Abstract
Background
An association between higher serum calcium (Ca2+) levels and breast cancer has been previously reported. However, little is known regarding the relationship between serum Ca2+ levels and the expression of Ca2+ channels in the presence of breast microcalcifications.
Methods
A retrospective analysis of women newly diagnosed with breast microcalcifications was performed based on the Breast Imaging Reporting and Data System (BI-RADS). The expression of TRPC1, TRPC3, and TRPM7 using normal biopsy without microcalcifications (controls) and infiltrating ductal carcinoma with microcalcifications was evaluated.
Results
Data on 138 women were analyzed. Seventy percent of women had a BI-RADS score (1–3) corresponding to benign disease. Seventy-six percent of women with a BI-RADS score (4 or 5) were diagnosed with breast cancer, 56% were cancers in situ, and 93% were infiltrating ductal carcinomas. No difference in the distribution of corrected serum Ca2+ levels between BI-RADS scores (1–3) and BI-RADS scores (4–5) (P = 0.82) was observed. Serum Ca2+ levels were similar in women without cancer and women diagnosed with breast cancer (P = 0.94). However, the expression of TRPM7 and TRPC1, but not TRPC3, Ca2+ channels were increased in infiltrating ductal carcinoma samples with microcalcifications when compared with age-matched controls without calcification or cancer.
Conclusion
We observed an increase in the expression of TRPM7 and TRPC1 Ca2+ channels in infiltrating ductal carcinoma samples with microcalcifications, whereas no change in serum Ca2+ levels was observed. Together these data suggest that increased expression of these channels might lead to an increase in intracellular Ca2+ levels thereby restoring serum Ca2+ levels, but these can contribute to the breast microcalcifications. However, future studies exploring the intracellular Ca2+ levels as well as the role of TRPM7 and TRPC1 function according to BI-RADS scores are needed.
Introduction
Mammary microcalcifications occur in 30% to 50% of breast cancer patients,Citation1 which are frequently associated with a poor survival.Citation2 The presence of microcalcifications in the breasts has been correlated with an increased risk of synchronous and ensuing breast carcinoma;Citation3 however, the mechanism is not known. Elevated serum calcium (Ca2+) levels have been shown to increase the risk of breast cancer. For example, Almquist et alCitation4 found that high serum Ca2+ increased the risk of breast cancer among overweight and postmenopausal women. A follow-up of this cohort revealed higher serum Ca2+ levels were associated with cancer aggressiveness.Citation5 Consistent with these studies, in a large case control study, we found that women newly diagnosed with breast cancer had significantly higher serum Ca2+ levels than women without cancer.Citation6 In addition, our results also show that postmenopausal women with breast tumors (>2 cm) have higher serum Ca2+ levels than women with smaller tumors,Citation7 suggesting that Ca2+ levels play an important role in either the initiation or progression of breast cancer.
In breast cancer cells, intracellular Ca2+ levels are maintained via two separate classes of Ca2+ channels that have distinct modes of activation. One of the mechanisms is activated by store depletion per se, where release of Ca2+ from the endoplasmic reticulum (ER) initiates Ca2+ influx, which is termed as store operated Ca2+ entry (SOCE). Importantly, both TRPC1 and Orai1 have been identified as SOCE channels in different cells; however, their function in breast cancer, especially with microcalcification, is not clear. The other Ca2+ entry mechanism is more diverse and is mediated by various mechanisms that include receptor activation followed by the generation of second messengers, or they can be activated just by a decrease in serum magnesium levels. Some members of the TRPC channels, especially TRPC3 have been shown to be activated by receptor activation, whereas TRPM7 is activated by changes in serum magnesium levels. In addition, some of these TRP channels have been reported to be correlated with cell proliferation and survival.Citation8,Citation9 TRPM7 and TRPC1 channels have been shown to be altered in breast cancer cell lines,Citation10,Citation11 and thus could play a role in breast carcinogenesis; however, its expression in breast cancer tissues with microcalcifications remains unknown.
Several studies have examined microcalcifications from the pathological and radiological perspectives; however, the association between serum Ca2+ levels and the presence of breast microcalcifications is still unknown. The aim of this study was to investigate whether serum Ca2+ levels correlate with the Breast Imaging Reporting and Data System (BI-RADS) scores, and to examine the expression of these TRP channels in tissue samples from control or infiltrating ductal carcinoma with microcalcifications.
Methods
Study design
We reviewed the medical charts of women with incident breast microcalcifications between February 2004 and August 2011. Patients were identified using International Classification of Diseases-9 code 793.81 for mammographic microcalcifications at Sanford Health, North Dakota, USA. The study was approved by the Institutional Review Boards of the Hospital and the University of North Dakota.
Data collection
Information on age at diagnosis of microcalcifications, date of microcalcification diagnosis, BI-RADS score ascertained from the pathology report, presence of carcinoma, histology, serum Ca2+, albumin, sodium, vitamin D, and the date of serum calcium measurement were obtained from the electronic medical records.
The exclusion criteria consisted of male gender, diagnosis of any cancer, chronic kidney disease, primary hyperparathyroidism, thiazides drug use, date of serum Ca2+ was more than 12 months before microcalcifications diagnosis, and diagnosis outside the study period.
Eligible cases were 219 women with confirmed breast microcalcifications from the pathology report. Thirty-six women were excluded; 14 women had missing values for serum Ca2+, 11 had a missing BI-RADS score, 11 were missing both, and 45 serum calcium measurements were done more than a year from microcalcifications diagnosis, which left 138 eligible patients.
If the serum albumin level was <4 g/dL, the total serum Ca2+ was adjusted by a standard formula:Citation15
If the serum albumin was ≥4 g/dL or if the albumin was not measured, then the total serum Ca2+ was used.
We used the BI-RADS from the American College of Radiology to categorize the types of breast microcalcifications.Citation12–Citation14 BI-RADS scores (1, 2, and 3) correspond to benign disease and (4 and 5) to high-risk of breast cancer. Bent et alCitation3 have shown that the positive predictive value for breast carcinoma based on BI-RADS valuation groups were: category 2 and 3 (0%), category 4A (13%), category 4B (36%), category 4C (79%), and category 5 (100%).
Immunohistochemistry and imaging
Paraffin embedded tissues from age-matched controls without cancer and infiltrating ductal carcinomas with microcalcifications were obtained, and 10–12 μm thick cryosections were performed. Hematoxylin and eosin staining was performed on the sections using the standard procedure (Sigma-Aldrich, St Louis, MO, USA). For fluorescent confocal imaging, sections from respective samples were permeabilized at room temperature with 0.1% Triton™ X-100 (Sigma-Aldrich) in phosphate buffered saline (pH 7.4), blocked (10% donkey serum and 5% bovine serum albumin in phosphate buffered saline), and were probed overnight with respective primary antibodies in a hydrated chamber maintained at 4°C. Following incubation with primary antibodies, the slides were washed and reacted with fluorophore-conjugated respective secondary antibodies for 1 hour at room temperature. Thereafter, the slides were washed and coverslip mounted using VECTASHIELD® HardSet Mounting Media with DAPI (Vector Laboratories, Inc, Burlingame, CA, USA). Images were acquired at 63× magnifications using a confocal laser-scanning LSM 510 Meta Confocal Microscope (Carl Zeiss Microscopy, LLC, Thornwood, NY, USA). Total fluorescence from each section was quantified using the ImageJ program (NIH, Bethesda, MD), and increase in fluorescence range was determined.
Quantitative real-time polymerase chain reaction
TRPC1 and TRPM7 messenger ribonucleic acid (mRNA) expressions were assessed with real-time polymerase chain reaction (PCR) using commercially available primers (OriGene Technologies, Inc, Rockville, MD, USA). Total ribonucleic acid (RNA) was isolated from 10 μm thick pooled paraffin embedded tissues (two each) from each condition (control and breast cancer with microcalcification) using the QIAGEN kit (Qiagen, USA). Individual complementary deoxyribonucleic acid (cDNA) was transcribed from 0.5 μg of total RNA with iScript cDNA (Bio-Rad Laboratories, Hercules, CA, USA). An equal amount of cDNA template was added to iQ SYBR green super mix together with the appropriate primers at 0.2 μM each. Quantitative PCR was performed using an iCycler iQ™ (Bio-Rad Laboratories) real-time detection system following the specifications of the manufacturer. The relative level of mRNA was interpolated from a standard curve prepared by serially diluting the cDNA reaction. Glyceraldehyde 3-phosphate dehydrogenase was used for normalization of the transcripts. Specificity of PCR product formation was confirmed by monitoring melting peaks.
Statistical analysis
Crude mean values were computed for all continuous variables and frequency distributions were calculated for all categorical variables. We compared women who were diagnosed with breast microcalcifications based on their BI-RADS scores (1, 2, and 3) versus (4 and 5) on demographic and other variables using the Wilcoxon signed-rank test for continuous variables and the chi-square test for categorical variables. Logistic regression was used to assess whether serum Ca2+, as a continuous variable, predicts higher BI-RADS score. All statistical tests were two-tailed with P < 0.05 considered to be significant. Statistics were performed using SAS (version 9.3 Users Guide; SAS Institute Inc, Cary, NC, USA).
Results
Participants were 138 women with newly-diagnosed breast microcalcifications (). The majority (70%) had a BI-RADS score of 1, 2, or 3, whereas the rest had a BI-RADS score of 4 or 5. Importantly, 76% of women with high BI-RADS scores (4 or 5) were diagnosed with breast cancer, suggesting that higher BI-RADS scores correlated with breast cancer. Additionally, 56% of these breast cancers were cancers in situ, and 93% of the cancers were infiltrating ductal carcinomas. We did not observe a significant difference in the distribution of corrected serum Ca2+ between BI-RADS scores 1, 2, or 3, and BI-RADS scores 4 or 5 (9.54 ± 0.4 versus 9.56 ± 0.5; P-value = 0.82), or between women who were diagnosed with breast cancer versus women who were cancer free (P-value = 0.94). Comparing women with BI-RADS scores of 4 or 5 to women with BI-RADS scores of 1, 2, or 3, the age-adjusted odds ratio for corrected serum Ca2+ was 1.05 (95% confidence interval: 0.46–2.41) (). Furthermore, the level of serum 25-hydroxyvitamin D, which is important for the absorption of Ca2+, was increased in higher BI-RADS scores (4, 5) compared to women with BI-RADS scores of 1, 2, or 3, suggesting that perhaps an increase in intracellular Ca2+ is the reason that no alteration in serum Ca2+ is observed.
Table 1 Descriptive characteristics of newly diagnosed breast microcalcifications by BI-RADS scores
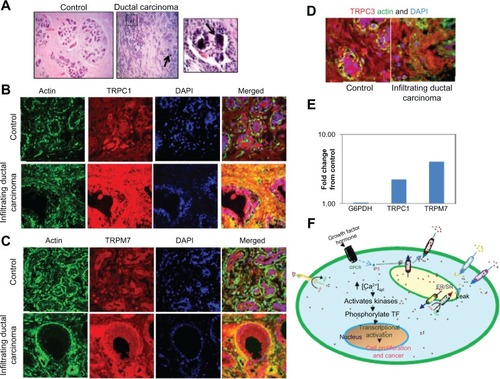
Since Ca2+ entry is dependent on various Ca2+ channels, we studied the expression/localization of various Ca2+ channels that have distinct modes of activation. In tissue samples with no cancer, localization of the endogenous TRPC1 protein displayed a uniform plasma membrane along with some subplasma membrane staining of both acinar and ductal cells (). Conversely, a significant increase (~30%) in TRPC1 expression was observed in the infiltrating ductal carcinoma tissue samples that had microcalcification, whereas no increase in actin (used as control) staining was observed. Tissues without the TRPC1 antibody showed no staining (). To further establish the expression of receptor operated Ca2+ channels, expression of TRPC3 was observed. As indicated in , no change in expression levels of TRPC3 was observed in control or infiltrating ductal carcinoma with microcalcification. TRPM7, which has been shown to be activated by decrease in serum magnesium levels and is increased in breast cancer, was also evaluated. As indicated in , increased staining of TRPM7 was also observed in infiltrating ductal carcinoma patients that exhibited microcalcification (~40% increase) when compared with control samples, further suggesting that these channels can play a role in breast cancer associated with microcalcifications. To validate these results, RNA was isolated from polled samples (two each) from control and breast cancer and cDNA was obtained. Finally real-time PCR was performed to confirm the expression of TRPC1, which showed a twofold increase in expression in breast cancer cells. Similarly, a fourfold increase in TRPM7 expression was observed in breast cancer samples when compared with controls.
Figure 1 TRPC1 and TRPM7 expression is increased in infiltrating ductal carcinomas. Hematoxylin and eosin staining of control and ductal carcinoma samples. Respective images were taken at 10× zoom; microcalcification has been shown with an arrow, and also a magnified image showing microcalcification is provided on the right (A). Confocal images showing endogenous TRPC1 (B), TRPM7 (C), TRPC3 (D), and actin staining in age-matched controls (without cancer or microcalcifications) and infiltrating ductal carcinoma samples with microcalcifications were observed. Anti-TRPC1, Anti-TRPC3, or Anti-TRPM7 antibodies followed by rhodamine-conjugated secondary antibodies were used to detect endogenous TRPC1, TRPC3, and TRPM7 protein. Antiactin antibodies followed by FITC-labeled secondary antibodies were used to detect actin levels. The images provided are from at least two to three individual sections, and total fluorescence was evaluated using the Zeiss software (Carl Zeiss Microscopy, LLC, Thornwood, NY, USA). Fold change in the expression of G6PDH, TRPC1, and TRPM7 in controls, as well as breast cancer samples were observed (E). A model for the activation of various channels and their role in increasing cytosolic calcium that leads to cancer is shown (F).
Discussion
In this study of women diagnosed with incident breast micro-calcifications that were confirmed in the pathology report, we did not find an association between serum Ca2+ levels and BI-RADS scores, or breast cancer with microcalcifications. Our data also showed that the overall incidence of breast cancer was 23.2%, which is in accordance with the positive predictive value for breast cancer reported by other investigators (21%–42%).Citation3,Citation16–Citation19 Of the malignancies found, 62% involved infiltrating ductal carcinoma, whereas the remaining 34% represented invasive cancer. The proportion of these histopathologic findings is similar to those of previously published reports.Citation15,Citation17,Citation18
To understand why no alterations in serum Ca2+ levels were observed, we focused our attention on various Ca2+ channels. Importantly, Cheung et alCitation20 reported that in cell cultures, the deposition of calcification crystals on the cell membrane leads to an acute Ca2+ response by increasing intracellular Ca2+ concentration, suggesting that microcalcification stimulates Ca2+ channels on the membrane. Additionally, the latter on a sustained elevation in intracellular Ca2+ levels was also observed, which could be due to crystal ingestion and perhaps its dissolution. Last but not least, experiments where cells are treated with crystals that are too large to endocytose only revealed a transient rise in intracellular Ca2+,Citation20 indicating that crystal deposition per se causes an influx of extracellular Ca2+. This is consistent with our findings, which showed that expression of TRPM7 and TRPC1, but not TRPC3 channels were increased in infiltrating ductal carcinoma samples when compared with age-matched controls without cancer.
In response to many extracellular signals, mammalian cells can trigger an elevation of intracellular Ca2+ by increasing the entry of Ca2+ from the extracellular through the Ca2+ channels, thereby decreasing serum Ca2+ levels. Intracellular Ca2+ could also be released simultaneously from intracellular stores as a consequence of 1,4,5-inositol triphosphate generation.Citation21,Citation22 Binding of 1,4,5-inositol triphosphate to its receptor in the ER triggers a release of Ca2+ from the internal ER stores that further activates plasma membrane Ca2+ channels, a phenomenon known as receptor operated Ca2+ entry ().Citation23 Although the ion channels that regulate the receptor operated calcium channels in breast cancer cells are unknown, previous studies have shown that TRPC3 can be activated by this mechanism. In addition, store depletion per se has been shown to activate SOCE, and both Orai1 and TRPC1 channels can function as SOCE channels in different cells.Citation24 Thus, an increase in the expression of these channels could facilitate an increase in intracellular Ca2+ that can lead to breast cancer. Since the expression of TRPC1 was increased in these cells, we hypothesize that TRPC1 could significantly contribute to increased intracellular Ca2+ to induce cell proliferation. TRPM7 channel has also been linked to cell proliferation and survival of breast cancer cells, which is consistent with our results, where increased expression of TRPM7 was observed in the ductal carcinoma samples with microcalcifications.
Holme et alCitation25 hypothesized that an abnormality in Ca2+ pumps or Ca2+ regulatory proteins might result in the deposition of microcalcifications. Consistent with these results, calcium-sensing receptors (CaR) have also been suggested to be involved in calcifications in the vascular smooth muscle cells.Citation26 Importantly, CaR has been shown to play a key role in the regulation of extracellular Ca2+ homeostasis in various tissues including breast tissues.Citation27 Although the role of CaR in microcalcifications of breast tissues has not yet been established, it can be hypothesized that it may have an impact on microcalcification genesis. Alteration in this Ca2+ homeostasis, due to aberrant activation of CaR, can lead to microcalcification, and more research is needed to establish this link. However, an increase in microcalcification can be ultimately responsible for the intake of Ca2+ via the TRPC1 channels that will lead to an increase in cell proliferation.
We acknowledge some limitations; this study was restricted to women with recent data on Ca2+, which raises the possibility of selection bias. However, extending the period for Ca2+ measurement from the microcalcifications diagnosis did not result in an association between serum Ca2+ and BI-RADS scores. It is possible that our results could be influenced by differences in vitamin D levels. Although serum Ca2+ levels might increase with serum levels of vitamin D, this correlation is most evident in the setting of vitamin D insufficiency and excess.Citation28 Conversely, this is the first study to assess serum Ca2+ levels in the presence of breast microcalcifications and to quantify two distinct Ca2+ channels in ductal carcinoma samples in the presence of microcalcifications. Finally, although TRPC1 and TRPM7 channels were overexpressed in this study, their activity is still unknown. Future studies should explore the function of these channels using wet tissue samples and measure intracellular Ca2+ levels according to BI-RADS scores. This information will be critical in understanding the etiology of ductal carcinoma and in exploring potential therapeutic interventions to treat breast cancer.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We thank Ms Mary Kara for assistance with the electronic medical records data. We also thank Dr Donald Sens for providing us with control and breast cancer tissue samples. The lab work was supported by National Institutes of Health Grants DE017102 and 5P20RR017699 (to BBS).
References
- MorganMPCookeMMMcCarthyGMMicrocalcifications associated with breast cancer: an epiphenomenon or biologically significant feature of selected tumors?J Mammary Gland Biol Neoplasia200510218118716025224
- MorganMPCookeMMChristophersonPAWestfallPRMcCarthyGMCalcium hydroxyapatite promotes mitogenesis and matrix metalloproteinase expression in human breast cancer cell linesMol Carcinog200132311111711746823
- BentCKBassettLWD’OrsiCJSayreJWThe positive predictive value of BI-RADS microcalcification descriptors and final assessment categoriesAJR Am J Roentgenol201019451378138320410428
- AlmquistMAnagnostakiLBondesonLSerum calcium and tumour aggressiveness in breast cancer: a prospective study of 7847 womenEur J Cancer Prev200918535436019593149
- AlmquistMManjerJBondesonLBondesonAGSerum calcium and breast cancer risk: results from a prospective cohort of 7,846 womenCancer Causes Control200718659560217410477
- MartinEMillerMKrebsbachLBealJRSchwartzGGSahmounAESerum calcium levels are elevated among women with untreated postmenopausal breast cancerCancer Causes Control201021225125719856117
- ThawSSSahmounASchwartzGGSerum calcium, tumor size, and hormone receptor status in women with untreated breast cancerCancer Biol Ther201213746747122406994
- YeeNSZhouWLeeMYeeRKTargeted silencing of TRPM7 ion channel induces replicative senescence and produces enhanced cytotoxicity with gemcitabine in pancreatic adenocarcinomaCancer Lett201231819910522166235
- GuilbertAGautierMDhennin-DuthilleIHarenNSevestreHOua-did-AhidouchHEvidence that TRPM7 is required for breast cancer cell proliferationAm J Physiol Cell Physiol20092973C493C50219515901
- DavisFMPetersAAGriceDMNon-stimulated, agonist-stimulated and store-operated Ca2+ influx in MDA-MB-468 breast cancer cells and the effect of EGF-induced EMT on calcium entryPLoS One201275e3692322666335
- Ouadid-AhidouchHDhennin-DuthilleIGautierMSevestreHAhidouchATRP calcium channel and breast cancer: expression, role and correlation with clinical parametersBull Cancer201299665566422640890
- MasroorIEffectiveness of assigning BI-RADS category-3 to breast lesion with respect to follow-upJ Coll Physicians Surg Pak200818420921218474152
- American College of RadiologyIllustrated Breast Imaging Reporting and Data System (BI-RADS)3rd edReston, VAAmerican College of Radiology1998
- CardenosaGMendelsonEBassettLAppropriate imaging work-up of breast microcalcifications. American College of Radiology. ACR Appropriateness CriteriaRadiology2000Suppl 21597398011037525
- BairdGSIonized calciumClin Chim Acta20114129–1069670121238441
- BurnsideESOchsnerJFowlerKJUse of Microcalcification Descriptors In BI-RADS, 4th ed to stratify risk of malignancyRadiology2007242238839517255409
- LibermanLAbramsonAFSquiresFBGlassmanJRMorrisEADershawDDThe breast imaging reporting and data system: positive predictive value of mammographic features and final assessment categoriesAJR Am J Roentgenol1998171135409648759
- UematsuTKasamiMYuenSUsefulness and limitations of the Japan Mammography Guidelines for the categorization of microcalcificationsBreast Cancer200815429129718288569
- KettritzUMorackGDeckerTStereotactic vacuum-assisted breast biopsies in 500 women with microcalcifications: radiological and pathological correlationsEur J Radiol200555227027616036159
- CheungHSStoryMTMcCartyDJMitogenic effects of hydroxyapatite and calcium pyrophosphate dihydrate crystals on cultured mammalian cellsArthritis Rheum19842766686746329235
- RasmussenHThe calcium messenger system (2)N Engl J Med198631418116411703007987
- RasmussenHThe calcium messenger system (1)N Engl J Med198631417109411012870434
- PaniBBollimunthaSSinghBBThe TR (i)P to Ca2+ signaling just got STIMy: an update on STIM1 activated TRPC channelsFront Biosci201217805823
- ChengKTOngHLLiuXAmbudkarISContribution of TRPC1 and Orai1 to Ca(2+) entry activated by store depletionAdv Exp Med Biol201170443544921290310
- HolmeTCReisMMThompsonAIs mammographic microcalcification of biological significance?Eur J Surg Oncol19931932502538390947
- AlamMUKirtonJPWilkinsonFLCalcification is associated with loss of functional calcium-sensing receptor in vascular smooth muscle cellsCardiovasc Res200981226026818852253
- SaidakZMentaverriRBrownEMThe role of the calcium-sensing receptor in the development and progression of cancerEndocr Rev200930217819519237714
- SkinnerHGLitzelmanKSchwartzGGRecent clinical trials of vitamin D3 supplementation and serum calcium levels in humans: Implications for vitamin D-based chemopreventionCurrent Opin Investig Drugs2010116678687