Abstract
Background
We previously studied the noninferiority of anastrozole (ANZ) versus ANZ followed by letrozole (A-LTZ) due to reimbursement policy. We found that patients with A-LTZ had better overall survival (OS) than did patients with ANZ alone. This study aimed to prove that patients with A-LTZ also had better OS than patients with letrozole (LTZ) alone.
Methods
All medical records of the breast cancer patients taking LTZ with or without ANZ between 2004 and 2013 were reviewed. All patients were divided into two groups: the LTZ group included patients treated with LTZ alone, and the A-LTZ group included patients treated with ANZ who were automatically changed to LTZ due to change of the reimbursement policy.
Results
From 359 cases, there were 179 cases in the LTZ group and 180 cases in the A-LTZ group. The mean age of patients in the LTZ group was 53.7 years and in the A-LTZ group was 54.2 years. The distribution of clinical stages among the LTZ group versus the A-LTZ group was 21 versus 4 (stage 1), 86 versus 116 (stage 2), 55 versus 46 (stage 3), and 17 versus 14 (stage 4), respectively. Among the LTZ patients, 63.7% took aromatase inhibitor monotherapy and 36.3% had a switching strategy, while in the A-LTZ group, 53.9% took AI monotherapy and 46.1% had a switching strategy. OS of the A-LTZ group was longer than that of the LTZ group.
Conclusion
The patients in A-LTZ, taking ANZ followed by LTZ had better OS than those in LTZ, taking LTZ alone.
Introduction
One of the standard treatments for estrogen receptor-positive breast cancer patients is endocrine therapy. It can be used as an adjuvant for the early stageCitation1 or a palliative for the advanced disease.Citation2 In Thailand, the available oral antiestrogen drugs include tamoxifen, anastrozole (ANZ), letrozole (LTZ), and exemestane. For premenopausal patients, tamoxifen seems to be the drug of choice, while aromatase inhibitors (AIs) have been used for postmenopausal patients.Citation2–Citation8 AIs are divided into two groups: nonsteroidal, which includes ANZ and LTZ, and steroidal, which includes exemestane. A switching strategy using an AI and tamoxifen is as effective as the AI monotherapy.Citation9,Citation10
In Thailand, some breast cancer patients receiving ANZ were automatically switched to LTZ due to the change of the reimbursement policy since 2008. We studied the outcome of this switching treatment to prove the noninferiority, but found that patients with ANZ followed by LTZ (A-LTZ) had better overall survival (OS) than patients with ANZ alone.Citation11 Some studies have shown no difference in OS among AIs, either nonsteroidal or steroidal,Citation12–Citation17 some cases changed from ANZ to LTZ due to early adverse effect of ANZ, but no clear superiority of LTZ was demonstrated.Citation7–Citation10,Citation17–Citation21 One study showed LTZ seemed to be superior to ANZ, but no clear benefit was demonstrated.Citation21 From our previous study,Citation11 we could not conclude whether A-LTZ was superior or similar to LTZ. So we designed the study comparing patients receiving LTZ and patients receiving A-LTZ during the same period.
Materials and methods
All medical records of the breast cancer patients taking LTZ between 2004 and 2013 were reviewed. AI therapy included two types of strategy: AI monotherapy for 5 years; or 2–3 years of tamoxifen followed by 2–3 years of AI, up to total of 5 years. All collected patients were divided into two groups: the LTZ group included patients taking LTZ with or without tamoxifen; and the A-LTZ group included patients taking ANZ who were automatically changed to LTZ, either in an AI monotherapy strategy or AI-tamoxifen switching strategy, due to the change of the reimbursement policy. Demographic data, type of reimbursement, endocrine therapy, and OS were reviewed and analyzed.
Demographic data was analyzed using Excel® 2007 (Microsoft Corp, Redmond, WA, USA). Survival data was analyzed using Stata version 10.1 (StataCorp LP, College Station, TX, USA). OS was analyzed using a Cox regression model and presented as Kaplan–Meier estimates with hazard ratios (HR) and 95% confidence interval (CI). The LTZ and A-LTZ groups were compared using logrank test. A P-value <0.05 was considered statistically significant.
This study was reviewed and approved by the Khon Kaen University Ethics Committee for Human Research and was based on the Declaration of Helsinki and the International Conference on Harmonisation (ICH) Good Clinical Practice Guidelines.
Results
The medical records of 359 patients with invasive breast cancer treated with LTZ with or without ANZ were reviewed. There were 25 stage 1 patients (mean age 53.6±11.7 years), 202 stage 2 patients (mean age 53.8±9.9 years), 101 stage 3 patients (mean age 54.5±9.8 years), and 31 stage 4 patients (mean age 53.6±10.0 years). In 180 cases (50.1%) out of 359 cases, ANZ was replaced with LTZ. The mean age of the LTZ group was 53.7 years and of the A-LTZ group was 54.2 years. The distribution of clinical stages among the LTZ group versus the A-LTZ group was 21 versus 4 (stage 1), 86 versus 116 (stage 2), 55 versus 46 (stage 3), and 17 versus 14 (stage 4), respectively. Among the LTZ patients, 63.7% took AI monotherapy and 36.3% had a switching strategy, while among the A-LTZ patients, 53.9% took AI monotherapy and 46.1% had a switching strategy (). Within the A-LTZ group, the average duration of ANZ was 18.7 months. Stage 4 patients took the shortest duration of ANZ, of only 8.7 months, while stage 1 patients took the longest duration, of 24.2 months ().
The OS of breast cancer patients in the LTZ and A-LTZ groups was analyzed by Cox regression model and presented as Kaplan–Meier survival curve with a HR of 0.6824 (), and the OS of the A-LTZ group was found to be significantly better than that of the LTZ group (P= 0.0386). When the OS was stratified by each stage (), only stage 4 patients in the A-LTZ group had a significantly better OS than stage-matched patients in the LTZ group (P=0.0114); the Kaplan–Meier survival curve was shown, with a HR of 0.3083, in .
Figure 1 Kaplan–Meier survival curve of the LTZ and the A-LTZ groups of patients (all stages).
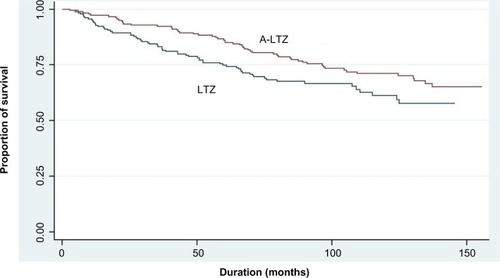
Figure 2 Kaplan–Meier survival curve of stage 4 LTZ and A-LTZ patients.
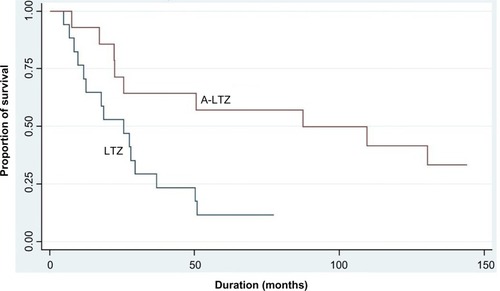
Table 1 Comparison of demographic data between the LTZ and the A-LTZ groups of patients
Table 2 Survival analysis between the LTZ and the A-LTZ groups of patients
Discussion
In Thailand, there are three major medical reimbursement systems: the Civil Servant Medical Benefit Scheme (CSMBS) for government officers, Social Security for employees, and Universal Coverage for all the remaining Thai people. On average, government officers have higher socioeconomic status than most Thai people. The proportions of CSMBS and non-CSMBS patients in the LTZ group (50:50) were higher than in the A-LTZ group (46:54). We classified the reimbursement systems into CSMBS and non-CSMBS because higher socioeconomic status might have a longer survival than lower socioeconomic status. In comparison, in our previous study,Citation11 the proportions of CSMBS and non-CSMBS patients, respectively, in the ANZ group were nearly the same as those in the A-LTZ group (45:55 [ANZ] versus 46:54 [A-LTZ]). Although the OS of both the LTZ and ANZ groups differed significantly from the A-LTZ group, the HR of LTZ (0.6824) was higher than that of the ANZ (0.5515) group.
Some studiesCitation9,Citation10,Citation13,Citation22 have reported the superiority of AI to tamoxifen but no significant difference in disease-free survival between the switching strategy compared with AI monotherapy strategy. However, AI produced more adverse events than tamoxifen, so we had to consider the survival benefit between the two strategies.Citation19,Citation22–Citation24 The difference of HR between LTZ and ANZ groups might be affected by the proportions of AI monotherapy and switching strategy (64:36 versus 54:46).Citation11
This study confirmed that treatment with A-LTZ should lengthen the survival of hormone-sensitive breast cancer patients better than LTZ or ANZ. The efficacy of LTZ was shown to be the same as ANZ in several studies,Citation12–Citation14 although some studies have shown LTZ might be superior to ANZ in terms of quality of life, due to lower incidence of adverse events.Citation25,Citation26 But there were no data about sequential therapy of ANZ and LTZ. Both drugs were classified in the same group – nonsteroidal AI. So it seemed to be irrational using both drugs sequentially, even with their different chemical structure.Citation14 We observed the shortest duration of ANZ usage with narrowest standard deviation in stage 4 patients of the A-LTZ group, which might relate to statistical significance in the OS difference between the two groups. However, this retrospective study with small number of patients can reach limited conclusions. Further prospective study with larger number of patients should be performed to confirm the better outcome of sequential therapy of ANZ and LTZ.
Conclusion
The patients in the A-LTZ group, taking A-LTZ, seemed to have better OS than those in the LTZ group, taking LTZ alone.
Disclosure
The authors report no conflicts of interest in this work.
References
- ConnorCAttaiDAdjuvant endocrine therapy for the surgeon: options, side effects, and their managementAnn Surg Oncol201320103188319323975307
- SmithIEDowsettMAromatase inhibitors in breast cancerNew Engl J Med2003348242431244212802030
- ForbesJFCuzickJBuzdarAHowellATobiasJSBaumMArimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists’ GroupEffect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trialLancet Oncol200891455318083636
- CuzickJSestakIBaumMATAC/LATTE InvestigatorsEffect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trialLancet Oncol201011121135114121087898
- DaviesCPanHGodwinJAdjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative GroupLong-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trialLancet2013381986980581623219286
- GossPEIngleJNMartinoSA randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancerN Engl J Med2003349191793180214551341
- JakeszRJonatWGnantMABCSG and the GABGSwitching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABC SG trial 8 and ARNO 95 trialLancet2005366948445546216084253
- BursteinHJPrestrudAASeidenfeldJAmerican Society of Clinical OncologyAmerican Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancerJ Clin Oncol201028233784379620625130
- van de VeldeCJReaDSeynaeveCAdjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trialLancet2011377976232133121247627
- MouridsenHGiobbie-HurderAGoldhirschABIG 1-98 Collaborative GroupLetrozole therapy alone or in sequence with tamoxifen in women with breast cancerN Engl J Med2009361876677619692688
- AphinivesPVachirodomDThanapaisalCRangsrikajeeDSomintaraOEffects of switching from anastrozole to letrozole, due to reimbursement policy, on the outcome of breast cancer therapyBreast Cancer (Dove Med Press)2014614515025249759
- DixonJMRenshawLLangridgeCAnastrozole and letrozole: an investigation and comparison of quality of life and tolerabilityBreast Cancer Res Treat2011125374174920821047
- RiemsmaRForbesCAKesselsASystematic review of aromatase inhibitors in the first-line treatment for hormone sensitive advanced or metastatic breast cancerBreast Cancer Res Treat2010123192420535542
- GeislerJDifferences between the non-steroidal aromatase inhibitors anastrozole and letrozole – of clinical importance?Br J Cancer201110471059106621364577
- GossPEIngleJNPritchardKIExemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27 – a randomized controlled phase III trialJ Clin Oncol201331111398140423358971
- EllisMJSumanVJHoogJRandomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype – ACOSOG Z1031J Clin Oncol201129172342234921555689
- FabianCJThe what, why and how of aromatase inhibitors: hormonal agents for treatment and prevention of breast cancerInt J Clin Pract200761122051206317892469
- BeresfordMTumurIChakrabartiJBardenJRaoNMakrisAA qualitative systematic review of the evidence base for non-cross-resistance between steroidal and non-steroidal aromatase inhibitors in metastatic breast cancerClin Oncol (R Coll Radiol)201123320921521134732
- FonteinDBSeynaeveCHadjiPSpecific adverse events predict survival benefit in patients treated with tamoxifen or aromatase inhibitors: an international tamoxifen exemestane adjuvant multinational trial analysisJ Clin Oncol201331182257226423610112
- FrieseCRPiniTMLiYAdjuvant endocrine therapy initiation and persistence in a diverse sample of patients with breast cancerBreast Cancer Res Treat2013138393193923542957
- BerryJAre all aromatase inhibitors the same? A review of controlled clinical trials in breast cancerClin Ther200527111671168416368441
- ThürlimannBKeshaviahACoatesASBreast International Group (BIG) 1-98 Collaborative GroupA comparison of letrozole and tamoxifen in postmenopausal women with early breast cancerN Engl J Med2005353262747275716382061
- AmirESerugaBNiraulaSCarlssonLOcañaAToxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysisJ Natl Cancer Inst2011103171299130921743022
- FallowfieldLCellaDCuzickJFrancisSLockerGHowellAQuality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) Adjuvant Breast Cancer TrialJ Clin Oncol200422214261427115514369
- ThomasRExamining quality of life issues in relation to endocrine therapy for breast cancerAm J Clin Oncol2003264S40S4412902876
- ThomasRGodwardSMakrisABloomfieldDMoodyAMWilliamsMGiving patients a choice improves quality of life: a multi-centre, investigator-blind, randomised, crossover study comparing letrozole with anastrozoleClin Oncol (R Coll Radiol)200416748549115490811
