Abstract
Background
The immune system appears to play a crucial role in how breast cancer responds to chemotherapy. In this study, we investigated a peripheral marker of immune and inflammation named the neutrophil to albumin ratio (NAR) to explore its potential relationship with pathological complete response (pCR) in locally advanced breast cancer patients who underwent neoadjuvant chemotherapy (NAC).
Methods
We conducted a retrospective analysis of 212 consecutive breast cancer patients who received NAC. The NAR was calculated by examining the complete blood cell count and albumin level in peripheral blood before starting NAC. Through ROC curve analysis, we determined the optimal cutoff value for NAR as 0.0877. We used Pearson’s chi-square test or Fisher’s exact test to evaluate the relationship between NAR and pCR, as well as other clinical and pathological characteristics. Logistic regression models were employed for univariate and multivariate analyses.
Results
The results of both univariate and multivariate logistic regression analyses showed that NAR was associated with tumor pathological regression. The NAR high group had a higher pCR rate compared to the NAR low group (OR 3.127 [95% CI 1.545–6.328]; p = 0.002).
Conclusion
According to this study, it was observed that patients with breast cancer who had high levels of NAR were more likely to achieve pCR when undergoing NAC.
Introduction
Breast cancer is the most common type of cancer among adult women worldwide, accounting for 31% of all female cancers globally. It also has the highest incidence rate among all tumors.Citation1 Currently, neoadjuvant chemotherapy (NAC) is widely used as the standard treatment for locally advanced and inflammatory breast cancer. The goal of NAC is to reduce the stage of cancer and increase the likelihood of breast-conserving surgery. Additionally, it can provide insights into an individual’s sensitivity to cancer treatment.Citation2 It has been suggested that achieving a pathological complete response (pCR) after NAC can be a favorable indicator for disease-free survival (DFS) and overall survival (OS), especially for patients with more aggressive subtypes such as triple-negative or human epidermal growth factor receptor 2 (HER2)-positive breast cancer.Citation3,Citation4 Breast cancer patients exhibit significant variability in their response to NAC due to the heterogeneity of tumors.Citation5
There is growing evidence suggesting a connection between inflammation and the development of cancer, with the host’s systemic inflammatory response playing a crucial role in cancer progression.Citation6–8 Many studies are currently underway to understand the role of the peripheral immune system in breast cancer, particularly in response to NAC. Some hematological and nutritional parameters, such as the neutrophil to lymphocyte ratio (NLR), prognostic nutrition index (PNI), and systemic immune-inflammation index (SII), have been developed to predict the development of breast cancer.Citation9–11 Additionally, albumin, the primary protein found in human serum, serves as an indicator of a patient’s nutritional status. Numerous studies have established a correlation between serum albumin levels and survival rates in various types of cancer, including breast cancer, gastric cancer, and colorectal cancer.Citation12–14 The neutrophil to albumin ratio (NAR), introduced by Bernard et al is a new biomarker that reflects both systemic inflammation and nutritional status.Citation15 It is calculated by dividing the absolute neutrophil count by the serum albumin concentration. This unique index related to cancer-associated inflammation combines information on the body’s inflammatory responses and nutritional status. Research has shown that this biomarker can predict the degree of malignancy and prognosis of patients with lung cancer and oral cancer.Citation16,Citation17 Additionally, NAR considers both pre-treatment markers of inflammation and malnutrition. In a retrospective study, NAR was found to be useful in predicting relapse-free survival in patients with gastrointestinal stromal tumors.Citation18 Moreover, NAR can independently predict a complete pathological response in patients who have undergone neoadjuvant chemoradiotherapy for rectal cancer.Citation19 However, there is a lack of reliable data on the potential use of this biomarker in predicting pCR in breast cancer patients undergoing NAC. In this study, we investigated whether the baseline NAR could identify breast cancer patients who are more likely to achieve pCR after NAC, and whether the predictive value of this biomarker is independent of other clinical factors.
Methods
Patient Enrollment
A total of 212 female breast cancer patients who underwent NAC were identified retrospectively from November 2019 to June 2023 using the inpatient databases from Peking University Shenzhen Hospital. The enrollment criteria were as follows: (1) confirmed diagnosis of breast adenocarcinoma through pathology; (2) patients who received NAC at our hospital and completed radical resection for breast cancer; (3) initially diagnosed with T3–4 or N+ disease; (4) patients undergoing standard adjuvant chemotherapy treatment. The exclusion criteria were as follows: (1) bilateral breast cancer; (2) presence of metastatic disease before or during preoperative treatment, or any other active malignancies; (3) incomplete case information. Tumor stage was classified according to the 2010 AJCC staging system. The current study was conducted in accordance with the ethical standards of the World Medical Association Declaration of Helsinki. Institutional Review Board approval was obtained from independent ethics committees at Peking University Shenzhen Hospital, with a waiver of informed consent as this research was retrospective and did not involve accessing any identifying patient data.
Data Collection
NAR is calculated based on laboratory test results from peripheral blood samples obtained within 7 days before the first cycle of NAC. All blood cell assessments were performed centrally in our institutional laboratory following standardized operative procedures. The NAR was calculated as the neutrophil count divided by the albumin (g/L) level. Breast cancer biopsies and surgical specimens were processed for immunohistochemistry (IHC). Tumors were considered estrogen receptor (ER) or progesterone receptor (PR) positive if more than 1% of cells showed nuclear receptor staining. Ki-67 was used as a cutoff point of 14% to distinguish between luminal A and luminal B tumors. The nuclear grade was assessed according to the Nottingham grading system. HER2 positivity was defined according to the ASCO/CAP guidelines.Citation20 Tumor molecular subtypes were classified as Luminal A, Luminal B, HER2-enriched/ hormone receptor (HR)-positive, HER2-enriched/HR-Negative, and Triple-negative, as previously described.
Patient Treatments
Primary tumors were staged according to the eighth edition of the breast cancer UICC-TNM staging system. Ultrasound, molybdenum target, and MRI were used to assess the tumor condition before NAC. The decision to use NAC was made based on the NCCN guideline and patients’ tolerance to chemotherapy. The NAC regimen consists of anthracycline and taxane based chemotherapy, with HER2-positive patients receiving anti-HER2 therapy. The NAC regimens adhered to standard clinical practices and were divided into two groups: (1) regimens based on anthracyclines and taxanes used in the HER2 negative group, such as the AC-T regimen, and (2) chemotherapy and anti-HER2 regimens used in the HER2 positive group, including TCbHP, THP, and AC-THP regimens. Specific dosing instructions for each regimen can be found in Supplementary Table 1. Surgical treatment was performed within 3–4 weeks after the completion of the prescribed cycle of NAC.
Evaluation of Pathological Response
After completing neoadjuvant chemotherapy, the decision to perform breast-conserving surgery or total mastectomy was based on the patient’s condition of breast cancer. If separated tumor cells were found in the sentinel lymph nodes, a complete axillary lymph node dissection would be performed. Each surgical specimen was reviewed by experienced pathologists to determine the extent of tumor regression. Pathological complete response was defined as the complete absence of invasive breast cancer in both the breast and axillary lymph nodes in the surgical specimen following NAC (ypT0/ypTis, ypN0). Noninvasive breast residuals (ductal carcinoma-in-situ) were allowed.Citation21
Statistical Analysis
Statistical analyses were performed using SPSS 20.0 software (IBM, Chicago, IL, USA), GraphPad Prism 7 software (GraphPad Software, Inc, San Diego, CA, USA). The receiver operating curve (ROC) analysis was applied to calculate the area under the ROC curve (AUC), which was used to determine the optimal cutoff value of NAR. The curve is drawn with true positive rate (sensitivity) as the ordinate, false positive rate (1-specificity) as the abscissa, and Youden index (sensitivity + specificity −1). The maximum value was defined as the optimal cut-off value. Comparison between groups was performed using χ2 test or Fisher’s test. The logistic regression model was used for univariate analysis, and the variables with p value less than 0.05 in univariate analysis were subjected to multivariate analysis. Odds Ratio (OR) was reported with the corresponding 95% confidence intervals (95% CI). p < 0.05 was considered statistically significant.
Results
Characteristics and Distribution of the Study Population
presents the clinicopathologic characteristics of the 212 patients included in this study. The median age of all patients was 46 years old (range 26–68), and the median tumor size was 34 mm (range 9–96). At the time of diagnosis, the majority of cases (74.5%) had a T stage of cT2, and 58.0% had an N stage of cN1. Among these patients, 71.2% were determined to be HR-positive, 56.6% were HER2-negative, and 94.3% exhibited Ki-67 > 14%.
Table 1 Clinicopathologic Features of Patients Involved in This Study
Relationship Between Baseline Characteristics and NAR
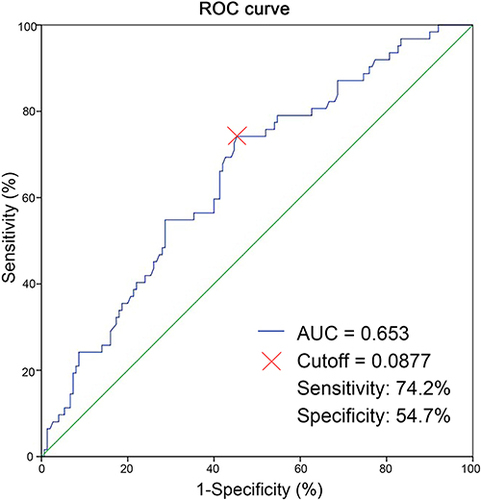
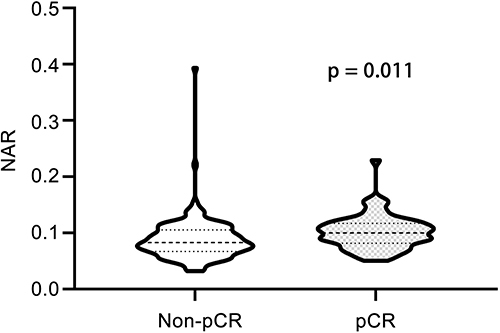
The optimal cutoff value for the NAR was determined to be 0.0877, which corresponded to the maximum sensitivity and specificity (0.742 and 0.547, respectively) of the NAR in predicting pCR, as indicated by ROC analysis. The Youden index was calculated to be 0.289 (). Based on the optimal cutoff values, patients were divided into a low NAR group (NAR ≤ 0.0877) and a high NAR group (NAR > 0.0877). Analysis of NAR levels in relation to various clinicopathological features revealed that patients over 50 years old (p = 0.043), those with positive HER2 status (p = 0.008) and chemotherapy regimen including anti-HER2 (p = 0.012) were more likely to belong to the high NAR group (). A total of 62 patients (29.2%) achieved pCR. The majority of these patients were HR negative (p = 0.001), HER2 positive (p < 0.001), and received a combination of chemotherapy and targeted therapy (p < 0.001). Additionally, these patients had higher NAR levels (p < 0.001) (). In , it is evident that the NAR values in the pCR group were notably higher compared to the non-pCR group (p = 0.011).
Table 2 Relationships Between NAR and Patient Characteristics
Table 3 Relationships Between pCR and Patient Characteristics
NAR Predicting pCR
As shown in , univariate logistic regression analysis indicated that HR status (OR 0.342 [95% CI 0.182–0.644]; p < 0.001), HER2 status (OR 5.194 [95% CI 2.720–9.919]; p < 0.001), chemotherapy regimen (OR 5.357 [95% CI 2.803–10.240]; p < 0.001) and NAR levels (OR 3.467 [95% CI 1.804–6.664]; p < 0.001) were associated with pCR. Multivariate analysis of predictive factors for pCR demonstrated significant positive correlations with negative HR status (OR 0.396 [95% CI 0.196–0.799]; p = 0.010), chemotherapy regimen including anti-HER2 (OR 4.326 [95% CI 2.198–8.515]; p < 0.001) and high NAR levels (OR 3.127 [95% CI 1.545–6.328]; p = 0.002). Both univariate and multivariate logistic regression analyses revealed that NAR was associated with tumor pathological regression, with a higher pCR rate observed in the high NAR group compared to the low NAR group.
Table 4 Association of Patient/Tumor Characteristics to pCR in Univariate and Multivariate Analysis
Discussion
In recent years, there has been a rise in both the incidence and mortality rates of breast cancer in China. As a result, new neoadjuvant treatment approaches have emerged with the goal of enhancing the overall tumor resection rate and ultimately improving survival rates. It has become increasingly crucial to accurately predict the effectiveness of NAC in advance, as this can significantly impact clinical decision-making. Inflammatory markers have been identified as the most reliable indicators of the body’s immune function and nutritional status. Therefore, our study aimed to explore the correlation between the inflammatory marker NAR and pCR in breast cancer patients. Additionally, we aimed to establish an optimal cut-off value for assessing the outcome of NAC based on NAR levels. As found in this study, breast cancer patients with high peripheral NAR levels at the beginning of NAC are more likely to achieve a pCR. We conducted a retrospective analysis on 212 patients undergoing NAC to calculate the NAR in their peripheral blood. Through ROC curve analysis, we established a cut-off value. Our study revealed that older patients (age > 50 years) and those in the chemotherapy regimen including anti-HER2 subgroup were more prevalent in the high-NAR group. This suggests that these patients may exhibit a stronger systemic inflammatory response, potentially leading to a higher likelihood of achieving pCR after NAC. Both univariate and multivariate logistic regression analyses indicated a correlation between NAR levels and tumor pathological regression. The high-NAR group (NAR > 0.0877) demonstrated a higher pCR rate compared to the low-NAR group (NAR ≤ 0.0877). This suggests that a higher pre-NAC NAR level is closely associated with the pCR rate in breast cancer patients.
Researchers have been investigating different tumor and patient characteristics to pinpoint factors that can predict pCR in various types of cancer.Citation22,Citation23 It has been shown that certain chemotherapy drugs and oncolytic viruses can trigger the release of antigens and pro-immunogenic factors, leading to immune activation and enhancing anti-cancer responses through immunogenic cell death induction.Citation24 Blood cell counts and albumin, as markers of inflammation and immune response, may provide additional information about the treatment response in cancer patients. Several studies have suggested that biomarkers reflecting systemic immunoinflammatory responses can indicate the balance between inhibiting and promoting tumor progression.Citation25 Examples include the neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio, which have been utilized to forecast the prognosis of various cancers, including breast cancer.Citation26 A recently published study demonstrated that the immune-inflammatory index, which considers pre-treatment peripheral blood platelet, neutrophil, and lymphocyte counts, can identify an elderly cancer patient population with a poor prognosis.Citation27 Other research has highlighted the significance of albumin in assessing the body’s nutritional status. Albumin also plays a role in antioxidative stress, enhancing microcirculation, and regulating systemic inflammatory responses.Citation28 In the advanced stages of malignant tumors, patients often experience decreased albumin levels due to malnutrition and tumor consumption. Reduced albumin levels are frequently linked to compromised liver function and immune deficiency, which can lead to tumor recurrence, metastasis, and ultimately, a poor prognosis. By combining various biomarkers, a more precise characterization of patients’ peripheral immune phenotype can be achieved.Citation29
Several studies have also examined the immune and inflammatory markers in response to NAC in breast cancer.Citation30,Citation31 In certain studies, peripheral indicators of immune and inflammatory balance have been linked to survival or treatment response. However, despite the numerous published studies to date, there is still no clear evidence regarding the role of peripheral systemic immunity. This is partly due to the lack of standardized cut-off values and the limited number of patients included in the studies. Fridlender et al discovered that tumor-associated neutrophils from the early tumor stage exhibited a pro-tumorigenic phenotype, acting as tumor-killing cells that produce high levels of hydrogen peroxide (H2O2), tumor necrosis factor (TNF)-α, and NO in a mouse model.Citation32 Additionally, there is evidence suggesting that neutrophils can counteract tumor cells through the production of reactive oxygen species (ROS). Neutrophils containing ROS have been found to strongly suppress IL-17-producing γδ T cells, which are crucial for shaping the immune suppressive microenvironment in various solid tumors.Citation33,Citation34 In our research, we found that a high NAR level was often linked to elevated neutrophil levels and decreased serum albumin levels. This correlation suggested a higher chance of achieving a pCR, which is consistent with current perspectives. Nevertheless, the precise mechanism underlying the connection between NAR and chemotherapy response warrants further investigation.
By clarifying the relationship between the NAR and the outcomes of neoadjuvant therapy, doctors can better assess the effectiveness of NAC and make necessary adjustments to treatment plans promptly. Since the NAR can be easily obtained from preoperative laboratory tests, we suggest that it holds significant potential for application in routine clinical practices and oncological research. Our study aimed to investigate the predictive value of NAR for pCR in 212 breast cancer patients who were candidates for NAC. All blood cell counts were performed in our institution’s laboratory. To the best of our knowledge, the NAR may indicate a link between inflammation and immune responses in peripheral blood, assisting in determining the host’s inflammatory status.
We have shown that the NAR can independently pCR. To our knowledge, this is the first study to explore the clinical significance of NAR in predicting treatment response in breast cancer patients undergoing NAC. However, there are certain limitations to our study, including its retrospective nature and the relatively small number of patients included. Additionally, this study was conducted in a single institution, which may limit the generalizability of the findings to other patient cohorts. Therefore, it is important to confirm these findings in a larger subgroup of patients from multiple institutions. One potential avenue for future research based on these findings is the exploration of combining anti-inflammatory drugs with standard chemotherapy to shift NAR from an inflammatory phenotype to an immunogenic phenotype, potentially improving the pCR rate.
Conclusion
Our research findings reveal that among 212 breast cancer patients undergoing NAC, older patients (age > 50 years) and those who received a combination of chemotherapy and targeted therapy were more likely to exhibit high levels of NAR. Moreover, a higher NAR was identified as an independent predictor of achieving pCR, suggesting that the NAR, as an inflammation marker, can serve as an indicator of the immune and nutritional status of the body. It is essential to validate and confirm the utility of NAR as a potential predictor of pCR in breast cancer patients by applying the Results of this study to other patient cohorts.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Data Sharing Statement
The original contributions presented in the study are included in the article material, further inquiries can be directed to the corresponding author.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
- Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from national surgical adjuvant breast and bowel project B-18. J Clin Oncol. 1997;15(7):2483–2493. doi:10.1200/JCO.1997.15.7.2483
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi:10.1016/S0140-6736(13)62422-8
- Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–1281. doi:10.1200/JCO.2007.14.4147
- Guo L, Kong D, Liu J, et al. Breast cancer heterogeneity and its implication in personalized precision therapy. Exp Hematol Oncol. 2023;12(1):3. doi:10.1186/s40164-022-00363-1
- Mantovani A. Cancer: inflaming metastasis. Nature. 2009;457(7225):36–37. doi:10.1038/457036b
- Wiseman MJ. Nutrition and cancer: prevention and survival. Br J Nutr. 2019;122(5):481–487. doi:10.1017/S0007114518002222
- Fernandes JV, Cobucci RN, Jatoba CA, et al. The role of the mediators of inflammation in cancer development. Pathol Oncol Res. 2015;21(3):527–534. doi:10.1007/s12253-015-9913-z
- Chen L, Bai P, Kong X, et al. Prognostic nutritional index (PNI) in patients with breast cancer treated with neoadjuvant chemotherapy as a useful prognostic indicator. Front Cell Dev Biol. 2021;9:656741. doi:10.3389/fcell.2021.656741
- Ethier JL, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19(1):2.
- Jiang C, Lu Y, Zhang S, Huang Y. systemic immune-inflammation index is superior to neutrophil to lymphocyte ratio in prognostic assessment of breast cancer patients undergoing neoadjuvant chemotherapy. Biomed Res Int. 2020;2020:7961568. doi:10.1155/2020/7961568
- Lis CG, Grutsch JF, Vashi PG, Lammersfeld CA. Is serum albumin an independent predictor of survival in patients with breast cancer? JPEN J Parenter Enteral Nutr. 2003;27(1):10–15. doi:10.1177/014860710302700110
- Boonpipattanapong T, Chewatanakornkul S. Preoperative carcinoembryonic antigen and albumin in predicting survival in patients with colon and rectal carcinomas. J Clin Gastroenterol. 2006;40(7):592–595. doi:10.1097/00004836-200608000-00006
- Onate-Ocana LF, Aiello-Crocifoglio V, Gallardo-Rincon D, et al. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol. 2007;14(2):381–389. doi:10.1245/s10434-006-9093-x
- Feng C, Yu H, Lei H, et al. A prognostic model using the neutrophil-albumin ratio and PG-SGA to predict overall survival in advanced palliative lung cancer. BMC Palliat Care. 2022;21(1):81. doi:10.1186/s12904-022-00972-x
- Yu YY, Lin YT, Chuang HC, et al. Prognostic utility of neutrophil-to-albumin ratio in surgically treated oral squamous cell carcinoma. Head Neck. 2023;45(11):2839–2850. doi:10.1002/hed.27511
- Varim C, Celik FD, Sunu C, et al. The role of neutrophil albumin ratio in predicting the stage of non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2022;26(8):2900–2905. doi:10.26355/eurrev_202204_28621
- Li R, Sun Z, Song S, et al. NARFIB: a novel prognostic score based on the neutrophil-to-albumin ratio and fibrinogen can predict the prognosis of gastrointestinal stromal tumors. Cancer Manag Res. 2020;12:11183–11190. doi:10.2147/CMAR.S281375
- Tawfik B, Mokdad AA, Patel PM, Li HC, Huerta S. The neutrophil to albumin ratio as a predictor of pathological complete response in rectal cancer patients following neoadjuvant chemoradiation. Anticancer Drugs. 2016;27(9):879–883. doi:10.1097/CAD.0000000000000411
- Wolff AC, Hammond M, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36(20):2105–2122. doi:10.1200/JCO.2018.77.8738
- Pennisi A, Kieber-Emmons T, Makhoul I, Hutchins L. Relevance of pathological complete response after neoadjuvant therapy for breast cancer. Breast Cancer. 2016;10:103–106. doi:10.4137/BCBCR.S33163
- Patel PM, Harris K, Huerta S. Clinical and molecular diagnosis of pathologic complete response in rectal cancer. Expert Rev Mol Diagn. 2015;15(11):1505–1516. doi:10.1586/14737159.2015.1091728
- Ramzan Z, Nassri AB, Huerta S. Genotypic characteristics of resistant tumors to pre-operative ionizing radiation in rectal cancer. World J Gastrointest Oncol. 2014;6(7):194–210. doi:10.4251/wjgo.v6.i7.194
- Davola ME, Cormier O, Vito A, et al. Oncolytic BHV-1 is sufficient to induce immunogenic cell death and synergizes with low-dose chemotherapy to dampen immunosuppressive T regulatory cells. Cancers. 2023;15(4):1295. doi:10.3390/cancers15041295
- Marchetti C, D’Indinosante M, Bottoni C, et al. NLR and BRCA mutational status in patients with high grade serous advanced ovarian cancer. Sci Rep. 2021;11(1):11125. doi:10.1038/s41598-021-90361-w
- Xie H, Ruan G, Ge Y, et al. Inflammatory burden as a prognostic biomarker for cancer. Clin Nutr. 2022;41(6):1236–1243. doi:10.1016/j.clnu.2022.04.019
- Fornarini G, Rebuzzi SE, Buti S, et al. A novel immunotherapy prognostic score for patients with pretreated advanced urInary TrAct CArcinoma from the subgroup analysis of the SAUL study: the ITACA score. Minerva Urol Nephrol. 2023;75(3):308–318. doi:10.23736/S2724-6051.22.05135-7
- Zhang CL, Gao MQ, Jiang XC, et al. Research progress and value of albumin-related inflammatory markers in the prognosis of non-small cell lung cancer: a review of clinical evidence. Ann Med. 2023;55(1):1294–1307. doi:10.1080/07853890.2023.2192047
- Konecny AJ, Mage P, Tyznik AJ, Prlic M, Mair F. 50-color phenotyping of the human immune system with in-depth assessment of T cells and dendritic cells. bioRxiv. 2023. doi:10.1101/2023.12.14.571745
- Zhang X, Hu M, Li S, et al. Clinical study on Yanghe decoction in improving neo-adjuvant chemotherapy efficacy and immune function of breast cancer patients. Medicine. 2022;101(10):e29031. doi:10.1097/MD.0000000000029031
- Jiang C, Xiu Y, Yu X, et al. Prognostic value of a modified systemic inflammation score in breast cancer patients who underwent neoadjuvant chemotherapy. BMC Cancer. 2022;22(1):1249. doi:10.1186/s12885-022-10291-2
- Mishalian I, Bayuh R, Levy L, et al. Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer Immunol Immunother. 2013;62(11):1745–1756. doi:10.1007/s00262-013-1476-9
- Chen HC, Eling N, Martinez-Jimenez CP, et al. IL-7-dependent compositional changes within the gammadelta T cell pool in lymph nodes during ageing lead to an unbalanced anti-tumour response. EMBO Rep. 2019;20(8):e47379. doi:10.15252/embr.201847379
- Mensurado S, Rei M, Lanca T, et al. Tumor-associated neutrophils suppress pro-tumoral IL-17+ gammadelta T cells through induction of oxidative stress. PLoS Biol. 2018;16(5):e2004990. doi:10.1371/journal.pbio.2004990