Abstract
Breast cancer is a leading cause of cancer-related deaths in females. Triple-negative breast cancer (TNBC) subtype is the most aggressive form of breast cancer that lacks biomarkers and effective targeted therapies. Its high degree of heterogeneity as well as innate and acquired resistance to treatment creates further barriers in achieving positive clinical outcomes in TNBC. Thus, development of novel treatment approaches in TNBC is of high clinical significance. Multimodality approaches with targeted agents and radiotherapy (RT) are promising for increasing efficacy of treatment and circumventing resistance. Here we examined anticancer effects of the Aurora Kinase B (AURKB) inhibitor AZD1152 as a single agent and in combination with RT using various TNBC cell lines, MDA-MB-468, MDA-MB-231 and SUM-159. We observed that AZD1152 alone effectively inhibited colony formation in TNBC cell lines. The combination of AZD1152 at IC50 concentrations together with ionizing radiation further reduced colony formation as compared to the single agent treatment. Our data support the notion that inhibition of the AURKB pathway is a promising strategy for treatment and radiosensitization of TNBC and warrants further translational studies.
Plain Language Summary
Breast cancer is a leading cause of cancer death in women globally. The triple negative breast cancer subtype confers the poorest oncologic outcomes and requires novel treatment approaches. Development of new therapeutics as well as combination treatments with radiation are crucial. Aurora Kinase B (AURKB) protein regulates cell division that is often altered in breast cancer, contributing to tumor pathogenesis. This study examined the combination of an AURKB inhibitor, AZD1152, with radiation therapy, compared to single-agent treatments, in treating triple negative breast cancer cells. Our results show that AZD1152 and ionizing radiation alone were able to delay cancer cell proliferation effectively. However, their combination further significantly inhibited cell proliferation compared to single-agent treatments. This suggests that further studies on this combination would be valuable in developing novel treatment strategies for breast cancer.
Introduction
Breast cancer is the world’s most prevalent cancer and a leading cause of cancer-related mortality in females, with 2.3 million women diagnosed and 685,000 deaths globally in 2020.Citation1 Some of its subtypes, such as triple negative breast cancer (TNBC), represent more aggressive phenotypes. Lack of common biomarkers and effective targeted therapies, as well as innate and/or acquired resistance to radiotherapy (RT) and chemotherapyCitation2–4 are important contributors to poor oncologic outcomes in TNBC. Developing new treatment strategies is therefore of critical importance. RT is a key modality for local disease control of breast cancer in the adjuvant and metastatic settings. It might also have advantages in the neoadjuvant setting to reduce tumour burden, facilitate surgical resection and induce chemosensitization of cancer.Citation5,Citation6 Inherited or induced radioresistance is the major barrier to the effective control of disease and this may be overcome by the use of radiosensitizing agents.Citation7 Combination treatments with chemotherapeutic or targeted agents and RT to synergize effects of each individual treatment can potentially improve anti-tumour activity and oncologic outcomes.Citation7–10
RT is known to induce genomic instability.Citation7,Citation11 Compounds that target proteins controlling genomic stability might enhance effects of RT and help in overcoming radioresistance. One such protein, Aurora kinase B (AURKB), has been shown to be overexpressed in TNBC,Citation12 associated with increased familial breast cancer risks,Citation13 reported as a prognostic factor for TNBCCitation14 and associated with resistance to chemotherapy.Citation15 AURKB plays a role in metaphase and is an essential component of the chromosome passenger complex, chromosome segregation and cytokinesis; dysregulation of which causes abnormal cell division and aneuploidy, a hallmark of cancer.Citation16 Various compounds have been developed to target AURKB in cancer. One such highly selective AURKB inhibitor, AZD1152 (Barasertib), has been shown to be an effective antineoplastic agent in breast cancerCitation17–19 acting through induction of mitotic catastrophe, polyploidy and apoptosis.Citation17 Therefore, combination of RT with an AURKB inhibitor may further enhance genomic instability in TNBC and eventually cell death. AZD1152 has been shown to enhance radiosensitivity in other cancers, such as prostateCitation20 and colon cancers.Citation21,Citation22 Recently, an abstract has been published, suggesting that Barasertib-HQPA induces radiosensitization in murine 4T1 TNBC cells.Citation23 However, to our knowledge, the anticancer effects of the combination of AURKB inhibition by AZD1152 or other agents with radiation in human breast cancer has never been studied. In this study, we aimed to test if the combination of RT with AZD1152 leads to enhanced anticancer effects in TNBC cell lines.
Materials and Methods
The MDA-MB-468 cell line was obtained from Dr. Ann Chambers (London Health Science Centre, London, Canada) and was cultured in α-MEM + 10% fetal bovine serum (FBS). The MDA-MB-231 cell line was obtained from Dr. Ann Chambers (London Health Science Centre) and was cultured in DMEM:F12 + 10% FBS. The SUM-159 cell line (Asterand Inc., Detroit, MI) was cultured in HAMS:F12 + 5% FBS, 0.5% insulin, 0.1% hydrocortisone and 1% HEPES. All cell lines were authenticated by the short tandem repeat (STR) profiling (using 9 markers). AZD1152 (Cat. No. S1147) was obtained from SelleckChem (Pennsylvania, USA). RT was performed using the Cobalt-60 radiation unit at the London Regional Cancer Program. Doses of RT and drug concentrations were selected by determining the ID50 or IC50 respectively for each cell line using dose response curves. Colony formation assays (CFA) were performed as previously described.Citation24 Briefly, cells were seeded in 6 well plates at colony forming density (52 cells/cm2 for MDA-MB-231, SUM159, and 104 cells/cm2 for MDA-MB-468). Adhered cells were treated with RT 16–20 hours later followed immediately by drug or vehicle control-supplemented media at the indicated doses, then grown for 7–14 days prior to fixation with acetone:methanol (1:1 vol/vol) and stained with 0.5% crystal violet in ddH2O. Plates were imaged, colonies consisting of ≥50 cells were counted, and numbers were compared to vehicle control. Experiments were performed at least in triplicate. Synergy was assessed using SynergyFinder software (version 3.0)Citation25 using the Bliss score.
Statistical analysis was performed using GraphPad Prism Software (Dotmatics, San Diego, USA). IC50/ID50 doses of compounds and RT were calculated from single-agent dose response curves using a nonlinear regression model. Comparison of control, single agent and combination effects for CFA was performed using Two-Way ANOVA. Statistical significance was defined as p≤0.05.
Results
Effects of Combination Treatment with AZD1152 and Radiation in TNBC Cell Lines
We determined IC50 (for AZD1152) for each cell line using CFA (). Based on our previous data, we used RT doses close to the therapeutically relevant dose of ID50 (2Gy of RT with MDA-MB-231 and MDA-MB-468 cells and 5Gy of RT with SUM-159 cells). Since different cell lines exhibited a different IC50 to AZD1152, we used a concentration of 15 nM for MDA-MB-231, 14nM for MDA-MB-468, and 124nM for SUM-159 cells. We then assessed efficacy of and differences in the combinatorial effect of AZD1152 and RT using these treatment parameters.
Figure 1 Effects of single agent AZD1152 at increasing concentrations on colony formation in TNBC cell lines. Dose response curves were generated for AZD1152 in studied cell lines. IC50 values were determined using a nonlinear regression model in GraphPad Prism software.
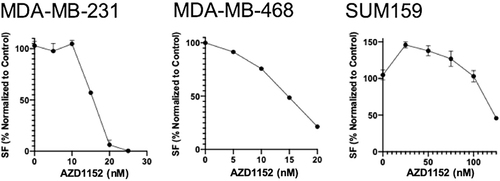
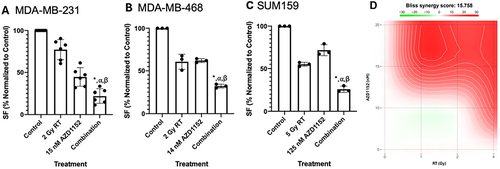
In MDA-MB-231 (), single agent treatment with AZD1152 resulted in a 46.9% (standard deviation, SD = 7.3%) decrease in colony formation (CF); 2 Gy of RT reduced CF by 17.5 ± 4.1%, while simultaneous combination treatment resulted in a 70.6 ± 3.7% decrease in CF compared to control (p≤0.05). This translated to a 2.8- and 1.8-fold decrease in CF with the combination treatment compared to RT or AZD1152 only treatments respectively. In MDA-MB-468 (), single agent treatment with AZD1152 resulted in a 38.0% (SD = 2.2%) decrease in CF; 2 Gy of RT reduced CF by 39.3 ± 8.8%, while simultaneous combination treatment resulted in a 67.9 ± 2.2% decrease in CF compared to control (p≤0.05). This translated to a 1.9- and 1.9-fold decrease in CF with the combination treatment compared to RT or AZD1152 only treatments respectively. In SUM-159 (), single agent treatment with AZD1152 resulted in a 28.3% (SD = 6.6%) decrease in CF; 5 Gy of RT reduced CF by 45.0 ± 2.3%, while simultaneous combination treatment resulted in a 74.3 ± 3.6% decrease in CF compared to control (p≤0.05). This translated to a 2.2- and 2.8-fold decrease in CF with the combination treatment compared to RT or AZD1152 only treatments respectively. To confirm synergistic effect of the combination, we investigated dose response matrices of different concentrations of the drug and doses of RT using the CFA (N = 3) in MDA-MB-231 cells. The analysis of the data using the SynergyFinder software suggested synergy (Bliss score of 15.8) between the drug and RT in providing an antiproliferative effect in TNBC cell lines ().
Figure 2 Effects of AZD1152 in combination with RT in TNBC cell lines. Combination treatment of (A) MDA-MB-231; (B) MDA-MB-468; (C) SUM159 cells with a single concentration of AZD1152 and a single dose of RT. Treatment with RT or AZD1152 alone results in reduced colony formation with further significant reduction of proliferation by a combination treatment relative to single agent treatment (p≤0.05 compared to control (*), RT only (⍺) or (β) AZD1152 only treatment); (D) Synergy plot using various concentrations of AZD1152 and doses of RT in MDA-MB-231 cells using SynergyFinder, where the intensity of red indicates a higher degree of synergy (n = 3).
Discussion
Targeting the axis of control of genomic stability appears to be a promising avenue for enhancing anticancer effects of RT. One of the promising targets for development of novel therapies in cancer is AURKB. It is localized to the centromeres and microtubules and is involved in the processes of chromosome alignment, kinetochore orientation and cytokinesis.Citation22 AURKB is often aberrantly expressed in breast cancerCitation26 and, if dysregulated, has been shown to induce genomic instability.Citation17 AURKB inhibition exacerbates genomic instability by targeting the microtubules or microtubule-organizing centres.Citation27 Inhibition of AURKB by AZD1152 has been shown to induce mitotic catastrophe (polyploidy, multinucleation and micronuclei formation) and sensitize cancer cells to RT.Citation20–22,Citation28 RT itself is known to induce genomic instability.Citation7,Citation10,Citation11 Compounds that target proteins controlling genomic stability might enhance effects of RT and help in overcoming radioresistance. Hence, we investigated if the combination of AURKB inhibition with RT could further enhance anti-cancer effects of RT in TNBC.
In the current study, we assessed effects of the AURKB inhibitor AZD1152 in combination with RT in TNBC. We show that AZD1152 leads to a decrease in TNBC cell proliferation in various cell lines in vitro. Moreover, we observed that the combination of the drug and RT significantly decreases breast cancer cell proliferation compared to a single agent treatment. Our data are in agreement with the previously published reports in other cancer types. AZD1152 has been shown to enhance radiosensitivity and counteraction of radioresistance in colon,Citation21 lung,Citation22,Citation28 gynecological Citation29 and androgen-resistant prostate cancerCitation20 cells. In non-small cell lung carcinoma cells, low concentration AZD1152 treatment during irradiation affected repopulation during RT.Citation28 These findings suggest that concomitant AURKB inhibition and RT may be a promising strategy for fast repopulating tumors, such as TNBC. This is an important observation, since in clinical trials in myeloid malignancies and solid tumours, while showing promising efficacy, AZD1152 (Barasertib), induced dose-limiting myelotoxicity limiting its subsequent clinical development.Citation18,Citation30–32 Hence, combining low dose AURKB with RT might provide promising results in terms of decreasing drug toxicity, increasing its efficacy through RT-mediated chemosensitization and increasing RT efficacy. These multimodality treatment approaches are emphasized as a promising avenue for clinical developmentCitation8,Citation9 and under investigation in clinical trials in patients with TNBC.Citation33–35
Overall, this study confirms that inhibition of the AURKB axis is a promising strategy for therapeutic development, including multimodality treatment strategies to enhance sensitivity to and anticancer effects of RT in TNBC. Several non-specific or pan-aurora kinase inhibitors have also been investigated in Phase 1 and 2 clinical trials with limited clinical activity reported.Citation36–39 However selective AURKB inhibitors, such as AZD1152, show clinical promise as anticancer agents.Citation17 Clinical use of AZD1152 is being investigated with a limiting factor for clinical application being myelotoxicity. AZD1152 is a pro-drug of the highly potent and selective AURKB inhibitor AZD2811 and novel avenues are being explored to improve efficacy and tolerability of AZD2811, such as use of nanoparticles for drug delivery.Citation32,Citation40–42
The limitations of this study include the use of immortalized cancer cells that might not well represent the heterogeneity of TNBC and the lack of in vivo data regarding this combination. Future studies utilizing patient-derived in vitro and in vivo models would help to better capture the heterogeneity observed in TNBC patients and would better recapitulate patient responses to this combination. This manuscript provides the first evidence for the potential role of combining AURKB inhibition with RT in the treatment of TNBC using immortalized human cancer cell lines. Further translational studies of the combination treatments with AURKB inhibition and RT are warranted to facilitate development of clinical trials and new treatments in TNBC. This multimodality treatment strategy might be found to be useful in various clinical scenarios, such as in the neoadjuvant setting where surgical removal of the primary tumor might be facilitated by enhancing its response and making it smaller; or in a metastatic setting where the combination of this radiosensitizing drug with radiation would provide better control of the metastatic deposits.
Conclusion
In conclusion, AURKB inhibition appears to be a promising target for the treatment of TNBC as a single agent or in combination with RT. Further translational and clinical studies of AZD1152 and its analogues would shed light on the clinical application of AURKB inhibition in the treatment of TNBC as a single agent or in combination with other chemo and radiotherapeutic approaches.
Disclosure
The authors declare no conflicts of interest in this work.
Acknowledgments
A.P. lab research is supported by the Cancer Research Society (CRS; Grant #942549)/Canadian Institutes of Health Research (CIHR; Funding Reference #184668) /Institute of Cancer Research (ICR), Operating Grant 2022 Competition, the Young Investigator Startup Grant, Department of Surgery, Western University and the London Regional Cancer Program Catalyst Grant for Translational Cancer Research, Western University (London, ON). A. P. was supported by a Clinical Scientist Award, Department of Surgery, Western University (London, ON) and the Opportunities Fund of the Academic Health Sciences Centre Alternative Funding Plan of the Academic Medical Organization of Southwestern Ontario (AMOSO; Project #F20-007). S.P. was supported by Breast Cancer Canada and by an Ontario Graduate Scholarship (OGS). H.A. is supported by Breast Cancer Canada. We would like to thank Dr. Vasudeva Bhat for his input regarding the experimental design and in the critical review of this manuscript.
References
- Arnold M, Morgan E, Rumgay H, et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast. 2022;66:15–23.
- Mahmoud R, Ordóñez-Morán P, Allegrucci C. Challenges for triple negative breast cancer treatment: defeating heterogeneity and cancer stemness. Cancers. 2022;14(17):4280. doi:10.3390/cancers14174280
- Burguin A, Diorio C, Durocher F. Breast cancer treatments: updates and new challenges. J Pers Med. 2021;11(8):808. doi:10.3390/jpm11080808
- Moran MS. Radiation therapy in the locoregional treatment of triple-negative breast cancer. Lancet Oncol. 2015;16(3):e113–e122. doi:10.1016/S1470-2045(14)71104-0
- Ahmed M, Jozsa F, Douek M. A systematic review of neo-adjuvant radiotherapy in the treatment of breast cancer. Ecancermedicalscience. 2021;15:1175. doi:10.3332/ecancer.2021.1175
- Sousa C, Cruz M, Neto A, et al. Neoadjuvant radiotherapy in the approach of locally advanced breast cancer. ESMO Open. 2020;4(Suppl 2):e000640.
- Bhat V, Pellizzari S, Allan AL, et al. Radiotherapy and radiosensitization in breast cancer: molecular targets and clinical applications. Crit Rev Oncol Hematol. 2022;169:103566. doi:10.1016/j.critrevonc.2021.103566
- Ahmad SS, Crittenden MR, Tran PT, et al. Clinical development of novel drug-radiotherapy combinations. Clin Cancer Res. 2019;25(5):1455–1461. doi:10.1158/1078-0432.CCR-18-2466
- Sharma RA, Plummer R, Stock JK, et al. Clinical development of new drug-radiotherapy combinations. Nat Rev Clin Oncol. 2016;13(10):627–642.
- Parsyan A, Cruickshank J, Hodgson K, et al. Anticancer effects of radiation therapy combined with Polo-Like Kinase 4 (PLK4) inhibitor CFI-400945 in triple negative breast cancer. Breast. 2021;58:6–9. doi:10.1016/j.breast.2021.03.011
- Huang R-X, Zhou P-K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct Target Ther. 2020;5(1):60. doi:10.1038/s41392-020-0150-x
- Alam MS, Sultana A, Wang G, Haque Mollah MN. Gene expression profile analysis to discover molecular signatures for early diagnosis and therapies of triple-negative breast cancer. Front Mol Biosci. 2022;9:1049741. doi:10.3389/fmolb.2022.1049741
- Tchatchou S, Wirtenberger M, Hemminki K, et al. Aurora kinases A and B and familial breast cancer risk. Cancer Lett. 2007;247(2):266–272. doi:10.1016/j.canlet.2006.05.002
- Yuan K, Wu M, Lyu S, Li Y. Identification of prognostic genes for early basal-like breast cancer with weighted gene co-expression network analysis. Medicine. 2022;101(42):e30581. doi:10.1097/MD.0000000000030581
- Liu M, Li Y, Zhang C, Zhang Q. Role of Aurora kinase B in regulating resistance to paclitaxel in breast cancer cells. Hum Cell. 2022;35(2):678–693. doi:10.1007/s13577-022-00675-8
- Ahmed A, Shamsi A, Mohammad T, Hasan GM, Islam A, Hassan MI. Aurora B kinase: a potential drug target for cancer therapy. J Cancer Res Clin Oncol. 2021;147(8):2187–2198. doi:10.1007/s00432-021-03669-5
- Gully CP, Zhang F, Chen J, et al. Antineoplastic effects of an Aurora B kinase inhibitor in breast cancer. Mol Cancer. 2010;9(1):42. doi:10.1186/1476-4598-9-42
- Falchook GS, Bastida CC, Kurzrock R. Aurora kinase inhibitors in oncology clinical trials: current state of the progress. Semin Oncol. 2015;42(6):832–848. doi:10.1053/j.seminoncol.2015.09.022
- Zhang J, Lin X, Wu L, et al. Aurora B induces epithelial-mesenchymal transition by stabilizing Snail1 to promote basal-like breast cancer metastasis. Oncogene. 2020;39(12):2550–2567. doi:10.1038/s41388-020-1165-z
- Niermann KJ, Moretti L, Giacalone NJ, et al. Enhanced radiosensitivity of androgen-resistant prostate cancer: AZD1152-mediated Aurora kinase B inhibition. Radiat Res. 2011;175(4):444–451. doi:10.1667/RR2317.1
- Tao Y, Leteur C, Calderaro J, et al. The Aurora B kinase inhibitor AZD1152 sensitizes cancer cells to fractionated irradiation and induces mitotic catastrophe. Cell Cycle. 2009;8(19):3172–3181. doi:10.4161/cc.8.19.9729
- Tao Y, Zhang P, Girdler F, et al. Enhancement of radiation response in p53-deficient cancer cells by the Aurora-B kinase inhibitor AZD1152. Oncogene. 2008;27(23):3244–3255. doi:10.1038/sj.onc.1210990
- Jungles KM, Wang Z, Bishop C, et al. Targeting Aurora kinase B (AURKB) as a radiosensitizing strategy in syngeneic models of triple negative breast cancer (TNBC) (Abstract 708). Cancer Resear. 2024;84(6_Supplement):708. doi:10.1158/1538-7445.AM2024-708
- Mason JM, Lin DC, Wei X, et al. Functional characterization of CFI-400945, a Polo-like kinase 4 inhibitor, as a potential anticancer agent. Cancer Cell. 2014;26(2):163–176. doi:10.1016/j.ccr.2014.05.006
- Ianevski A, Giri AK, Aittokallio T. SynergyFinder 3.0: an interactive analysis and consensus interpretation of multi-drug synergies across multiple samples. Nucleic Acids Res. 2022;50(W1):W739–W743. doi:10.1093/nar/gkac382
- Yang J, Ikezoe T, Nishioka C, et al. AZD1152, a novel and selective Aurora B kinase inhibitor, induces growth arrest, apoptosis, and sensitization for tubulin depolymerizing agent or topoisomerase II inhibitor in human acute leukemia cells in vitro and in vivo. Blood. 2007;110(6):2034–2040. doi:10.1182/blood-2007-02-073700
- Borah NA, Reddy MM. Aurora kinase B inhibition: a potential therapeutic strategy for cancer. Molecules. 2021;26(7):1981. doi:10.3390/molecules26071981
- Sak A, Stuschke M, Groneberg M, Kübler D, Pöttgen C, Eberhardt WE. Inhibiting the Aurora B kinase potently suppresses repopulation during fractionated irradiation of human lung cancer cell lines. Int J Radiat Oncol Biol Phys. 2012;84(2):492–499. doi:10.1016/j.ijrobp.2011.12.021
- Marampon F, Gravina GL, Popov VM, et al. Close correlation between MEK/ERK and Aurora-B signaling pathways in sustaining tumorigenic potential and radioresistance of gynecological cancer cell lines. Int J Oncol. 2014;44(1):285–294. doi:10.3892/ijo.2013.2167
- Schwartz GK, Carvajal RD, Midgley R, et al. Phase I study of barasertib (AZD1152), a selective inhibitor of Aurora B kinase, in patients with advanced solid tumors. Invest New Drugs. 2013;31(2):370–380. doi:10.1007/s10637-012-9825-7
- Cheung CH, Sarvagalla S, Lee JY, Huang YC, Coumar MS. Aurora kinase inhibitor patents and agents in clinical testing: an update (2011–2013). Expert Opin Ther Pat. 2014;24(9):1021–1038. doi:10.1517/13543776.2014.931374
- Burris HA, Wang JS-Z, Johnson ML, et al. A Phase I, open-label, first-time-in-patient dose escalation and expansion study to assess the safety, tolerability, and pharmacokinetics of nanoparticle encapsulated Aurora B kinase inhibitor AZD2811 in patients with advanced solid tumours. J clin oncol. 2017;35(15_suppl):TPS2608. doi:10.1200/JCO.2017.35.15_suppl.TPS2608
- Loap P, Loirat D, Berger F, et al. Combination of olaparib and radiation therapy for triple negative breast cancer: preliminary results of the RADIOPARP Phase 1 trial. Int J Radiat Oncol Biol Phys. 2021;109(2):436–440. doi:10.1016/j.ijrobp.2020.09.032
- Loap P, Loirat D, Berger F, et al. Concurrent olaparib and radiotherapy in patients with triple-negative breast cancer: the Phase 1 olaparib and radiation therapy for triple-negative breast cancer trial. JAMA Oncol. 2022;8(12):1802–1808. doi:10.1001/jamaoncol.2022.5074
- Ho AY, Barker CA, Arnold BB, et al. A Phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer. 2020;126(4):850–860. doi:10.1002/cncr.32599
- Bavetsias V, Linardopoulos S. Aurora Kinase Inhibitors: current Status and Outlook. Front Oncol. 2015;5:278. doi:10.3389/fonc.2015.00278
- Schöffski P, Jones SF, Dumez H, et al. Phase I, open-label, multicentre, dose-escalation, pharmacokinetic and pharmacodynamic trial of the oral Aurora kinase inhibitor PF-03814735 in advanced solid tumours. Eur J Cancer. 2011;47(15):2256–2264. doi:10.1016/j.ejca.2011.07.008
- Schöffski P, Besse B, Gauler T, et al. Efficacy and safety of biweekly i.v. administrations of the Aurora kinase inhibitor danusertib hydrochloride in independent cohorts of patients with advanced or metastatic breast, ovarian, colorectal, pancreatic, small-cell and non-small-cell lung cancer: a multi-tumour, multi-institutional Phase II study. Ann Oncol. 2015;26(3):598–607. doi:10.1093/annonc/mdu566
- Carducci M, Shaheen M, Markman B, et al. A phase 1, first-in-human study of AMG 900, an orally administered pan-Aurora kinase inhibitor, in adult patients with advanced solid tumors. Invest New Drugs. 2018;36(6):1060–1071. doi:10.1007/s10637-018-0625-6
- Floc’h N, Ashton S, Taylor P, et al. Optimizing therapeutic effect of aurora B inhibition in acute myeloid leukemia with AZD2811 nanoparticles. Mol Cancer Ther. 2017;16(6):1031–1040. doi:10.1158/1535-7163.MCT-16-0580
- Floc’h N, Ashton S, Ferguson D, et al. Modeling dose and schedule effects of AZD2811 nanoparticles targeting aurora B kinase for treatment of diffuse large B-cell lymphoma. Mol Cancer Ther. 2019;18(5):909–919. doi:10.1158/1535-7163.MCT-18-0577
- Ashton S, Song YH, Nolan J, et al. Aurora kinase inhibitor nanoparticles target tumors with favorable therapeutic index in vivo. Sci Transl Med. 2016;8(325):325ra317. doi:10.1126/scitranslmed.aad2355
