Abstract
Objective
To evaluate, from the perspective of the Brazilian public health care system, the cost-effectiveness of lapatinib plus capecitabine (LAP/CAP) versus capecitabine alone (CAP) or trastuzumab plus capecitabine (TRAST/CAP) in the treatment of women with human epidermal growth factor receptor-2-positive metastatic breast cancer previously treated with trastuzumab.
Methods
An economic model was developed to compare costs and clinical outcomes over a 5-year time horizon. Both costs and outcomes were discounted at a 5% rate, in accordance with Brazilian pharmacoeconomic guidelines. Clinical inputs were determined using indirect treatment comparisons. Costs were derived from public reimbursement databases and reported in 2010 Brazilian real (R$1 = USD$0.52). Clinical outcomes included progression-free survival years (PFYs), life-years (LYs) and quality-adjusted life-years (QALYs). The economic outcome was the incremental cost per LY, PFY, or QALY gained. The impact of variations in individual inputs (eg, drug cost, drug effectiveness) was examined using one-way sensitivity analyses. Overall model robustness was tested using probabilistic sensitivity analyses, varying the ranges of all input parameters within their standard distributions.
Results
Expected cost per patient was R$41,195 for CAP, R$95,256 for LAP/CAP, and R$113,686 for TRAST/CAP. Respective LYs were 1.406, 1.695, and 1.465; PFYs were 0.473, 0.711, and 0.612; and QALYS were 0.769, 0.958, and 0.827. LAP/CAP dominated TRAST/CAP for all outcomes. Incremental cost-effectiveness ratios of LAP/CAP over CAP were R$186,563 for LYs, R$226,403 for PFYs, and R$284,864 for QALYs. Results remained unchanged in one-way sensitivity analyses. In probabilistic analyses, LAP/CAP was dominant over TRAST/CAP in 93.5% of simulations.
Conclusion
LAP/CAP increases survival for women with human epidermal growth factor receptor-2-positive metastatic breast cancer. LAP/CAP is cost-effective against TRAST/CAP (ie, produces more benefits at a lower cost) and can be considered cost-effective over CAP at a willingness-to-pay of about R$290,000 (US$151,000) per QALY gained.
Introduction
Worldwide, breast cancer is the most frequent malignancy in women.Citation1 Each year, there are 50,000 new cases in Brazil; however, little information is available within this country.Citation2,Citation3 Metastases constitute a major problem, since early detection and treatment is associated with better patient outcomes. Unfortunately, as many as 10% of women already have distant metastases on diagnosis.Citation4 Additionally, 40% of those treated locally for breast cancers suffer recurrence within 5 years.Citation5
Women who overexpress the human epidermal growth factor receptor-2 (HER2) protein have worse outcomes that those who do not.Citation6 Overexpression occurs in 15%–22% of breast cancers.Citation7,Citation8 Drugs have been developed that specifically target HER2-positive breast cancers, including trastuzumab and lapatinib.Citation6–Citation9 They have recently been incorporated into major international treatment guidelines.Citation10,Citation11
The use of trastuzumab in combination with capecitabine (TRAST/CAP) has been evaluated in clinical research in women with metastatic breast cancer (MBC) and produced favorable results.Citation12 However, when first-line treatments for MBC fail and the disease progresses, other therapies must be instituted. Although not currently approved by the Brazilian National Agency of Health Surveillance (ANVISA) for use after disease progression is observed, observational data support clinical benefits with TRAST/CAP continuation.Citation13 Another treatment option currently includes lapatinib combined with capecitabine (LAP/CAP), which is also supported by clinical evidence.Citation14 In addition, LAP/CAP is approved for use in Brazil in patients with MBC following initial treatment with TRAST/CAP.
The recently created National Committee to Incorporate Technologies (CONITEC) from the Brazilian Ministry of Health has positively recommended the use of trastuzumab as both early- and late-stage treatment for breast cancer.Citation15,Citation16 The basis for this decision was related to the positive clinical results supporting the use of trastuzumab in such disease. Another important criterion used in health decision making in Brazil is related to the economics supporting the cost-effectiveness of appraised technologies.Citation17
A review of the literature located a study from the UK that examined the cost-effectiveness of LAP/CAP and TRAST/CAP in MBC.Citation18 The authors concluded, after considering a wide variety of sensitivity analyses, that LAP/CAP was dominant (ie, produced more benefits at a lower cost) over TRAST/CAP in treating women with HER2-positive MBC who had received prior therapy with trastuzumab. Although it seems intuitive to transfer results observed in one country to another, with health economic evaluations, such a practice is not recommended by international guidelines.Citation19 Thus, their cost-effectiveness in Brazil is currently unknown.
Therefore, the aim of this study was to determine the cost-effectiveness of LAP/CAP, TRAST/CAP, and capecitabine alone (CAP) in Brazilian women with HER2-positive MBC after failing trastuzumab therapy, from the economic perspective of the Brazilian public health care system.
Material and methods
Study perspective and type, target population, and interventions
This study was developed from the economic perspective of the Brazilian Public Health Care System (Sistema Unico de Saude). A cost-effectiveness analysis similar to that of Delea et al was undertaken,Citation18 using an economic model to simulate treatments and their consequences over a 5-year time horizon. The population was a hypothetical cohort of Brazilian women with HER2-positive MBC in whom trastuzumab therapy had failed. Treatments included LAP/CAP, TRAST/CAP, and CAP administered in monthly cycles.
Model structure and parameters
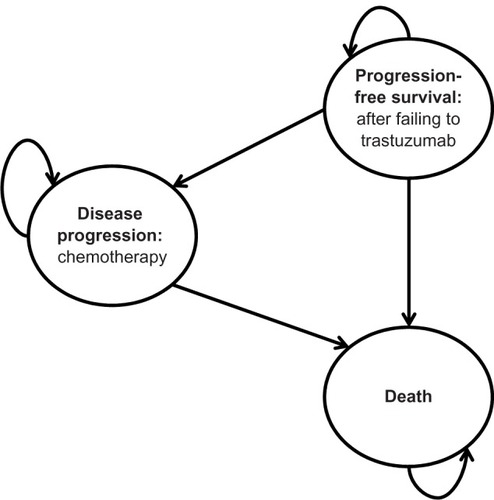
To simulate the natural history of the disease and incorporate Brazilian patterns of care, a mathematical model was constructed with guidance from a panel of local experts. Three basic health states were considered, which included progression-free survival (PFS; after trastuzumab failure), disease progression (supportive care), and death. depicts the mathematical model structure. All patients entered the analysis in the PFS state and in the course of the analysis could migrate to other states or remain in the PFS state, according to literature-based probabilities. The proportion of women in the three health states was estimated mathematically in the same manner as Delea et al using Weibull distributions or by fitting curves to survival functions with input parameters for the calculations derived from the literature ().Citation18 The model represents a partitioned survival analysis, which the proportion of patients in each health state over the course of time was estimated based on empirical or fitted survival functions for PFS and overall survival (OS). Such an approach is similar to a Markov model, but does not explicitly make use of transition probabilities. Further, costs and quality of life were assumed to be conditional upon treatment and state, and were calculated by multiplying expected time in each health state by their corresponding costs and estimated utilities. Resulting treatment costs and consequences were calculated by multiplying each expected health state proportions (ie, PFS and disease progression) by their corresponding cost and utility estimates. Monthly cycles were applied.
Table 1 Clinical, humanistic, and economic estimates used as inputs in the model
The clinical outcomes of interest in this analysis were life-years of survival (LYs), PFS years, and quality-adjusted life-years (QALYs). Since the analytic time horizon in the base case analysis was more than 1 year, a 5% discount rate was applied to these outcomes as well as costs, in accordance with the Brazilian guidelines for health economic evaluations.Citation17
Clinical, safety, and utility data used in the model
Clinical inputs, such as efficacy rates (ie, transition probabilities) and frequency of adverse events (which were restricted to grade 3–4 events), were derived from a search of the literature. Inputs and their sources are presented in .Citation14,Citation18,Citation20–Citation25
Two clinical studies formed the body of evidence to support the current cost-effectiveness analysis. Those were the EGF100151 and GBG 26/BIG 3–05 clinical trials, which demonstrated the clinical benefits of adding HER2-targeted therapy with lapatinib or trastuzumab to capecitabine monotherapy in patients with MBC who had progressed while receiving trastuzumab.Citation20,Citation21 Final survival analysis from both EGF100151 and GBG 26/BIG 3–05 clinical trials were considered as model input data.Citation22,Citation23
As previously described, the analytical approach used here was similar to that of Delea et al.Citation18 Estimates for treatment effectiveness were obtained from the Weibull survival functions of CAP calculated in Delea et al,Citation18 by applying treatment relative efficacy data from both EGF100151 and GBG 26/BIG 3–05 clinical trials to project the effects of LAP/CAP and TRAST/CAP on OS and PFS. CAP Weibull survival function was estimated from the EGF100151-study data using accelerated failure time regression.Citation18 A detailed methodology of the Weibull function is described elsewhere.Citation26
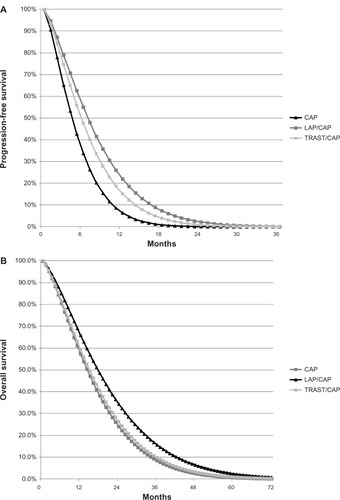
The current study used the hazard ratio (HR) for PFS between LAP/CAP and CAP from the EGF100151 trial (0.55; 95% confidence interval 0.40–0.74) and between TRAST/CAP and CAP from the GBG 26/BIG 03–05 trial (0.69; 95% confidence interval 0.48–0.97).Citation20,Citation21 To estimate an HR for OS, a slightly different approach was required since patients receiving CAP could change to another active treatment, thereby confounding results. Consequently, for OS, the combination therapy arm of the EGF100151 trial was used as the referent treatment, because it was not confounded by crossover, and there was no practical method to adjust the survival curve for monotherapy directly (eg, excluding the patients who crossed over might bias the result if patients who crossed over differed from those who did not with respect to prognostic factors). Therefore, the Weibull parameters reported by Delea et al were used in order to estimate the OS curve for CAP.Citation18 Then, the HR for OS with CAP versus LAP/CAP was that based on the adjusted Cox proportional hazards regression analysis entered as a time-dependent covariate and baseline prognostic factors included as covariates (HR = 1.33; 95% confidence interval 1.06–1.67; P = 0.013).Citation22 OS for TRAST/CAP versus CAP was obtained by applying the HR for OS from the GBG 26/BIG 03–05 trial (HR = 0.94; 95% confidence interval 0.65–1.35; P = 0.734) to the estimated OS curve for CAP.Citation23 Weibull-estimated PFS and OS curves are displayed in .
Figure 2 Weibull-estimated (A) progression-free survival and (B) overall survival curves.
Utility data were derived from EQ-5D™ (EuroQoL Group, Rotterdam, The Netherlands) mean preprogression measures from patients in the EGF100151 trial.Citation24 Estimated decrements in utility associated with progression were 32% (0.22 in absolute terms) using data from a study of societal preferences for different stages of MBC.Citation25
Resource use and treatment costs
Only direct medical costs were considered including drugs, medical care, hospital care, and tests/imaging. Costs were grouped into medication treatment (per cycle), supportive care, management of adverse events, and disease progression (ie, supportive care). The types and quantities of resources used during these activities were defined based on the opinions of experts.
summarizes the cost inputs and their sources. Unit costs of drugs were obtained from the Health Price Database of the Brazilian Ministry of Health.Citation27 The average price that would be paid for these drugs was used. All other cost inputs were obtained from the Management System of Procedures and Medications of the Brazilian Public Health Care System (SIGTAP).Citation28 In Brazil, oncology treatments for individuals covered by public health care are conferred by the Authorization for High Complexity Procedures (APAC) from the Brazilian Ministry of Health. Such procedures contemplate monthly reimbursement packages for public hospitals or oncology treatment centers to manage patients with specific conditions and include: drugs, hospital stays, medical/surgical procedures, and laboratory tests.
In the CAP arm, the drug was infused in a dose of 2500 mg/m2/day on the first to the 14th day of the 21-day cycle.Citation20 A standard mean body surface of 1.7 m2 was assumed. Those in the TRAST/CAP arm received capecitabine in the same manner plus a 30-minute infusion of 6 mg/kg trastuzumab every 21 days.Citation21 As a conservative approach, it was assumed that there would be no wastage of trastuzumab. Patients in the LAP/CAP arm received capecitabine as above, but at a dose of 2000 mg/m2/day plus 1250 mg/day of lapatinib (orally).Citation20 According to expert opinion, when disease progression occurred, the costs associated with treating that progression were considered only for the first 3 months. After that time, the costs of palliative chemotherapy were included, which consisted of a monthly rate of reimbursement conferred by APAC. Monthly follow-up costs (ie, physician visit and monitoring) were incurred for both PFS and disease progression health states.
Expert opinion was also used to determine the treatment of adverse events. As mentioned above, only serious events (grades 3–4) were considered because they had implications for resource utilization. Costs for managing these events appear in .
Pharmacoeconomic outcomes
The pharmacoeconomic outcome was the incremental cost-effectiveness ratio (ICER) for each outcome of interest (ie, LYs, PFS years, and QALYs). Results were reported in 2010 Brazilian real (R$) and converted to US dollars (US$) for international comparisons of data using the 2012 monetary conversion rate (1R$ = 0.52US$). In the absence of a three-way head-to-head comparison, drugs were compared in a pairwise fashion, as was done by Delea et al in accordance with recommendations from the UK’s National Institute for Health and Clinical Excellence.Citation18,Citation29
Sensitivity analysis
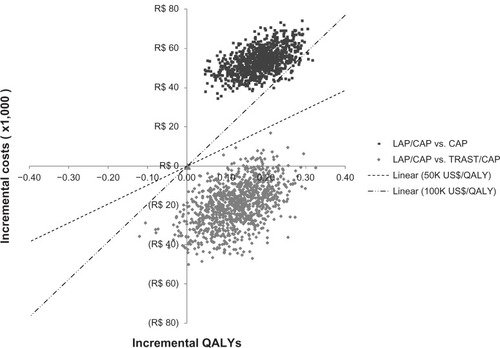
To examine the robustness of the model, one-way sensitivity analyses were conducted on all input parameters. Additionally, a probabilistic sensitivity analysis on the ICER was conducted. All inputs were varied simultaneously over 1000 iterations within various error distributions. Costs were varied ±10% using a gamma distribution, and clinical probabilities were varied assuming they were distributed as beta. A scatter plot was made of the outputs, categorized by quadrants as first described by Laupacis et al.Citation30 ICERs were categorized as: quadrant one (incremental effectiveness > 0 and incremental cost > 0); quadrant two (incremental effectiveness > 0 and incremental cost < 0); quadrant three (incremental effectiveness < 0 and incremental cost < 0), and quadrant four (incremental effectiveness < 0 and incremental cost > 0). ICERs that fall into quadrant two indicate dominance.
Results
Cost-effectiveness analysis
Summarized results for patients with metastatic HER2-positive breast cancer after failure with trastuzumab appear in . Compared with TRAST/CAP, all clinical outcomes for LAP/CAP were greater and its overall cost was lower. As a result, LAP/CAP dominated TRAST/CAP for all outcomes examined in this analysis. Compared with CAP, the clinical outcomes for LAP/CAP were again superior, with an increase in survival of 2.9 months. ICERs for the outcomes examined ranged from R$186,563 to R$284,864.
Table 2 Results of the cost-effectiveness analyses
Sensitivity analyses
displays the results of the numerous one-way sensitivity analyses conducted to examine model robustness. The results remained unchanged for the vast majority of analyses. Under the LAP/CAP versus CAP comparison, none of the model parameters was sensitive to assumed variations, where the ICERs ranged from R$210,979 to R$578,091 (base case was R$284,864). However, while comparing LAP/CAP versus TRAST/CAP, changes in only one model variable resulted in significant ICER change. The lower limit confidence interval estimate for the OS effect of the combination TRAST/CAP altered the cost-effectiveness rankings, where TRAST/CAP could then generate higher benefits and costs compared with LAP/CAP. ICER variations were 0.40 and R$554,030 when the OS HR (TRAST/CAP versus CAP) was 0.65.
Table 3 Results of individual one-way sensitivity analyses
The two clouds in depict the results of the multivariate sensitivity analyses. LAP/CAP was dominant over TRAST/CAP in 93.5% of the simulations and cost-effective in the majority of the remaining simulations, considering commonly accepted international cost-effectiveness threshold range (ie, between US$50,000 and US$100,000 per QALY gained). Compared with CAP, LAP/CAP had increased costs and increased outcomes, with 100% of the simulations falling into quadrant one. With a very few exceptions, all ICERs were greater than US$100,000 per QALY gained.
Discussion
In this analysis, the combined therapy of LAP/CAP was dominant (ie, had a lower cost and greater clinical benefit) over TRAST/CAP for treating women with HER2-positive MBC after failing trastuzumab. Sensitivity analyses confirmed this dominance as well as a high probability of cost-effectiveness for LAP/CAP. Consequently, under the clinical and economic premises computed here, TRAST/CAP should not be considered for routine use in Brazil for this indication, except in special cases (eg, intolerance). In addition, local regulatory permission for use of TRAST/CAP is currently not granted in these patients and clinical staging or therapeutic phasing. However, the use of trastuzumab following disease progression in practice may be present as per clinical decision. The current research shows that from a cost-effectiveness viewpoint, the use of LAP/CAP in HER2-positive MBC showing disease progression after trastuzumab is preferred, given improved clinical benefits at a lower cost compared with TRAST/CAP continuation.
When comparing results across jurisdictions, the current analysis produced results that were similar to those of Delea et al in the UK.Citation18 In both analyses, the higher benefits with LAP/CAP were associated with a lower HR for PFS of LAP/CAP (0.55) in comparison with TRAST/CAP (0.69). This means that patients receiving LAP/CAP spend more time in a condition with a higher health-related quality-of-life (ie, more time in preprogression versus postprogression health states). Projected numbers of PFS years were very close between studies, where minor discrepancies resulted from cycle length adjustments. In the study by Delea et al, a daily cycle was used,Citation18 whereas in the current analysis, a monthly cycle was applied. Moreover, final data on the OS HR for TRAST/CAP versus CAP was recently published,Citation23 and numerical differences from the previous data used by Delea et al (0.94 versus 0.76, respectively) resulted in further improvements in benefits with LAP/CAP versus TRAST/CAP when comparing LY and QALY results from the current analysis to those reported in Delea et al.Citation18
On the other hand, the two analyses differed significantly in terms of costs. Whereas in the UK the lower estimated cost with LAP/CAP versus TRAST/CAP was primarily reflected by savings from the use of oral rather than an intravenous HER2 therapy and lower capecitabine dose in the LAP/CAP combination versus TRAST/CAP, in the current cost-effectiveness analysis, differences in drug prices were the main factor influencing differences in treatment costs with LAP/CAP versus TRAST/CAP. In Brazil, according to reimbursement data, the monthly cost for LAP/CAP is around 35% lower than for TRAST/CAP. Thus, as described in , even with a 25% reduction in TRAST/CAP drug cost, LAP/CAP is still cost saving under the economic perspective of the public health care system.
In addition, LAP/CAP could be cost-effective compared to CAP with a willingness-to-pay of R$290,000 per LY gained (approximately US$151,000). In other studies of HER2-positive MBC, Delea et al calculated an ICER of £77,993 (US$121,061) for LAP/CAP versus CAP and Le and Hay reported an ICER of US$166,111 per QALY gained.Citation18,Citation31 Matter-Walstra et al estimated an ICER of £98,329 (US$152,626) for TRAST/CAP over CAP.Citation32 In a review of 23 cost-effectiveness ratios pertaining to treatment of early breast cancer, Chan et al reported that these ratios ranged from US$5020 to US$134,610 per QALY gained.Citation33 Therefore, it can be concluded that LAP/CAP does offer clinical advantages in terms of increased survival and quality of life, but at an increased cost that exceeds commonly accepted cost-effectiveness thresholds. Nonetheless, the ICERs reported here are similar to those in the literature associated with treating MBC.
The combined clinical and economic context of the decision must also be considered. MBC is an increasing problem in Brazil as elsewhere in the world. Budgets are strained and choices must be made. Using LAP/CAP in place of TRAST/CAP would be a cost-saving maneuver that would also increase survival time and its quality. As previously described, the benefits of LAP/CAP result mostly from patients spending more time in a condition with a higher health-related quality-of-life. In addition, the resulting overall treatment cost of LAP/CAP is lower compared to TRAST/CAP. Another advantage is that this drug may be administered orally, saving nursing time and creating other health care-related efficiencies. Furthermore, since ongoing research into the use of lapatinib as a single option or in conjunction with other agents has found benefit for these patients, an even greater advantage would be created.Citation34,Citation35 That is, on the equity side, an oral HER2-positive agent used alone or in combination with other therapies would obviate the need for intravenous administration, could be administered on an outpatient basis, and deliver multiple treatment options for patients with HER2-positive MBC.
However, despite the clear economic advantages described here of treating HER2-positive MBC patients with LAP/CAP following initial treatment with trastuzumab, the estimated monthly cost of LAP/CAP is higher than the available funding resources through the APAC system in Brazil and, as a result, the current funding rates covered by APAC in the metastatic setting need to be revised.
Limitations and assumptions
The patients within the studies used as inputs for this analysis were not identical to each other with respect to disease characteristics and treatment history. Although they were similar with respect to Eastern Cooperative Oncology Group status and Karnofsy index, their experiences with anthracyclines differed. In the study comparing TRAST/CAP to CAP, only 75 patients (48%) had been previously treated with anthracyclines.Citation21 On the other hand, in the study comparing LAP/CAP to CAP alone, 98% of patients received prior treatment with anthracyclines.Citation20 This difference suggests a worse prognosis for the lapatinib population.
Another difference observed between the studies concerns the dose of capecitabine used in combination treatment. In the lapatinib study, the dose of capecitabine in combined therapy was 2000 mg/m2/day compared to 2500 mg/m2/day in the trastuzumab study.Citation20,Citation21 It is unknown whether there would be a clinical effect resulting from this difference in terms of efficacy or safety. In colorectal cancer, a dose reduction has been associated with a reduction in the occurrence of adverse effects without compromising efficacy.Citation36
The differences in disease severity between study populations and different dosages of capecitabine could compromise the indirect comparison of treatment with LAP/CAP and TRAST/CAP. However, variations in the relative effectiveness of the treatments were evaluated in the probabilistic sensitivity analysis.
Additionally, the development of metastases in the central nervous system is common in women with MBC who have received trastuzumab.Citation37,Citation38 In the study evaluating the combination of LAP/CAP, a lower frequency of metastasis was observed in the combined treatment arm (4% versus 13%; P < 0.05).Citation20 On the other hand, brain metastases were observed more frequently in the combined treatment arm (eight versus five cases) in the clinical trial comparing TRAST/CAP with CAP, but this difference was not statistically significant.Citation21 Therefore, brain metastases and the cost of their treatment were not included in the current analysis.
Conclusion
In Brazil, combined therapy with LAP/CAP was found to be more cost-effective than TRAST/CAP in treating women with HER2-positive MBC who had previously been treated with trastuzumab. LAP/CAP would be cost-effective against CAP with a willingness-to-pay threshold of about R$290,000 (US$151,000) per QALY gained.
Disclosure
Dr Machado is an employee of GlaxoSmithKline Brasil Ltda. Dr Einarson received fees from GlaxoSmithKline for scientific review, methodological guidance, and writing of this manuscript.
Acknowledgments
This study was sponsored by GlaxoSmithKline Brasil Ltda.
References
- EdwardsBKBrownMLWingoPAAnnual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatmentJ Natl Cancer Inst200597191407142716204691
- Instituto Nacional do CancerEstimativa 2010: Incidencia de Cancer no Brasil [Cancer Incidence in Brazil: 2010 Estimates]Rio de JaneiroInstituto Nacional do Cancer2009 Portuguese
- LeeBLLiedkePEBarriosCHSimonSDFinkelsteinDMGossPEBreast cancer in Brazil: present status and future goalsLancet Oncol2012133e95e10222381937
- Fundacao Oncocentro de Sao PauloDados de cancer: registro hospitalar de cancer, 2001–2008 [Cancer data: hospital cancer registries, 2001–2008]2008 Available from: http://www.fosp.saude.sp.gov.br/html/fr_dados.htmlAccessed June 12, 2012 Portuguese
- Early Breast Cancer Trialists’ Collaborative GroupEffects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trialsLancet200536594721687171715894097
- RossJSSlodkowskaEASymmansWFPusztaiLRavdinPMHortobagyiGNThe HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicineOncologist200914432036819346299
- WolffACHammondMESchwartzJNAmerican Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancerJ Clin Oncol200725111814517159189
- MauriDPolyzosNPSalantiGPavlidisNIoannidisJPMultiple-treatments meta-analysis of chemotherapy and targeted therapies in advanced breast cancerJ Natl Cancer Inst2008100241780179119066278
- HarrisCAWardRLDobbinsTADrewAKPearsonSThe efficacy of HER2-targeted agents in metastatic breast cancer: a meta-analysisAnn Oncol20112261308131721119031
- CardosoFFallowfeldLCostaACastiglioneMSenkusELocally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-upAnn Oncol201122Suppl 6vi25vi3021908499
- National Comprehensive Cancer NetworkNCCN clinical practice guidelines in oncology72004 Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.aspAccessed June 11, 2012
- SlamonDJLeyland-JonesBShakSUse of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2N Engl J Med20013441178379211248153
- FabiAMetroGFerrettiGDo HER-2 positive metastatic breast cancer patients benefit from the use of trastuzumab beyond disease progression? A mono-institutional experience and systematic review of observational studiesBreast200817549950518450443
- GeyerCEForsterJLindquistDLapatinib plus capecitabine for HER2-positive advanced breast cancerN Engl J Med2006355262733274317192538
- Ministerio da Saude do BrasilTrastuzumabe para tratamento do cancer de mama inicial [Trastuzumab for the treatment of initial stage breast cancer]52012 Available from: http://portal.saude.gov.br/portal/arquivos/pdf/Relatorio_Trastuzumabe_ca_inicial.pdfAccessed June 18, 2012 Portuguese
- Ministerio da Saude do BrasilTrastuzumabe para tratamento do cancer de mama avancado [Trastuzumab for the treatment of advanced stage breast cancer]52012 Available from: http://portal.saude.gov.br/portal/arquivos/pdf/Relatorio_Trastuzumabe_ca_avancado.pdfAccessed June 18, 2012 Portuguese
- Ministerio da Saude do BrasilEstudos de avaliacao economica de technologias em saude [Economic evaluation studies of technologies in health]2009 Available from: http://www.ispor.org/PEguidelines/source/Economic-Evaluation-Guidelines-in-Brazil-Final-Version-2009.pdfAccessed March 13, 2012 Portuguese
- DeleaTETappendenPSofryginOCost-effectiveness of lapatinib plus capecitabine in women with HER2+ metastatic breast cancer who have received prior therapy with trastuzumabEur J Health Econ201213558960321701940
- DrummondMBarbieriMCookJTransferability of economic evaluations across jurisdictions: ISPOR Good Research Practices Task Force reportValue Health200912440941819900249
- CameronDCaseyMPressMA phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analysesBreast Cancer Res Treat2008112353354318188694
- von MinckwitzGdu BoisASchmidtMTrastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German breast group 26/breast international group 03–05 studyJ Clin Oncol200927121999200619289619
- CameronDCaseyMOlivaCNewstatBImwalleBGeyerCELapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase III randomized trialOncologist201015992493420736298
- von MinckwitzGSchwedlerKSchmidtMTrastuzumab beyond progression: overall survival analysis of the GBG 26/BIG 3–05 phase III study in HER2-positive breast cancerEur J Cancer201147152273228121741829
- ZhouXCellaDCameronDLapatinib plus capecitabine versus capecitabine alone for HER2+ (ErbB2+) metastatic breast cancer: quality-of-life assessmentBreast Cancer Res Treat2009117357758919153829
- LloydANafeesBNarewskaJDewildeSWatkinsJHealth state utilities for metastatic breast cancerBr J Cancer200695668369016967055
- CarrollKJOn the use and utility of the Weibull model in the analysis of survival dataControl Clin Trials200324668270114662274
- Ministerio da Saude do Brasil[Health Price Database – version 2.0.1] Available from: http://bps.saude.gov.br/login.cfm112011Accessed March 13, 2012 Portuguese
- Ministerio da Saude[SIGTAP – Management System for Medical Procedures]112008 Available from: http://sigtap.datasus.gov.br/tabela-unificada/app/sec/inicio.jspAccessed March 13, 2012 Portuguese
- National Institute for Health and Clinical ExcellenceGuide to the methods of technology appraisal12009 Available from: http://www.nice.org.uk/aboutnice/howwework/devnicetech/guidetothemethodsoftechnologyappraisal.jspAccessed March 13, 2012
- LaupacisAFeenyDDetskyASTugwellPXHow attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluationsCMAJ199214644734811306034
- LeQAHayJWCost-effectiveness analysis of lapatinib in HER-2-positive advanced breast cancerCancer2009115348949819117341
- Matter-WalstraKWDedesKJSchwenkglenksMBrauchliPSzucsTDPestalozziBCTrastuzumab beyond progression: a cost-utility analysisAnn Oncol201021112161216820444849
- ChanALLeungHWLuCLLinSJCost-effectiveness of trastuzumab as adjuvant therapy for early breast cancer: a systematic reviewAnn Pharmacother200943229630319193576
- HurvitzSAKakkarARole of lapatinib alone or in combination in the treatment of HER2-positive breast cancerBreast Cancer (London)2012413551
- JohnstonSPippenJJrPivotXLapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancerJ Clin Oncol200927335538554619786658
- CassidyJTwelvesCVan CutsemEFirst-line oral capecitabine therapy in metastatic colorectal cancer: a favorable safety profile compared with intravenous 5-fluorouracil/leucovorinAnn Oncol200213456657512056707
- LinNUBellonJRWinerEPCNS metastases in breast cancerJ Clin Oncol200422173608361715337811
- ClaytonAJDansonSJollySIncidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancerBr J Cancer200491463964315266327