Abstract
Background
Triple-negative breast cancer (TNBC) is the most aggressive malignancy. Psychological distress and elevated CXCL1 level have been reported to be closely associated with the poor prognosis and quality of life of patients with TNBC. In preclinical studies using xenograft mouse models, XIAOPI formula, a nationally approved drug prescribed to patients at high risk for breast cancer, inhibited CXCL1 expression and improved survival. Traditional Chinese medicine has unique advantages in improving patients’ emotional disorders and quality of life. However, the impact of XIAOPI formula on the serum level of CXCL1, psychological distress, and quality of life among patients with TNBC is currently unknown.
Methods
In this study, we designed a randomized, double-blind, placebo-controlled trial. Patients with TNBC were randomly assigned to receive either the XIAOPI formula or a placebo for three months. The primary outcomes include serum CXCL1 expression, Self-Rating Anxiety Scale (SAS), and the Self-Rating Depression Scale (SDS). Secondary outcomes included the Pittsburgh Sleep Quality Index (PSQI) and the Functional Assessment of Cancer Therapy-Breast (FACT-B).
Results
A total of 60 patients with TNBC were enrolled in the investigation. The results showed that the XIAOPI formula significantly decreased CXCL1 expression compared with the control group. Moreover, in comparison to the placebo, the XIAOPI formula increased FACT-B scores while decreasing SDS, SAS, and PSQI scores.
Conclusion
In patients with TNBC, XIAOPI formula may be effective in reducing CXCL1 levels, enhancing psychological well-being, and quality of life. While our research offers a natural alternative therapy that may enhance the prognosis of TNBC, future validation of its therapeutic effects will require large-scale, long-term clinical trials.
Clinical Registration Number
Registration website: www.chictr.org.cn, Registration date: 2018-1-19, Registration number: ChiCTR1800014535.
Introduction
The most recent international statistics compiled by the International Agency for Research on Cancer (IARC) indicate that breast cancer has the highest incidence rate of all malignancies. Globally, the incidence of breast cancer was estimated to have increased to approximately 2.26 million new cases in 2020, resulting in 0.68 million breast cancer-related deaths.Citation1 Triple-negative breast cancer (TNBC) accounts for 15–20% of all pathological subtypes and is considered to have the most unfavorable clinical outcomes and prognosis.Citation2 At present, research indicates that the five-year survival rate for triple-negative breast cancer is approximately 77%.Citation3 This figure is significantly constrained by the slow advancements in targeted drugs. How to improve quality of life and clinical prognosis has emerged as a great challenge for oncologists.
Current research indicates that TNBC is closely associated with human immunity. TNBC is associated with increased tumor mutation burden, T cell infiltration, and PD-L1 expression.Citation4 Additionally, a poor prognosis and a microenvironment that suppresses the immune system are associated with a high infiltration of tumor-associated macrophages (TAMs) in triple-negative breast cancer.Citation5 Our previous study has demonstrated that CXCL1 is the highest chemokine secreted by TAMs.Citation6 In bladder cancer, it has been shown that urinary CXCL1 levels are significantly elevated in patients with invasive phenotypes than in those with noninvasive tumors and normal controls.Citation7 Whereas, CXCL1 demonstrates predictive power for the survival of patients with gastric cancer as an independent variable,Citation8 CXCL1 upregulation is significantly correlated with the progression and advanced stage of gastric cancer. Moreover, it was discovered that CXCL1 derived from TAMs recruited CXCR2+ myeloid-derived suppressor cells (MDSCs) to form a premetastatic niche, which finally led to liver metastasis in colorectal cancer.Citation9 In breast cancer, TAM-derived CXCL1 can facilitate metastasis by activating the NF-κB/SOX4 pathway.Citation6 Meanwhile, bioinformatic analysis and clinical investigation suggest that CXCL1 upregulation is significantly associated with TNBC, lymph node metastasis, and poor overall survival. In addition, a meta-analysis involving 2265 patients with cancer revealed that elevated CXCL1 expression in cancer is a risk factor indicating poor overall survival, an advanced TNM stage, and lymph node metastasis; thus, CXCL1 may be a promising prognostic biomarker for predicting cancer prognosis.Citation10
In addition to the immunosuppressive microenvironment, patients with TNBC have a significantly elevated likelihood of developing psychological distress, such as anxiety and depression, when compared to other breast cancer subtypes.Citation11 Psychological disorders have been demonstrated to adversely affect the adherence to treatment and survival rates of patients with cancer.Citation12 In addition, psychological disorders may contribute to the recurrence and metastasis of breast cancer through their ability to exacerbate tumor metabolism, immune suppression, oxidative stress, and glucocorticoid secretion, among other mechanisms.
Therefore, TNBC is frequently correlated with a decline in health-related quality of life (HRQoL).Citation13,Citation14 In addition, poor sleep quality is also prevalent in patients with TNBC due to the cytotoxic therapies and the fear of a poor prognosis.Citation15 Notably, it has been reported that the economic burden of patients with TNBC is a significant factor influencing their psychological distress and quality of life.Citation16 Given that TNBC typically occurs in younger individuals, the economic burden on the TNBC population is likely to be even more substantial. Therefore, it is critical and essential to identify an economic and effective way to enhance the psychological well-being and quality of life of patients diagnosed with TNBC.
Traditional Chinese medicine (TCM) is widely recognized for its efficacy and cost-effectiveness in enhancing the clinical prognosis and life quality of patients with cancer. In a prospective cohort study, it was revealed that the implementation of TCM could reduce the disease-related recurrence and metastasis rate by 11%.Citation17 The 2-year rate of invasive disease-free survival (DFS) in the TCM-exposed group was 88.7%, and in the non-exposed group was 82.5%.Citation17 At the same time, several TCM formulations have been discovered to inhibit the growth or metastasis of TNBC in mouse xenografts. It has been reported that the Shuganhuazheng formula inhibits TNBC growth by suppressing HIF-1 and Akt expression.Citation18 Tubeimu extracts were discovered to inhibit TNBC metastasis by decreasing levels of integrin β1, β8, and Rho GTPase activating protein 5.Citation19 Current evidence regarding the anti-tumor activity of TCM and natural medicine in TNBC is summarized in a literature review.Citation20 The results demonstrated that TCM formulations and plant medicine inhibited multiple processes of TNBC, including growth, proliferation, migration, invasion, and metastasis. Meanwhile, multiple pathways, such as PI3K/Akt/mTOR, MAPK, and Wnt/β-catenin, are involved in the molecular mechanisms of the anti-tumor activities of TCM. And not only that, TCM has also been proven to have advantages in improving emotional disorders and quality of life in cancer patients. In 2023, a meta-analysis of 9 randomized controlled trials involved 789 participants stated that a therapeutic regimen involving TCM could better improve the depression status and it can be an add-on therapy for postoperative depression in breast cancer patients. The results showed the intervention group was better at decreasing the score of the Hamilton rating scale for depression (HAMD) (mean difference, MD = −4.21, 95% CI −5.54 to −2.88) and SDS (MD = −12.03, 95% CI −15.94 to −8.13).Citation21 Another meta-analysis showed that TCM, when used in conjunction with the conventional Western medicine, could effectively improve the quality of life of breast cancer patients measured by rating scales (standardized mean difference, SMD = 1.29, P=0.01) and ranking scales (relative risk ratio, RR = 1.53, P=0.02) compared with the single-used conventional Western medicine treatment.Citation22
The XIAOPI formula is an officially approved drug for mammary hyperplasia treatment by the National Medical Products Administration of China. Previous studies demonstrated that the formula is effective in relieving atypical hyperplasia, mastalgia, and lump growth.Citation23 Meanwhile, the formulation exhibits minimal adverse effects during treatment. Our previous animal study demonstrated that the XIAOPI formula could inhibit TNBC growth and metastasis by suppressing TAMs and CXCL1.Citation24 Additionally, it was discovered that the XIAOPI formula enhances breast cancer chemosensitivity by inhibiting CXCL1/HMGB1-mediated autophagy.Citation25 Furthermore, XIAOPI formula can inhibit the pre-metastatic niche formation in TNBC by suppressing TAMs/CXCL1-mediated MDSC accumulation.Citation24 Intriguingly, the number of cancer stem cells in TNBC was also significantly reduced after XIAOPI administration.Citation26 These results collectively indicate that the XIAOPI formula could potentially enhance the clinical prognosis and quality of life of patients with TNBC. Further investigation is urgently required to validate its clinical efficacy in enhancing life quality and decreasing CXCL1 levels in patients with TNBC.
A randomized controlled trial was conducted in the current study to investigate the potential clinical effects of the XIAOPI formula on reducing the CXCL1 level and improving selected outcomes, namely anxiety, depression, sleep quality and life quality in patients with TNBC.
Methods
Study Design
This was a preliminary randomized, double-blind, placebo-controlled clinical trial. The Institutional Research Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine granted approval for the study (No. ZF2018-097). The study protocol is registered at the Chinese Clinical Trials Registry (Registration website: www.chictr.org.cn, Registration date: 2018-1-19, Registration number: ChiCTR1800014535). The protocols in this study adhere to the guidelines and regulations of China for good clinical practice as well as the World Medical Association Declaration of Helsinki. All participants provided signed, written informed consent before participating in this research (Supplementary Material - CONSORT 2010 checklist).
Randomization was performed using computer-generated numbers and stratified by center at a ratio of 1:1. An independent statistician with no clinical involvement in the trial prepared the randomization sequence and allocation concealment. The group designations remained unknown to all investigators, staff, participants, pharmacists, and sponsor personnel who were involved in the treatment administration, drug allocation, or clinical evaluation. The XIAOPI medication/placebo was administered in a blinded manner as capsules, which were identically packaged.
Population
The following were the inclusion criteria for the study: (a) Patients included in the study were women aged ≥ 18 years and ≤ 60 years; (b) histologically diagnosed as invasive breast cancer with pathological subtypes as triple negative, including ER-, PR-, and HER2-; (c) complete surgery and chemotherapy and follow up for at least 5 years; (d) absence of dysphagia and ability to take TCM formulas orally; (e) provided written informed consent in accordance with the requirements of the local ethics committee; (f) and an Eastern Cooperative Oncology Group (ECOG) score of 0 or 1.
The exclusion criteria for this study included the following: (a) metastatic breast cancer; patients with recurrence and metastasis during follow-up; (b) family history of endometrial cancer or any other kind of gynecologic cancer; (c) patients who were pregnant, lactating, menopausal, or had an intention to become pregnant; (d) patients who had received hormone replacement therapy, contraceptives, or androgen treatment within the previous three months; (e) patients who had also been administered other TCM formulas or botanical drugs; (f) individuals who presented with dysphagia, chronic diarrhea, bowel obstruction and other difficulties in taking formulas; (g) allergic to any phytochemical in the formula; (h) severe comorbidity that indicating intolerance to TCM formula treatment; psychiatric disorders or other diseases leading to noncompliance to the therapy; (i) patients suspected of alcohol or drug abuse; (j) patients with cognitive impairment or limited education who are unable to comprehend and complete the scales.
Intervention and Comparison
Patients with TNBC who completed surgery and chemotherapy were included and randomly assigned (1:1) to either the XIAOPI group (treatment group) or the placebo group (control group). The XIAOPI formula consists of 10 herbs, including Epimedium brevicornum (Chinese name YIN YANG HUO, YYH), Cistanche deserticola (Chinese name ROU CONG RONG, RCR), Leonurus heterophyllus (Chinese name YI MU CAO, YMC), Salvia miltiorrhiza (Chinese name DAN SHEN, DS), Curcuma Aromatica (Chinese name YU JIN, YJ), Rhizoma Curcumae (Chinese name E ZHU, EZ), Ligustrum lucidum (Chinese name NV ZHEN ZI, NZZ), Radix Polygoni Multiflori Praeparata (Chinese name HE SHOU WU, HSW), Crassostrea gigas (Chinese name MU LI, ML), and Carapax Trionycis (Chinese name BIE JIA, BJ). The formulation was manufactured and supplied by Guangdong QIJI Pharmaceutical Company Ltd. (No. 180,101). Placebo was made of maltodextrin and had a taste and appearance similar to XIAOPI formula. All patients were administered either 8.5 g of the XIAOPI formula or placebo treatment three times daily for three months. The dosage used in the current study was compared to that in mammary hyperplasia treatment. The administration of the medication was halted during the menstrual period for the respective patients. The drug treatment was discontinued for patients who experienced severe adverse events during the trial period.
Outcome Measures
The serum level of CXCL1 was detected. CXCL1 concentrations were measured in serum samples collected from the patients both prior to and following the treatment using the Human CXCL1 ELISA Kit (SEA041Hu, USCN Business) in accordance with the ELISA assay protocol.
The anxiety and depression levels of the patients were assessed using the Self-Rating Anxiety Scale (SAS),Citation27 and the Self-Rating Depression Scale (SDS),Citation28 respectively, prior to and following the treatment. Each scale consists of 20 parameters, with each item rating from 1 to 4. The scores for each item were then added and multiplied by 1.25 to obtain the final score. In accordance with the Chinese population, a high score (≥ 50) on the SAS or SDS was defined as positive for anxiety or depression, respectively.
The secondary outcomes included life quality improvement and sleep quality in patients with TNBC, which were assessed using the Functional Assessment of Cancer Therapy-Breast (FACT-B)Citation29 and the Pittsburgh Sleep Quality Index (PSQI) questionnaires,Citation30 respectively. As an instrument for assessing the physiological, emotional, and social functioning of patients with breast cancer, the FACT-B questionnaire is universally utilized. The questionnaire includes the FACT-G, an evaluation of the life quality of patients with general cancer, and a subscale for breast cancer. The FACT-G contains subscales including physical (7 items), functional (7 items), emotional (6 items), and social well-being (7 items). The breast cancer subscale comprises 9 items. The total score of FACT-B is 144. The higher the score, the better the quality of life for the patient.
The PSQI was used to evaluate the sleep quality of patients. PSQI has 7 items, including subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping pills, and daytime dysfunctions. The aggregate of the individual item scores (from 0 to 3) gives the overall scores, which range from 0 to 21. A score greater than 7 indicated poor sleep quality.
Clinicopathological Information Collection
The clinical information of patients enrolled in the trial was obtained from the electronic medical record system of the Guangdong Provincial Hospital of Chinese Medicine. This information comprised age, menstrual status, body mass index (BMI), ECOG score, and parameters of routine blood tests and biochemical analysis. Pathological sections were diagnosed by two independent pathologists. The parameters included tumor size, lymph node metastasis, histological grade, ER, PR, HER2, and vascular invasion. ER and PR were defined as positive when their expression was ≥ 1%. HER2 positive status was confirmed as 3+ by immunohistochemistry or + by fluorescence in situ hybridization (FISH). Breast cancer staging was performed in accordance with the seventh edition of American Joint Committee on Cancer. The previous treatment strategies of each patient were obtained from the database maintained by the multidisciplinary team.
Statistical Analysis
Baseline demographic and clinical characteristics were calculated using t-tests for continuous and ordinal variables. Chi-squared tests were used to compare the nominal variables. Comparisons of scale scores at different phases of treatment were performed using analysis of variance tests for paired samples. For the individual time points, between-group analyses were conducted using t-tests or Mann–Whitney U-tests. Differences were considered statistically significant at P < 0.05. All statistical analyses were conducted utilizing version 26.0 of the SPSS software for Windows.
Results
Demographic and Clinical Characteristics of Patients with TNBC
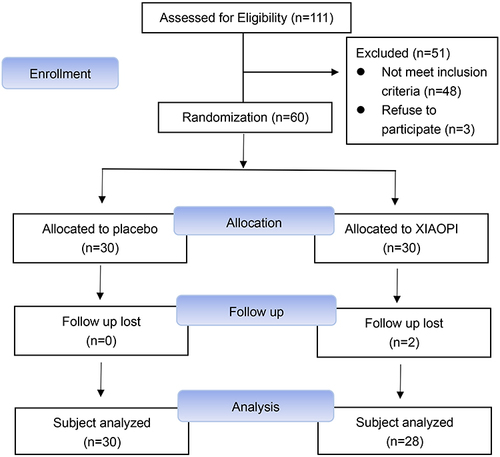
A total of 60 patients with cancer went through the screening process between June 2018 and May 2020. Among these patients, 30 were randomly assigned to the treatment group and the remaining 30 to the control group. The CONSORT flow diagram is presented in . provides a summary of the demographic characteristics and pathological parameters of the control and treatment groups at baseline. The mean age was 47.1 ± 10.4 years, with 23 women (38.3%) being at least 50 years old; 80% of patients were non-obese (BMI < 25), and the rest were obese (BMI ≥ 25). Thirty-two patients (53.3%) were premenopausal, 26 (43.3%) were postmenopausal, and the remaining 2 (3.3%) were unknown. While implementing randomization, there were no baseline differences observed between the control and treatment groups in terms of age, BMI, menopause status, age at menarche, number of pregnancies, age at first birth, number of births, and lactation time (P > 0.05).
Table 1 Demographic and Clinical Characteristics of Enrolled Participants
Histologically, the pathological grading of breast cancer comprised grade 1 (1.7%), grade 2 (36.7%), and grade 3 (61.7%). Furthermore, an equal distribution was observed between left-side (50.0%) and right-side (50.0%) breast cancer cases. Notably, a majority of patients, accounting for 91.7%, were diagnosed with Stage I or Stage II breast cancer. In relation to tumor size, 61.7% exhibited sizes exceeding 2 cm, while the remaining 38.3% manifested sizes less than 2 cm. Furthermore, a notable observation was made, indicating that 76.7% of the patients did not present with metastatic lymph nodes. Similarly, there were no differences in tumor histological grade, location, stage, tumor size, or lymph node status between the control and treatment groups (P > 0.05).
The prevalence of anxiety, depression, and poor sleep quality was balanced between the control and treatment groups at baseline (P > 0.05), as shown in .
Table 2 Prevalence of Anxiety, Depression, and Sleep Quality in TNBC Patients
Primary Outcomes
XIAOPI Formula Inhibits CXCL1 Expression in Patients with TNBC
To evaluate the effects of the XIAOPI formula on CXCL1 expression in patients with breast cancer, its level was detected in both groups prior to and subsequent to the administration of the drug. There were no statistically significant differences in CXCL1 expression at baseline between the treatment and control groups (P > 0.05), as shown in . However, following 3-month treatment, the downregulation of CXCL1 level (11.28 pg/mL) in the treatment group was significantly higher compared with the control group (0.73 pg/mL).
Table 3 Comparison of CXCL1 Expression of the Breast Patients Before and After XIAOPI Formula Intervention (`x±s, Pg/Ml)
XIAOPI Formula Decreases the SAS and SDS Scores of Patients with TNBC
To evaluate the effects of the XIAOPI formula on improving patients’ emotional well-being, the scores of SAS and SDS were compared before and after treatment. There were no significant differences in anxiety and depression between the treatment and control groups at baseline (P > 0.05), as shown in . However, SAS and SDS scores decreased after XIAOPI treatment, while they increased in the control group (Inter-groups comparison, P = 0.020 for SAS and P = 0.031 for SDS). These findings suggest that anxiety and depression were significantly improved by XIAOPI administration.
Table 4 Comparison of the SAS, SDS, PSQI and FACT-B Scores of the Breast Patients Before and After XIAOPI Formula Intervention (`x±s, Scores)
Secondary Outcomes
XIAOPI Formula Decreases the PSQI Scores of Patients with TNBC
To evaluate the effects of the XIAOPI formula on enhancing the sleep quality of patients, the scores of PSQI were compared before and after treatment. There were no significant differences in PSQI between the intervention and control groups at baseline (P > 0.05), as shown in . Following XIAOPI administration, PSQI scores decreased by 2.04 points, while they decreased by 0.039 points in the control group (P = 0.019). In addition, patients who received XIAOPI treatment exhibited more substantial enhancements in subjective sleep quality at postintervention when compared to those in the control group (P = 0.027).
XIAOPI Formula Increases the FACT-B Scores of Patients with TNBC
Additionally, the life quality of patients with breast cancer in both groups was evaluated prior to and following drug administration. As shown in , the FACT-B scores at baseline were similar between the treatment and control groups. After the 3-month XIAOPI intervention, the FACT-B total scores of patients in the treatment group improved by 8.48 points, however, there was no difference in the control group. And there was a significant difference between the two groups (P = 0.005). A cumulative effect of XIAOPI treatment on anxiety, depression, sleep disturbances, and quality of life in patients with breast cancer was observed from baseline to three months.
Associations between life quality, sleep disturbances, anxiety, depression, and CXCL1
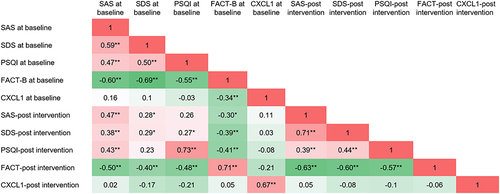
As illustrated in , noteworthy positive correlations were identified among sleep quality, anxiety, and depression in patients, both prior to the commencement of treatment (P < 0.01) and following a 3-month intervention (P < 0.01). Supplementary analysis was performed to explore associations between SDS, SAS, PSQI, and FACT-B scores before and after treatment. It was observed that SDS, SAS, and PSQI scores exhibited negative correlations with FACT-B scores (P < 0.01). Moreover, the level of CXCL1 was also negatively correlated with FACT-B scores prior to treatment (P < 0.01).
XIAOPI Formula Can Improve DFS in Patients with TNBC
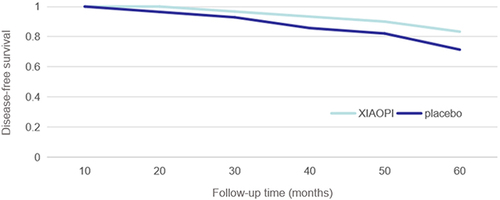
After a 60-month follow-up period, patients in the XIAOPI group continued to take the XIAOPI formula beyond the conclusion of the trial, for an average duration of 23.6 months. In contrast, patients in the placebo group exhibited poor compliance, with an average post-trial XIAOPI formula intake period of 3.7 months. Disease progression among patients was assessed at 10, 20, 30, 40, 50, and 60 months from the trial’s commencement. At the conclusion of the 60-month period, 5 patients in the XIAOPI formula group experienced relapse and metastasis, resulting in zero all-cause deaths. Conversely, 6 patients in the placebo group exhibited relapse and metastasis, with 2 fatalities. illustrates a statistically significant improvement in DFS within the XIAOPI formula group compared to the placebo group (P < 0.05).
Discussion
The results of our study demonstrated that the XIAOPI formula could reduce the CXCL1 serum level in patients with TNBC. In a prior study, we also established that the XIAOPI formula could inhibit premetastatic niche formation in breast cancer by decreasing CXCL1.Citation24 Meanwhile, cancer stem cells were also decreased following XIAOPI treatment,Citation26 and the formula elevated the chemosensitivity of breast cancer cells, accompanied by a reduction in CXCL1.Citation25 All of these results however, were derived from mouse xenografts. This is the first clinical study to our knowledge to investigate the effects of the XIAOPI formula on CXCL1 levels. Encouragingly, our findings indicate that XIAOPI can decrease CXCL1 levels following administration for three months, indicating its potential for overcoming cancer metastasis and improving clinical outcomes in patients with TNBC. Notwithstanding these findings, it is imperative to conduct additional clinical trials with larger and more diverse patient cohorts to substantiate and validate the obtained results.
Our results indicated that depression is common in patients with breast cancer (54.0%), which was slightly higher than that found in a previous meta-study (32.2%).Citation31 However, the current study reveals a reduced prevalence of anxiety compared to a prior investigation, where it was reported to have reached 41.9%.Citation32 Interestingly, we found a strong correlation between depression and anxiety before and after treatment, indicating a co-occurrence of these conditions and their negative impacts on the quality of life of patients, which was comparable to the previous report.Citation33 Patients diagnosed with breast cancer often experience suboptimal sleep quality, including delayed sleep onset, early morning awakening, prolonged nocturnal waking periods, unrefreshing sleep, and daytime sleepiness.Citation34 Based on our study, the PSQI score was positively correlated with anxiety and depression and negatively correlated with quality of life. In addition to adverse effects, anxiety, depression, pain, the use of opioids, fatigue, and cancer treatment can all contribute to poor sleep quality.Citation35 In turn, the symptoms of anxiety and depression are likely to be worsened by poor sleep quality.Citation36 Furthermore, a negative correlation was observed between CXCL1 and FACT-B scores prior to treatment in the current investigation, suggesting that there might exist a feedback loop between high CXCL1 levels and poor life quality. Although a significant difference was not observed in physical status, social or family status, emotional status, functional status, or in terms of additional concerns in the control group compared to those in the treatment group, the XIAOPI formula is effective in enhancing the total FACT-B score of patients with breast cancer. This phenomenon could be ascribed to the limited patient sample size; hence, future studies should involve a larger number of participants. Simultaneously, further research is anticipated to explore the potential benefits of the XIAOPI formula in extending overall survival or DFS in patients with TNBC, given the constraints posed by the limited duration of observation.
Antidepressant agents are preferred for patients with depression in clinical settings. However, drug-drug interactions should be considered when applied to patients with cancer. Antidepressant drugs such as fluoxetine and paroxetine, for instance, may reduce the active metabolite of tamoxifen.Citation37 Additionally, the common adverse effects of antidepressants,Citation38 such as constipation, dizziness, or sickness, could reduce compliance with cancer treatment. Therefore, TCMs with low toxicity may be a reasonable choice for patients with breast cancer with depression. The fact that the XIAOPI formula was reported to have few side effects in our study indicates that it may be a viable alternative. Furthermore, compared with antidepressants, TCM formulas work on multiple targets for the treatment of depression, anxiety, sleep quality, and quality of life. Therefore, additional clinical research comparing the XIAOPI formula and antidepressants is warranted for validation. Indeed, increasing evidence indicates that TCM may be helpful for patients with cancer and depression. Chaihu Shugan San, Xiaoyao San, and acupuncture, for instance, are prevalent TCM treatments in the majority of Asian countries. Deng et al demonstrated that Chaihu Shugan San could effectively improve depression in patients with cancer.Citation39 Jiao et al reported that Xiaoyao San could relieve depression in patients during adjuvant chemotherapy after breast cancer surgery.Citation40 In their study, Liu et al discovered that needle-warming moxibustion could alleviate edema and improve depression, anxiety, and quality of life for patients with breast cancer after the surgery.Citation41 Similarly, our findings revealed that anxiety and depression scores also declined significantly by the XIAOPI formula. However, the majority of prior research incorporated nearly every subtype of breast cancer. Considering the impact of tumor heterogeneity on treatment efficacy, our research focused on the most challenging TNBC subtype. Therefore, our results provide greater guidance regarding the precise management of TNBC.
Meanwhile, prior research has demonstrated that psychological disturbances usually occur during periods of diagnosis, surgery, and adjuvant therapies.Citation42,Citation43 It is common for patients breast cancer to experience symptoms of anxiety and stress-related disorders shortly after diagnosis. This is an overwhelming response to a potentially life-threatening disease and the uncertainty surrounding the future.Citation44 Although most patients with breast cancer may adapt to the diagnosis over time, psychological stress may persist in specific subgroups, such as young women who have concerns about fertility or weight gain.Citation45 It has been reported that psychological disorders and low quality of life occur more frequently during periods of surgery, chemotherapy, and radiotherapy.Citation46–48 A study has identified a positive correlation between depression status and chemotherapy-induced myelosuppression in patients with breast cancer. This finding implies that monitoring depression status before chemotherapy may facilitate better management of adverse events.Citation49 Notably, patients diagnosed with breast cancer frequently turn to complementary and alternative therapies, primarily motivated by the anticipated benefits or a perceived inadequacy of conventional medicine in addressing their psychosocial needs.Citation50,Citation51 Our results revealed that the XIAOPI formula may enhance the psychological well-being and quality of life of patients with breast cancer during their recuperation periods. Furthermore, further investigation is warranted into the potential therapeutic value of the XIAOPI formula during the phases of diagnosis, surgery, and adjuvant therapies. Moreover, there is a need to determine the optimal dosage and duration of the XIAOPI formula interventions. A three-month treatment period of 8.5 g of XIAOPI formula was utilized in this investigation to evaluate its efficacy. However, existing literature suggests that the effectiveness of TCM formulas may vary based on varied dosages or extended intervention times.Citation52,Citation53 Furthermore, the therapeutic effectiveness of the XIAOPI formula in treating different levels or types of mental disorders remains unclear. It is significant to determine the best therapeutic dose and duration of the XIAOPI formula by conducting real-world and multi-center clinical trials. In addition, depressive symptoms are observed to be exceedingly prevalent among patients diagnosed with breast cancer; however, they are frequently disregarded. Therefore, early identification and management of emotional disorders in patients with breast cancer are critical for enhancing the quality of life and clinical outcomes.
As an observational metric, DFS within the XIAOPI formula group exhibited a notable and statistically significant superiority over that observed in the placebo group. This difference may be attributed to the prolonged adherence of patients in the XIAOPI formula group, who continued to diligently consume the XIAOPI formula well beyond the trial’s conclusion. The sustained commitment to the XIAOPI formula was contingent upon the patients’ perceptions of the drug’s efficacy and their willingness to persist in the intake of the TCM formula.
This study impressively demonstrated that in the XIAOPI group, not only are CXCL1 levels effectively reduced, but also mental well-being, sleep quality, quality of life and even patient survival appear to be superior. The clinical results are novel and consistent with previous preclinical animal studies, which will be likely to make a meaningful contribution to the advancement of the management of TNBC. The present study also has some limitations. It is important to note that this study was conducted at a single center, the sample size was small. Therefore, the conclusions drawn from this study should be further confirmed through larger-scale, multi-center, double-blind studies with more diverse patient cohorts. Additionally, assessment of psychological well-being and quality of life was only conducted through patient-reported outcome measures which could be quite subjective sometimes. Furthermore, the therapeutic effects of the XIAOPI formula in treating different levels or types of mental disorders was not explored due to the sample size limitation.
Conclusion
In summary, our results suggest that the XIAOPI formula not only reduces CXCL1 levels effectively but also enhances psychological well-being, sleep quality, and overall quality of life for patients with TNBC. Therefore, it exhibits potential as a natural alternative therapy for improving the prognosis of TNBC. Moving forward, it is anticipated that the validation of therapeutic effects attributed to the XIAOPI formula will be established through large-scale and long-term clinical trials.
Abbreviations
TNBC, Triple-negative breast cancer; TAMs, Tumor-associated macrophages; CXCL1, C-X-C Motif Chemokine Ligand 1; TCM, Traditional Chinese medicine; SAS, Self-Rating Anxiety Scale; SDS, Self-Rating Depression Scale; PSQI, Pittsburgh sleep Quality Index; FACT-B, Functional Assessment of Cancer Therapy–Breast; CONSORT, Consolidated Standards of Reporting Trials.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the declaration of Helsinki. All participants provided written informed consent and the protocol was approved by the Medical Ethics Committee of the Second Affiliated Hospital of Guangzhou University of Chinese Medicine (ZF2018-097-01).
Consent for Publication
All authors have consented to the publication of the manuscript.
Disclosure
The authors declare no potential conflicts of interest in this work.
Data Sharing Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
- Jia H, Truica CI, Wang B, et al. Immunotherapy for triple-negative breast cancer: existing challenges and exciting prospects. Drug Resist Updat. 2017;32:1–15. doi:10.1016/j.drup.2017.07.002
- Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2010;109(9):1721–1728. doi:10.1002/cncr.22618
- Gao C, Li H, Liu C, Xu X, Sun C. Tumor mutation burden and immune invasion characteristics in triple negative breast cancer: Genome high-throughput data analysis. Front Immunol. 2021;12:650491. doi:10.3389/fimmu.2021.650491
- Leeat K, Marc B, Diana M, et al. A structured tumor-immune microenvironment in triple negative breast cancer revealed by multiplexed ion beam imaging. Cell. 2018;174(6):1373–87.e19. doi:10.1016/j.cell.2018.08.039
- Wang N, Liu W, Zheng Y, et al. CXCL1 derived from tumor-associated macrophages promotes breast cancer metastasis via activating NF-κB/SOX4 signaling. Cell Death Dis. 2018;9(9):880. doi:10.1038/s41419-018-0876-3
- Burnier A, Shimizu Y, Dai Y, et al. CXCL1 is elevated in the urine of bladder cancer patients. Springerplus. 2015;4:610. doi:10.1186/s40064-015-1393-9
- Xiang Z, Jiang DP, Xia GG, et al. CXCL1 expression is correlated with Snail expression and affects the prognosis of patients with gastric cancer. Oncol Lett. 2015;10(4):2458–2464. doi:10.3892/ol.2015.3614
- Wang D, Sun H, Wei J, Cen B, DuBois RN. CXCL1 Is Critical for premetastatic niche formation and metastasis in colorectal cancer. Cancer Res. 2017;77(13):3655–3665. doi:10.1158/0008-5472.CAN-16-3199
- Zhang Z, Chen Y, Jiang Y, Luo Y, Zhang H, Zhan Y. Prognostic and clinicopathological significance of CXCL1 in cancers: a systematic review and meta-analysis. Cancer Biol Ther. 2019;20(11):1380–1388. doi:10.1080/15384047.2019.1647056
- Swiger KD, Sendecki JA, Guglielmino JE, et al. Abstract P5-17-06: emotional/psychological characteristics of women with triple-negative breast cancer: Do socioeconomic, demographic, and provider variables impact emotional change from diagnosis to post-treatment? Cancer Res. 2015;75(9_Supplement):P5-17-06-P5-17–06. doi:10.1158/1538-7445.SABCS14-P5-17-06
- Hass HG, Seywald M, Wöckel A, Muco B, Tanriverdi M, Stepien J. Psychological distress in breast cancer patients during oncological inpatient rehabilitation: incidence, triggering factors and correlation with treatment-induced side effects. Arch Gynecol Obstet. 2023;307(3):919–925. doi:10.1007/s00404-022-06657-3
- Shen A, Qiang W, Wang Y, Chen Y. Quality of life among breast cancer survivors with triple negative breast cancer--role of hope, self-efficacy and social support. Eur J Oncol Nurs. 2020;46:101771. doi:10.1016/j.ejon.2020.101771
- Vadaparampil ST, Christie J, Donovan KA, et al. Health-related quality of life in Black breast cancer survivors with and without triple-negative breast cancer (TNBC). Breast Cancer Res Treat. 2017;163(2):331–342. doi:10.1007/s10549-017-4173-0
- Soucise A, Vaughn C, Thompson CL, et al. Sleep quality, duration, and breast cancer aggressiveness. Breast Cancer Res Treat. 2017;164(1):169–178. doi:10.1007/s10549-017-4245-1
- Huang M, Haiderali A, Fox GE, et al. Economic and humanistic burden of triple-negative breast cancer: A systematic literature review. Pharmacoeconomics. 2022;40(5):519–558. doi:10.1007/s40273-021-01121-7
- Wang Y, Li JW, Qin YN, et al. Clinical observation on the effect of Chinese medicine-“TCM formula” intervention on recurrence and metastasis of triple negative breast cancer. Complement Ther Med. 2020;52:102456. doi:10.1016/j.ctim.2020.102456
- Wang B, Fei R, Yang Y, Jing N, Zhang Y. The Shuganhuazheng formula in triple-negative breast cancer: a study based on network pharmacology and in vivo experiments. Evid Based Complement Alternat Med. 2020;2020(2):1–10.
- Wang J, Yang X, Han H, Wang L, Hu K. Inhibition of growth and metastasis of triple-negative breast cancer targeted by Traditional Chinese Medicine Tubeimu in orthotopic mice models. Chin J Cancer Res. 2018;30(1):112. doi:10.21147/j.issn.1000-9604.2018.01.12
- Yang Z, Zhang Q, Yu L, Zhu J, Gao X, Gao X. The signaling pathways and targets of traditional Chinese medicine and natural medicine in triple-negative breast cancer. J Ethnopharmacol. 2020;264:113249. doi:10.1016/j.jep.2020.113249
- Wang Y, Liu SY, Zhang Y, et al. Effect of traditional Chinese medicine on postoperative depression of breast cancer: a systematic review and meta-analysis. Front Pharmacol. 2023;23(14):1019049. doi:10.3389/fphar.2023.1019049
- Bai X, Ta N, Gong GH, Zhang B, Wei CX, Emran TB. Effects of integrated Chinese traditional medicine and conventional western medicine on the quality of life of breast cancer patients: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2022;2022:3123878. doi:10.1155/2022/3123878
- Zou J, Bian W, Ren X, Liu F. Clinical research of xiaopi granule in treating hyperplasia of mammary glands with phlegm and blood stasis and chong ren dysfunction syndrome. World Chin Med. 2013;8(12):1439–1441.
- Zheng Y, Wang N, Wang S, Yang B, Wang ZJB. XIAOPI formula inhibits the pre-metastatic niche formation in breast cancer via suppressing TAMs/CXCL1 signaling. Cell Commun Signal. 2020;18(1):48. doi:10.1186/s12964-020-0520-6
- Wang N, Yang B, Muhetaer G, Wang S, Wang Z. XIAOPI formula promotes breast cancer chemosensitivity via inhibiting CXCL1/HMGB1-mediated autophagy. Biomed Pharmacother. 2019;120:109519. doi:10.1016/j.biopha.2019.109519
- Wang S, Liu X, Huang R, et al. XIAOPI formula inhibits breast cancer stem cells via suppressing tumor-associated macrophages/C-X-C Motif Chemokine Ligand 1 Pathway. Front Pharmacol. 2019;10:1371. doi:10.3389/fphar.2019.01371
- William WK Zung. A Rating Instrument for Anxiety Disorders. Psychosomatics. 1971;12(6): 371–379. doi:10.1016/S0033-3182(71)71479-0
- William WK Zung. A Self-Rating Depression Scale. Arch Gen Psychiatry. 1965;12:63–70. doi:10.1001/archpsyc.1965.01720310065008
- Brady M.J., Cella D.F., Mo F. Reliability and validity of the Functional Assessment of Cancer Therapy – Breast quality-of-life instrument. Journal of Clinical Oncology. 1997;15(3):974–986. doi:10.1200/JCO.1997.15.3.974
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4
- Pilevarzadeh M, Amirshahi M, Afsargharehbagh R, Rafiemanesh H, Hashemi SM, Balouchi A. Global prevalence of depression among breast cancer patients: a systematic review and meta-analysis. Breast Cancer Res Treat. 2019;176(3):519–533. doi:10.1007/s10549-019-05271-3
- Hashemi SM, Rafiemanesh H, Aghamohammadi T, et al. Prevalence of anxiety among breast cancer patients: a systematic review and meta-analysis. Breast Can. 2020;27(2):166–178. doi:10.1007/s12282-019-01031-9
- Wang X, Wang N, Zhong L, et al. Prognostic value of depression and anxiety on breast cancer recurrence and mortality: a systematic review and meta-analysis of 282,203 patients. Mol Psychiatry. 2020;25(12):3186–3197. doi:10.1038/s41380-020-00865-6
- Fox RS, Ancoli-Israel S, Roesch SC, et al. Sleep disturbance and cancer-related fatigue symptom cluster in breast cancer patients undergoing chemotherapy. Supt Care Cancer. 2020;28(2):845–855. doi:10.1007/s00520-019-04834-w
- El Kherchi O, Aquil A, Elkhoudri N, et al. Relationship between sleep quality and anxiety-depressive disorders in Moroccan women with breast cancer: a cross-sectional study. Iran J Public Health. 2023;52(7):1457–1465. doi:10.18502/ijph.v52i7.13247
- Lam CS, Yu BY, Cheung DST, et al. Sleep and Mood Disturbances during the COVID-19 Outbreak in an Urban Chinese Population in Hong Kong: a Longitudinal Study of the Second and Third Waves of the Outbreak. Int J Environ Res Public Health. 2021;18(16):8444. doi:10.3390/ijerph18168444
- Donneyong MM, Bykov K, Bosco-Levy P, Dong YH, Levin R, Gagne JJ. Risk of mortality with concomitant use of tamoxifen and selective serotonin reuptake inhibitors: multi-database cohort study. BMJ. 2016;354:i5014. doi:10.1136/bmj.i5014
- Carina R, Tibor S, Kristian B, Sarah M, Jürgen W, Siepmann T. Adverse Effects of Antidepressants for Chronic Pain: a Systematic Review and Meta-analysis. Front Neurol. 2017;8:307. doi:10.3389/fneur.2017.00307
- Deng HY, Wu SP, Wu XF, Liu D. Clinical study of Chaihu Shugan Powder in the treatment of cancer-related depression. Int J Trad Chin Med. 2022;44(2):150–153.
- Jiao J, Luo KH, Li YH, Li DF, Ang JY T. Clinical Observation on Modified Xiaoyao Powder Treatment for patients with depression syndrome of liver depression and spleen deficiency during adjuvant chemotherapy after breast cancer operation. Anti-Tumor Pharmacy. 2019;9(1):107–111.
- Liu XF, Zhang X, Jing K. Observational study on the efficacy of needle warming moxibustion in edema of the upper extremity and anxiety-depression of mammary cancer patients after operation. World Chinese Med. 2019;14(7):1856–1860.
- WKW S, Law BMH, MSN N, et al. Symptom clusters experienced by breast cancer patients at various treatment stages: a systematic review. Cancer Med. 2021;10(8):2531–2565. doi:10.1002/cam4.3794
- Bidstrup PE, Christensen J, Mertz BG, Rottmann N, Dalton SO, Johansen C. Trajectories of distress, anxiety, and depression among women with breast cancer: looking beyond the mean. Acta Oncol. 2015;54(5):789–796. doi:10.3109/0284186X.2014.1002571
- Heo J, Chun M, Oh YT, Noh OK, Kim L. Psychiatric comorbidities among breast cancer survivors in South Korea: a nationwide population-based study. Breast Cancer Res Treat. 2017;162(1):151–158. doi:10.1007/s10549-016-4097-0
- Carreira H, Williams R, Müller M, Harewood R, Stanway S, Bhaskaran K. Associations Between Breast Cancer Survivorship and Adverse Mental Health Outcomes: a Systematic Review. J Natl Cancer Inst. 2018;110(12):1311–1327. doi:10.1093/jnci/djy177
- Grocott B, Reynolds K, Logan G, Hebbard P, El-Gabalawy R. Breast cancer patient experiences of perioperative distress and anxiety: a qualitative study. Eur J Oncol Nurs. 2023;63:102299. doi:10.1016/j.ejon.2023.102299
- Fortunato L, Loreti A, Cortese G, et al. Regret and quality of life after mastectomy with or without reconstruction. Clin Breast Cancer. 2021;21(3):162–169. doi:10.1016/j.clbc.2019.11.005
- Grilo AM, Gomes AI, Monsanto F, Albino D, Augusto C, Pragana C. First day of radiotherapy for women with breast cancer: predictors of anxiety. Supt Care Cancer. 2020;28(3):1241–1248. doi:10.1007/s00520-019-04902-1
- Lv D, Lan B, Zhang L, Sun X, Yang M, Ma F. Association between depression and anxiety status of breast cancer patients before adjuvant chemotherapy and chemotherapy-induced adverse events. Cancer Med. 2023;12(4):4794–4800. doi:10.1002/cam4.5283
- Keene MR, Heslop IM, Sabesan SS, Glass BD. Complementary and alternative medicine use in cancer: a systematic review. Comp Ther Clin Pract. 2019;35:33–47. doi:10.1016/j.ctcp.2019.01.004
- Naing A, Stephen SK, Frenkel M, et al. Prevalence of complementary medicine use in a Phase 1 clinical trials program. Cancer. 2011;117(22):5142–5150. doi:10.1002/cncr.26164
- Zhou R, Zheng Y, An X, Jin D, Lian F, Tong X. Dosage modification of traditional Chinese medicine prescriptions: An analysis of two randomized controlled trials. Front Pharmacol. 2021;12:732698. doi:10.3389/fphar.2021.732698
- Li Z, Deng H, Guo X, et al. Effective dose/duration of natural flavonoid quercetin for treatment of diabetic nephropathy: a systematic review and meta-analysis of rodent data. Phytomedicine. 2022;105:154348. doi:10.1016/j.phymed.2022.154348