell invasion mediated by MMP-13 in MDA-MB-231 breast cancer cells but not MCF-7 cells,Citation47 and aggressive tumor behavior.Citation46 Notably, TLR9 expression in 124 breast cancer tissue specimens collected from women correlated with estrogen-receptor negative/progesterone-receptor negative status, high tumor grade, and NF-κB expression.Citation47 Conversely, overexpression of TLR9 in fibroblast cells was associated with low propensity toward metastatic breast cancer.Citation45 The conflicting pro- and anti-tumorigenic properties of TLR9 may be attributed to its role in both cell survival and apoptosis, respectively;Citation48–Citation50 therefore, targeting TLR9 requires evaluation and validation in preclinical studies. The role of TLRs in tumor development and inhibition may depend on the cell type, tumor subtype, tumor microenvironment, and the metabolic condition of the cell.
Collectively, these reports suggest that the TLR signaling pathway in breast tumor cells may play a supporting role in the secretion of proinflammatory cytokines/chemokines, aggressive tumor behavior (eg, NF-κB activity), cell proliferation, cell invasion, cell migration, and metastasis. Future inclusion of an adequate representation of breast tumor subtypes, breast cancer cell lines, transgenic animal models, and downstream signaling TLR markers will enhance our understanding of the biological mechanisms that mediate the role of the innate immunity pathway in breast tumorigenesis. Lastly, studies on the silencing of invasive TLRs (eg, TLR2) hold potential for targeted treatment strategies (see the “TLR pathway and breast cancer treatment” section below).
TLR signaling sequence variants and breast cancer risk
Six published reports have examined the relationship between TLR-associated sequence variants and breast cancer. In a pilot study, Etokebe et al did not observe any significant differences in the genotype frequencies of five TLRSNPs detected in TLR2 (T597C, T1350C), TLR3 (C1377T), TLR4 (rs4986790, A896G, Asp299Gly), and TLR9 (A1635G) between 130 breast cancer cases and 101 controls in a case-control study from Croatia.Citation51 Contrary to the findings of Etokebe et al, Theodoropoulos et al found that inheritance of the TLR4 rs4986790 Asp299Gly allele was linked to a modest increase in breast cancer risk (odds ratio [OR] = 1.67, 95% confidence interval [CI] = 1.17–2.38) among women from Athens, Greece.Citation52 However, in the same study, null findings were observed for the TLR4 Thr399Ile SNP.
In the Greek study, the increased susceptibility affiliated with the TLR4 Asp299Gly SNP may be attributed to a reduction in the recognition of TLR4-associated ligands, such as lipopolysaccharide, which is found in the cell wall of Gram-negative bacteria.Citation53 It is believed that individuals who possess the hyporeactive TLR4 variant allele may have reduced cytokine production, increased susceptibility to acute bacterial infections, and increased mortality.Citation54 Alternatively, the low-functioning TLR4 locus may be linked to a compromised inflammatory response that permits damaging persistent subclinical infection.Citation55–Citation58 In any event, genetic alterations attributed to the TLR4 Asp299Gly SNP may disturb the TLR signaling pathway and enhance proinflammatory networks that favor tumor growth.
The TLR4 Asp299Gly variant allele is 10–20 times more prevalent among women of African ancestry relative to those of other ancestries.Citation53 Moreover, this same variant is two to three times more common among African-Americans than among Caucasians http://www.ncbi.nlm.nih.gov/projects/SNP.Citation59 Despite this disparity and the relatively high breast cancer mortality rate among African-Americans and Africans,Citation59 the influence of the TLR4 Asp299Gly locus on breast cancer outcomes in these subgroups is currently unknown. Consequently, additional studies are warranted to evaluate the impact of polymorphic TLR4 and other innate immunity markers in relation to breast cancer risk and ultimately disease prognosis among women of African ancestry.
In a secondary analysis of data collected by the National Cancer Institute Cancer Genetic Susceptibility Breast Cancer Genome-Wide Association Study, Kimbro et al analyzed 127 TLR SNPs in relation to breast cancer risk among 1145 postmenopausal women of European ancestry with invasive breast cancer and 1142 controls from the Nurses’ Health Study.Citation60 Unfortunately, only one marker (TLR6 rs1039559 C/T) was marginally related to a reduction in breast cancer risk. However, this relationship did not remain significant after adjusting for multiple hypothesis testing. In two other large case-control studies, inheritance of the TLR1/TLR6 rs7696175 CC genotype was associated with a 1.6- and 4-fold increase in breast cancer risk among African-American (OR = 4.11; 95% CI = 1.28–13.24) and Chinese women (OR = 1.63; 95% CI = 1.10–2.41), respectively.Citation61,Citation62 However, this TLR1/TLR6 locus was not an important indicator of disease risk among European-American women.Citation61
An independent study analyzed the relationship between downstream TLR-associated SNPs and breast cancer susceptibility. Among 1536 evaluated SNPs, the additive genetic model of the IRAK3 rs1732877 T1471C SNP was linked to a modest 1.6-fold increase in breast cancer risk (OR = 1.63, 95% CI = 1.14–2.34) among Koreans.Citation63 Unfortunately, this downstream signaling marker did not remain significant after adjusting for multiple comparison bias.
Taken together, the epidemiologic evidence for the impact of genetic alterations in innate immunity markers relative to breast cancer is not very compelling. However, the lack of strong relationships may be partly attributed to the failure to evaluate higher order gene–gene and gene–environment interactions. For instance, exposure to environmental chemicals may alter innate immunity signaling activities and ultimately modify the relationship between genetic susceptibilities and breast cancer risk.Citation64 To date, studies have focused primarily on nine SNPs detected in TLR-associated genes (TLR1–4, TLR9, and IRAK3), ignoring hundreds of known and novel TLR downstream signaling markers. Future studies are needed to consider the full array of TLR-related sequence variants and environmental exposures in relation to breast cancer risk within large and racially/ethnically diverse multicenter studies.
TLR pathway and breast cancer treatment
Over the past 30 years, a few research efforts have taken advantage of genomic anomalies associated with TLRs to both identify candidate therapeutic targets and design treatment strategies against breast cancer. To date, agonists for TLR3 and 7 have been used to directly inhibit tumor growth in preclinical studies or to treat breast cancer in humans.Citation43 In addition, TLR3 and TLR4 expression or functional TLR4 sequence variants appear to influence tumor sensitivity to chemotherapy,Citation43,Citation71 albeit unequivocally.Citation72 Lastly, stimulation of TLR9 is the focus of many ongoing randomized clinical trials to treat breast cancer.
Synthetic double-stranded RNAs (dsRNAs), including polyinosinic:polycytidylic and polyadenylic:polyuridylic acid (Poly A:U) have been used in recent breast cancer preclinical and clinical trial studies.Citation65 The use of dsRNA Poly A:U, which targets TLR3 expressed on breast tumor cells, has resulted in favorable breast cancer outcomes (ie, an increase in overall survival and a decrease in metastasis) in two randomized clinical trials with 14 or 111 months of follow-up.Citation66,Citation67 Similarly, in a randomized trial of dsRNA treatment versus chemotherapy, Salaun et al demonstrated a decrease in metastatic relapse among TLR3 positive but not TLR3 negative breast cancer patients in the Poly A:U arm.Citation43 Among patients with a TLR3 positive tumor, women in the Poly A:U arm were three times more likely to remain metastasis free 15 years post-treatment than those treated with chemotherapy (56% [95% CI = 37–71] vs 20% [95% CI = 8–37]). The anti-tumorigenic properties of Poly A:U were supported with results using a breast adenocarcinoma cell line and an immune-compromised mouse model. Following exposure to Poly A:U, the HCC1806 cells underwent TLR3-dependent cell death. Further, subcutaneous growth of the HCC1806 cell lines was compromised by intravenous injection of Poly A:U within immune-compromised non-obese diabetic/severe combined immunodeficiency (NOD-SCID) mice relative to controls. Although the exact mechanisms are unclear, dsRNAs may elicit their antitumor properties against breast cancer by: reducing cell proliferation; inducing TLR3 (and TRIF)-dependent cell death, independent of dsRNA-dependent protein kinase; and promoting IRAK4 induction of IFN β, NF-κB, and – to a lesser extent – caspase-3 and -8 activation.Citation42,Citation43 Although there are no specific data for breast cancer, we cannot exclude the possibility that dsRNAs may potentiate the capacity of TLR3 to trigger a chemoattractant response (secretion of chemokines, macrophages, neutrophils, and lymphocytes) to eradicate the tumor or alter tumor vascularization.Citation68–Citation70 In any case, participation of innate and adaptive immunity is critical to the success of chemotherapy against breast cancer.
Although attention has been given to targeting the TLR signaling pathway for breast cancer treatment, it is important to note that this pathway influences sensitivity to some, but not all, chemotherapeutic strategies. In a breast cancer murine model, Stimulation of dendritic cell TLR4 in combination with systemic chemotherapy or local radiotherapy was shown to reduce tumor growth and prolong survival of tumor-bearing mice.Citation71 Apetoh et al demonstrated that breast cancer patients who carried a TLR4 loss of function SNP (TLR4299 Asp/Gly + Gly/Gly) were more likely to experience metastatic recurrence after chemotherapy and local radiation relative to carriers of the referent genotype.Citation71 In contrast, Szkandera et al did not observe a significant relationship between two commonly reported TLR4 SNPs (Asp299Gly and Thr399Ile) and pathological response to neoadjuvant anthracycline-based chemotherapy among breast cancer patients.Citation72 However, this null finding may be due to the small sample size (70 Caucasian female patients) as well as failure to consider other TLR-associated sequence variants.
In a subcutaneous setting, the administration of TLR5 ligand (Salmonella typhimurium flagellin) in the D2F2 mouse mammary tumor cell line and its antigenic sub-line with stably transfected ErbB-2 (DF2/E2) resulted in varied breast outcomes depending on the dosing regimen.Citation70 Tumor growth was inhibited only in the D2F2 cell line when flagellin was administered 8–10 days after tumor inoculation. However, accelerated tumor growth was observed for both cell lines when the ligand was administered at the time of inoculation. These findings suggest that the impact of TLR5 in relation to breast tumorigenesis is influenced by interactions among the tumor, the tumor microenvironment, and the host immune system.Citation73 In addition, a combination of TLR5 and TLR9 agonists (i.e., CpG oligonucleotides) [CpG ODN] administered 8–10 days after inoculation, inhibited tumor growth in D2F2/E2 cell lines. Additional studies are warranted on the stimulation of multiple TLRs and their joint effects on abrogating tumor growth.
TLR7 recognizes single-stranded RNA and triggers innate immune signaling in response to viral infections.Citation73 TLR7 is also activated by several low molecular weight compounds, including imiquimod, which has anti-tumorigenic properties: induction of cytokines (IFN-γ, IL-12), activation of tumor antigen-specific cytotoxic T-lymphocytes, and activation of myeloid dendritic cells with cytotoxic activity.Citation75–Citation77 A topical cream formulation of imiquimod has been approved by the US Food and Drug Administration for the successful treatment of superficial basal cell carcinoma, actinic keratosis, and genital warts.Citation65 Within ongoing cancer trials, topical application of imiquimod targets several cancers including breast cancer. For instance, the New York University School of Medicine is currently running a Phase II/III clinical trial with imiquimod for breast cancer patients with chest wall recurrence and skin metastasis. In addition, the Fred Hutchinson Cancer Research Center recently completed a Phase III trial of imiquimod in conjunction with a paclitaxel albumin-stabilized nanoparticle formulation.
Despite the conflicting roles of TLR9 in tumorigenesis, TLR9 agonists (eg, CpG ODN) have been studied as antitumor drugs either alone or in concert with other therapeutic strategies in preclinical and clinical studies.Citation65 Stimulation of TLR9 in CpG DNA triggers tumor invasion and migration in breast cancers using in vitro assays.Citation46 Currently, several clinical trials are capitalizing on the anti-tumorigenic properties of the TLR9 agonist CpG 7909 alone or in conjunction with trastuzumab.Citation65,Citation78–Citation81 It is speculated that TLR9 ligands may stimulate anti-tumorigenic activity by inducing apoptotic signals or by interfering with blood vessel formation and tumor growth; however, the exact mechanism for breast cancer remains unclear.Citation82
The growing understanding that TLR-associated targets are involved in breast tumorigenesis offers some justification using larger pharmacogenetic studies. However, such observational studies need to consider the full cadre of possible targets involved in the TLR signaling pathway (TLRs, adaptor/accessory proteins, IRAKs, MMPs, chemokines, cytokines, and caspases). Collectively, continuing efforts targeting the TLR signaling pathway may lead to more effective, noninvasive strategies that will improve breast cancer survival rates.
Conclusion
Breast cancer is a heterogeneous disease whose incidence and progression may vary according to genetic status and function of the tumor innate immunity system. Basic research on key proteins and genetic variants in the TLR signaling pathway will likely offer insights into the processes that promote and/or prevent breast tumorigenesis. Therapeutic targeting of the TLR signaling pathway may be used in conjunction with new or existing chemotherapy, radiation, or immunotherapy approaches to treat breast cancer. Future studies targeting multiple TLRs may lead to more effective therapeutic targets for drug development and treatment of breast cancer. Such effort may ultimately improve breast cancer survival rates among all women.
Disclosure
The authors declare no conflicts of interest in this work.
Acknowledgments
The preparation of this article, together with the program of research reported, was supported in part by the following grant/research support: Clinical Translational Science Pilot Grant to LRK; the JGBCC Bucks for Brains “Our Highest Potential” in Cancer Research Endowment to LRK; and grant P20-MD000175 NIH NCMHD to KSK.
References
- American Cancer SocietyCancer Facts and Figures 2013Atlanta, GeorgiaAmerican Cancer Society2013 Available at http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf. Accessed
- SiegelRNaishadhamDJemalACancer statistics, 2012CA Cancer J Clin2012621102922237781
- AhmedAWangJHRedmondHPSilencing of TLR4 Increases Tumor Progression and Lung Metastasis in a Murine Model of Breast CancerAnn Surg Oncol Epub8142012
- KimSHagemannADeMicheleAImmuno-modulatory gene polymorphisms and outcome in breast and ovarian cancerImmunol Invest2009383–432434019811442
- PensaSWatsonCJPoliVStat3 and the inflammation/acute phase response in involution and breast cancerJ Mammary Gland Biol Neoplasia200914212112919424782
- HojillaCVWoodGAKhokhaRInflammation and breast cancer: metalloproteinases as common effectors of inflammation and extracellular matrix breakdown in breast cancerBreast Cancer Res200810220518394187
- MedzhitovRJanewayCAJrInnate immunity: impact on the adaptive immune responseCurr Opin Immunol199791499039775
- HoebeKJanssenEBeutlerBThe interface between innate and adaptive immunityNat Immunol200451097197415454919
- KutikhinAGAssociation of polymorphisms in TLR genes and in genes of the Toll-like receptor signaling pathway with cancer riskHum Immunol201172111095111621872627
- KutikhinAGYuzhalinAEInherited variation in pattern recognition receptors and cancer: dangerous liaisons?Cancer Manag Res20124313822427729
- AhmadHGubbelsREhlersEKaposi sarcoma-associated herpesvirus degrades cellular Toll-interleukin-1 receptor domain-containing adaptor-inducing beta-interferon (TRIF)J Biol Chem2011 Mar 11286107865787221212282
- SayiAKohlerETollerIMTLR-2-activated B cells suppress Helicobacter-induced preneoplastic gastric immunopathology by inducing T regulatory-1 cellsJ Immunol2011 Jan 15186287889021149607
- KunduSDLeeCBillipsBKThe toll-like receptor pathway: a novel mechanism of infection-induced carcinogenesis of prostate epithelial cellsProstate2008 Feb 168222322918092352
- PrinzCSchwendySVolandPH pylori and gastric cancer: shifting the global burdenWorld J Gastroenterol200612345458546417006981
- MantovaniAMolecular pathways linking inflammation and cancerCurr Mol Med201010436937320455855
- MantovaniAGarlandaCAllavenaPMolecular pathways and targets in cancer-related inflammationAnn Med201042316117020384432
- KunduSDLeeCBillipsBKThe toll-like receptor pathway: a novel mechanism of infection-induced carcinogenesis of prostate epithelial cellsProstate200868222322918092352
- AmaranteMKWatanabeMAThe possible involvement of virus in breast cancerJ Cancer Res Clin Oncol2009135332933719009309
- LawsonJSGlennWKWhitakerNJBreast cancer as an infectious diseaseWomens Health (Lond Engl)2010615820088725
- JoshiDBuehringGCAre viruses associated with human breast cancer? Scrutinizing the molecular evidenceBreast Cancer Res Treat2012135111522274134
- LiXJiangSTappingRIToll-like receptor signaling in cell proliferation and survivalCytokine20104911919775907
- Rakoff-NahoumSMedzhitovRToll-like receptors and cancerNat Rev Cancer200991576319052556
- JingZZHeXBFangYXJiaHJZhouTModulation of the host toll-like receptor signaling pathways by virus infectionBing Du Xue Bao2012284453461 Chinese22978173
- TakedaKAkiraSToll-like receptorsCurr Protoc Immunol2007 Chapter 14:Unit 14.12
- PisetskyDSThe origin and properties of extracellular DNA: from PAMP to DAMPClin Immunol20121441324022659033
- AkiraSTakedaKToll-like receptor signallingNat Rev Immunol20044749951115229469
- AkiraSTakedaKKaishoTToll-like receptors: critical proteins linking innate and acquired immunityNat Immunol20012867568011477402
- BlasiusALBeutlerBIntracellular toll-like receptorsImmunity201032330531520346772
- LeeCAvalosAPloeghHAccessory molecules for Toll-like receptors and their functionNat Rev Immunol20121216817922301850
- MotshwenePGMoncrieffeMCGrossmannJGAn oligomeric signaling platform formed by the Toll-like receptor signal transducers MyD88 and IRAK-4J Biol Chem200928437254042541119592493
- LinSCLoYCWuHHelical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signallingNature2010465730088589020485341
- GayNJGangloffMO’NeillLAWhat the Myddosome structure tells us about the initiation of innate immunityTrends Immunol201132310410921269878
- BartonGMMedzhitovRToll-like receptor signaling pathwaysScience200330056251524152512791976
- SnoussiKMahfoudhWBouaouinaNAhmedSBHelalANChouchaneLGenetic variation in IL-8 associated with increased risk and poor prognosis of breast carcinomaHum Immunol2006671–2132116698420
- KarinMNuclear factor-kappaB in cancer development and progressionNature2006441709243143616724054
- O’NeillLABowieAGThe family of five: TIR-domain-containing adaptors in Toll-like receptor signallingNat Rev Immunol20077535336417457343
- HsuLCParkJMZhangKThe protein kinase PKR is required for macrophage apoptosis after activation of Toll-like receptor 4Nature2004428698034134515029200
- AliprantisAOYangRBWeissDSGodowskiPZychlinskyAThe apoptotic signaling pathway activated by Toll-like receptor-2EMBO J200019133325332610880445
- XieWWangYHuangYYangHWangJHuZToll-like receptor 2 mediates invasion via activating NF-kappaB in MDA-MB-231 breast cancer cellsBiochem Biophys Res Commun200937941027103219141294
- ParkBKZhangHZengQNF-kappaB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSFNat Med2007131626917159986
- KarinMCaoYGretenFRLiZWNF-kappaB in cancer: from innocent bystander to major culpritNat Rev Cancer20022430131012001991
- SalaunBCosteIRissoanMCLebecqueSJRennoTTLR3 can directly trigger apoptosis in human cancer cellsJ Immunol200617684894490116585585
- SalaunBZitvogelLAsselin-PaturelCTLR3 as a biomarker for the therapeutic efficacy of double-stranded RNA in breast cancerCancer Res20117151607161421343393
- YangHZhouHFengPReduced expression of Toll-like receptor 4 inhibits human breast cancer cells proliferation and inflammatory cytokines secretionJ Exp Clin Cancer Res2010299220618976
- González-ReyesSMarínLGonzálezLStudy of TLR3, TLR4 and TLR9 in breast carcinomas and their association with metastasisBMC Cancer201010166521129170
- BergerRFieglHGoebelGToll-like receptor 9 expression in breast and ovarian cancer is associated with poorly differentiated tumorsCancer Sci201010141059106620156214
- MerrellMAIlvesaroJMLehtonenNToll-like receptor 9 agonists promote cellular invasion by increasing matrix metalloproteinase activityMol Cancer Res20064743744716849519
- JózsefLKhreissTFilepJGCpG motifs in bacterial DNA delay apoptosis of neutrophil granulocytesFASEB J200418141776177815345690
- El AndaloussiASonabendAMHanYLesniakMSStimulation of TLR9 with CpG ODN enhances apoptosis of glioma and prolongs the survival of mice with experimental brain tumorsGlia200654652653516906541
- FischerSFRehmMBauerAToll-like receptor 9 signaling can sensitize fibroblasts for apoptosisImmunol Lett200597111512215626483
- EtokebeGEKnezevićJPetricevićBPavelićJVrbanecDDembićZSingle-nucleotide polymorphisms in genes encoding toll-like receptor -2, -3, -4, and -9 in case-control study with breast cancerGenet Test Mol Biomarkers200913672973419810822
- TheodoropoulosGESaridakisVKarantanosTToll-like receptors gene polymorphisms may confer increased susceptibility to breast cancer developmentBreast201221453453822560646
- FerwerdaBMcCallMBVerheijenKFunctional consequences of toll-like receptor 4 polymorphismsMol Med2008145–634635218231573
- SchröderNWSchumannRRSingle nucleotide polymorphisms of Toll-like receptors and susceptibility to infectious diseaseLancet Infect Dis20055315616415766650
- AgneseDMCalvanoJEHahmSJHuman toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of gram-negative infectionsJ Infect Dis2002186101522152512404174
- ChildNJYangIAPulletzMCPolymorphisms in Toll-like receptor 4 and the systemic inflammatory response syndromeBiochem Soc Trans200331Pt 365265312773175
- FaberJMeyerCUGemmerCHuman toll-like receptor 4 mutations are associated with susceptibility to invasive meningococcal disease in infancyPediatr Infect Dis J12006251808116395111
- FraudendorfHGelbrichWReimerWHeringBEffect of experimentally induced changes in blood flow on the heat and electrical conductivity of the skinActa Biol Med Ger1975345923931 German1199613
- SherrySTWardWHKholodovMdbSNP: the NCBI database of genetic variationNucleic Acids Res200129130831111125122
- KimbroKSOpreaGMBurnsBGInnate immunity-related sequence variants as predictors of breast cancer risk among women of African descentJ Clin Oncol201028Suppl15s Abstract 1581
- Barnholtz-SloanJSShettyPBGuanXFGFR2 and other loci identified in genome-wide association studies are associated with breast cancer in African-American and younger womenCarcinogenesis20103181417142320554749
- ChanMJiSLiawCAssociation of common genetic variants with breast cancer risk and clinicopathological characteristics in a Chinese populationBreast Cancer Res Treat2012136111222915072
- LeeJYParkAKLeeKMCandidate gene approach evaluates association between innate immunity genes and breast cancer risk in Korean womenCarcinogenesis20093091528153119372141
- BiXHameedMMiraniNPimentaEMAnariJBarnesBJLoss of interferon regulatory factor 5 (IRF5) expression in human ductal carcinoma correlates with disease stage and contributes to metastasisBreast Cancer Res2011136R11122053985
- GoutagnyNEstornesYHasanULebecqueSCauxCTargeting pattern recognition receptors in cancer immunotherapyTarget Oncol201271295422399234
- LacourJLacourFDucotBPolyadenylic-polyuridylic acid as adjuvant in the treatment of operable breast cancer: recent resultsEur J Surg Oncol19881443113163044833
- LaplancheAAlzieuLDelozierTPolyadenylic-polyuridylic acid plus locoregional radiotherapy versus chemotherapy with CMF in operable breast cancer: a 14 year follow-up analysis of a randomized trial of the Fédération Nationale des Centres de Lutte contre le Cancer (FNCLCC)Breast Cancer Res Treat200064218919111194454
- PaoneAGalliRGabelliniCToll-like receptor 3 regulates angiogenesis and apoptosis in prostate cancer cell lines through hypoxia-inducible factor 1 alphaNeoplasia201012753954920651983
- ZimmerSSteinmetzMAsdonkTActivation of endothelial toll-like receptor 3 impairs endothelial functionCirc Res2011108111358136621493895
- BergéMBonninPSulpiceESmall interfering RNAs induce target-independent inhibition of tumor growth and vasculature remodeling in a mouse model of hepatocellular carcinomaAm J Pathol201017763192320120971743
- ApetohLGhiringhelliFTesniereAToll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapyNat Med20071391050105917704786
- SzkanderaJAbsengerGDandachiNAnalysis of functional germline polymorphisms for prediction of response to anthracycline-based neoadjuvant chemotherapy in breast cancerMol Genet Genomics2012287975576422903472
- MaruyamaKSelmaniZIshiiHYamaguchiKInnate immunity and cancer therapyInt Immunopharmacol201111335035720955832
- DieboldSSKaishoTHemmiHAkiraSReis e SousaCInnate antiviral responses by means of TLR7-mediated recognition of single-stranded RNAScience200430356631529153114976261
- RechtsteinerGWargerTOsterlohPSchildHRadsakMPCutting edge: priming of CTL by transcutaneous peptide immunization with imiquimodJ Immunol200517452476248015728450
- PrinsRMCraftNBruhnKWThe TLR-7 agonist, imiquimod, enhances dendritic cell survival and promotes tumor antigen-specific T cell priming: relation to central nervous system antitumor immunityJ Immunol2006176115716416365406
- StaryGBangertCTauberMStrohalRKoppTStinglGTumoricidal activity of TLR7/8-activated inflammatory dendritic cellsJ Exp Med200720461441145117535975
- PfizerPF-3512676 (CpG 7909) injection for patients who completed an oncology study using PF-3512676 (CpG 7909)ClinicalTrials.gov [website on the Internet]Bethseda, MDUS National Library of Medicine2008 [updated March 11, 2009]. Available from: http://clinicaltrials.gov/show/NCT00043368. NLM identifier: NCT00043368Accessed February 21, 2013
- PfizerCPG 7909 plus Herceptin® in patients with metastatic breast cancerClinicalTrials.gov [website on the Internet]Bethseda, MDUS National Library of Medicine2002 [updated March 11, 2009]. Available from: http://clinicaltrials.gov/show/NCT00043394. NLM identifier: NCT00043394Accessed February 21, 2013
- PfizerSafety and efficacy study of the combination of CpG 7909 and Herceptin in patients with metastatic breast cancerClinicalTrials.gov [website on the Internet]Bethseda, MDUS National Library of Medicine2002 [updated May 26, 2011]. Available from: http://clinicaltrials.gov/show/NCT00031278. NLM identifier: NCT00031278Accessed February 21, 2013
- Ohio State University Comprehensive Cancer CenterAgatolimod and trastuzumab in treating patients with locally advanced or metastatic breast cancerClinicalTrials.gov [website on the Internet]Bethseda, MDUS National Library of Medicine2009 [updated November 9, 2011]. Available from: http://clinicaltrials.gov/show/NCT00824733. NLM identifier: NCT00824733Accessed February 21, 2013
- VabulasRMBraedelSHilfNThe endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathwayJ Biol Chem200227723208472085311912201
- TangDKangRChehCWHMGB1 release and redox regulates autophagy and apoptosis in cancer cellsOncogene201029385299531020622903
- LotzeMTDeMarcoRADealing with death: HMGB1 as a novel target for cancer therapyCurr Opin Investig Drugs2003414051409
- LotzeMTTraceyKJHigh-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenalNat Rev Immunol2005533134215803152
- XieWHuangYGuoAWuWBacteria peptidoglycan promoted breast cancer cell invasiveness and adhesiveness by targeting toll-like receptor 2 in the cancer cellsPLoS One20105e1085020520770
- HiratsukaSWatanabeASakuraiYThe S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phaseNat Cell Biol200810111349135518820689
- ZhangGSunXLvHYangXKangXSerum amyloid A: A new potential serum marker correlated with the stage of breast cancerOncol Lett2012394094422741023
- SfondriniLRossiniABesussoDAntitumor activity of the TLR-5 ligand flagellin in mouse models of cancerJ Immunol2006176116624663016709820
- CaiZSanchezAShiZZhangTLiuMZhangDActivation of Toll-like receptor 5 on breast cancer cells by flagellin suppresses cell proliferation and tumor growthCancer Res2011 Apr 17172466247521427357