Abstract
A new breast cancer cell line (AMJ13) has been established from an Iraqi breast cancer patient. It is considered unique because it is the first for an Iraqi population, and is expected to be a useful tool in breast cancer research. The AMJ13 cell line was established from the primary tumor of a 70-year-old Iraqi woman with a histological diagnosis of infiltrating ductal carcinoma. The cells were morphologically characterized by light and scanning electron microscopy, and found to be elongated multipolar epithelial-like cells with a population doubling time of 22 hours. The anchorage-independent growth ability test showed that the cells were able to grow in semisolid agarose, confirming their transformed nature. Cytogenetic study of these cells showed chromosomal aberrations with many structural and numerical abnormalities, producing chromosomes of unknown origin called marker chromosomes. Immunocytochemistry showed that the estrogen receptor and the progesterone receptor were not expressed, and a weak positive result was found for HER2/neu gene expression. AMJ13 cells were positive for BRCA1 and BRCA2, as well as for vimentin. This cell line should be useful when testing new therapies for breast cancer in the Middle East.
Introduction
Breast cancer is the most common malignancy in women worldwide.Citation1 In Iraq, breast cancer accounts for about one-third of the cancers affecting women (32%) according to the Iraqi Cancer Registry, which also shows that breast cancer ranks first among the cancers affecting the Iraqi population.Citation2 In general, cell lines are an important tool in cancer research, their major benefit being that they provide an unlimited supply of a relatively homogeneous cell population capable of self-replication that can be widely distributed to facilitate comparative studies.Citation3 Human cancer cell line cultures are considered to be a model of physiological functioning in vivo; so these enable research on cancer cell biology and are useful for developing new strategies to prevent cancer cell growth and progression of the disease.Citation4 Important biomarkers that have been analyzed in detail in patients with breast cancer are the hormone receptors, including the estrogen receptor (ER) and progesterone receptor (PR), and the Her-neu2 gene.Citation4 Using these three parameters for molecular classification, breast cancers can be classified into five types that provide important information with regard to treatment.Citation5
Cells in culture can also be characterized for chromosomal aberrations, which are detected mainly by conventional cytogenetics.Citation6 Cytogenetic studies of mammary adenocarcinoma cell lines are essential for understanding the pathogenesis of these cancers.Citation7,Citation8 The implications of these chromosomal alterations has opened up a new and promising route toward better understanding of breast cancer.Citation9 The chromosomal alterations in such cancers are often numerous, and marker chromosomes often demonstrate hyperploidy.Citation10 The aim of this work was to establish a cell model to better characterize and understand breast cancer in Iraqi women and to develop more effective therapies for the disease.
Materials and methods
Clinical history
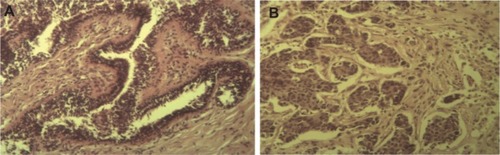
The patient from whom this cell line derived was a 70-year-old female who lived in the city of Al-Ameen in Baghdad. She was 160 cm in height, weighed 70 kg, and had had six children. She had undergone a right-sided mastectomy for breast cancer, but had no genetic or chronic diseases. The histological sample of her breast mass that was used to established our cell line showed poorly differentiated infiltrative ductal carcinoma (), which was graded 3/3 according to the Nottingham modification of the Bloom–Richardson system, with no vascular invasion seen. The nipple and resection margins were free of malignancy. All axillary lymph nodes (n=11) were free of malignancy. According to the TNM classification, clinical TNM staging of this patient is T2 N0 Mx. Paraffin sections were positive for the ER and PR and positive for the Her/Neu2 tumor suppressor gene. The study was approved by Iraqi Center for Cancer and Medical, Genetic, and Scientific Institutional Review Board, and had received the patient consent for the use of patient-derived cell line.
Figure 1 Histopathological sections of the original tumor of which the AMJ13 cell line was derived from.
Primary tumor processing and cell culture
The tumor specimen was washed in phosphate-buffered saline (PBS), minced into small pieces (approximately 2 mm in size), and digested with and without collagenase. The cell suspension was then filtered through sterile mesh to separate the undigested tissue, after which the filtrate was centrifuged at 1,500 rpm for 10 minutes. The pellet obtained was resuspended in Roswell Park Memorial Institute medium supplemented with 20% fetal bovine serum, 100 IU of penicillin, and 100 µg of streptomycin. The suspension was then transferred to a tissue culture flask and incubated at 37°C. The tumor cells were passaged every 3–4 days, and every few passages were frozen in liquid nitrogen. The serum requirement for further passages was 10%.
Analysis of morphology and morphometry
The cells were cultured on a Labtek tissue culture chamber slide, then fixed in 4% formalin for 10 minutes, rinsed gently in PBS, and stained with hematoxylin and eosin for study by light microscopy. For scanning electron microscopy, the cells were cultured on cover slips and fixed after confluency in 4% (v/v) glutaraldehyde in 0.1 M PBS buffer (pH 7.4) for 4 hours at 4°C. The fixed cells were washed in three changes of PBS buffer for 10 minutes each, and post fixed in 1% osmium tetroxide for 2 hours at 4°C. The samples were then washed in three changes of PBS buffer (pH 7.4) for 10 minutes each, dehydrated in ascending grades of ethanol (35%, 50%, 75%, 95%, and 100%), and dried in air for 2 hours. The specimens were viewed using a Phenom G2 pro desktop scanning electron microscope at an accelerating voltage of 10 kV.
Cell growth curve
The AMJ13 cell line was cultured in 30 mini Petri dishes (3 mL). The cells were counted using a Neubauer hemtocytometer at 24-hour intervals for 10 days at early passages of continuous cell culture. The population doubling time was determined using the following formula:
Anchorage-independent growth assay
The AMJ13 cells were detached from the flask by trypsinization. Next, 10,000 cells were prepared as single cell suspensions in 0.35% agar with 1 mL of culture medium, and seeded onto 1 mL of hard agar (1% prepared in culture medium) in a 12-well plate. The cells were then incubated at 37°C in a humidified 5% CO2 incubator for 41 days. The cell colonies were then fixed for observation, and images were taken using an inverted microscope. Colony diameters were quantified using the image analysis software MICROVISIBLE. The protocol was performed according to Voisin et alCitation12 with modifications.
Analysis of karyotype
The AMJ13 cells were karyotyped at passages 39 and 40. We used the protocol described by YassenCitation13 as modified by the cytogenetics laboratory at the Iraqi Center for Cancer and Medical Genetic Research. Briefly, confluent AMJ13 cell monolayers were treated with colcemid (Kreatech Biotechnology) for 1 hour and then dispersed with 0.5% trypsin. The harvested cells were washed in physiological solution, treated with 0.075 M KCl solution for 40 minutes at 37°C, and then fixed in a mixture of glacial acetic acid and methanol (1:6, v/v). The cell suspension was dropped onto a glass slide and stained with Giemsa dye to examine the slide and in preparation for chromosome G banding.
Immunocytochemistry
AMJ13 cells were cultured to a confluent monolayer on Labtek chamber slides, then fixed in 4% formalin for 10 minutes, rinsed gently in PBS, and incubated in a humid chamber, first with blocking reagents for 1 hour and then with selected primary antibodies, ie, mouse anti human-Her neu2, mouse anti human-PR, mouse anti human-ER BRCA1 and BRAC2, and vimentin (Santa Cruz Biotechnology), overnight at 4°C for detection of markers for breast cancer. The antibodies were diluted (1:50–100) according to the manufacturer’s recommendations. We used the standard protocol for the biotin–streptavidin staining kit (Santa Cruz Biotechnology).
Results
Establishment of primary cell culture
Several primary cultures were initiated using samples from Iraqi breast cancer patients, but only one sample (the AMJ13 cell line) could be successfully cultured. The first passage from the primary culture was done after 16 days, with subcultures repeated continuously for at least 2 years (March 2013 through April 2015) and the cells were subcultured every 3–4 days.
Morphology and morphometry of cultured cells
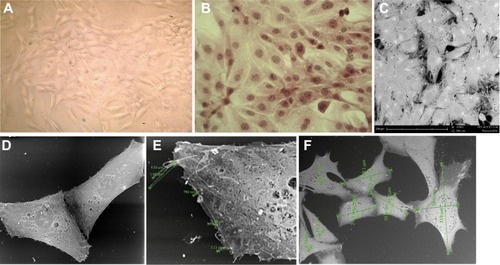
The cultured AMJ13 cells had an elongated multipolar epithelial-like cell shape, with nuclear polymorphism and multiple nuclei in most of the cells, as well as showing many cells with mitotic figures, which expressed the characteristics of cell morphology (). is a scanning electron photomicrograph showing AMJ13 cells with a characteristic polygonal epithelial squamous shape with many short and thin processes, with cells growing upon each other. Measurements taken by scanning electron microscopy indicated a mean cell length of 73.8 µm, a mean cell width of 33 µm, and a mean nucleus diameter of 11.1 µm. Microvilli were also present, and ranged from 0.792 µm to 5.98 µm in length ().
Figure 2 AMJ13 cell line morphology.
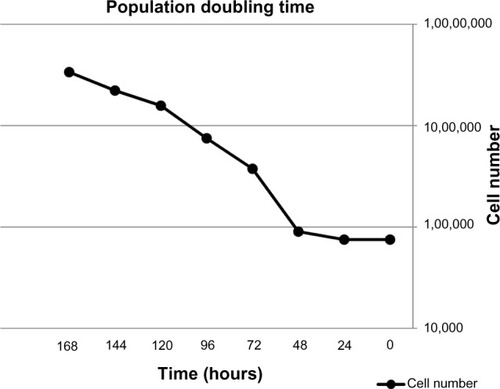
Growth curve and doubling time
The growth kinetics of the AMJ13 breast cancer cell line were studied at passage 10, and the cell growth curve is shown in . The population doubling time was calculated to be 22 hours.
Anchorage-independent growth assay
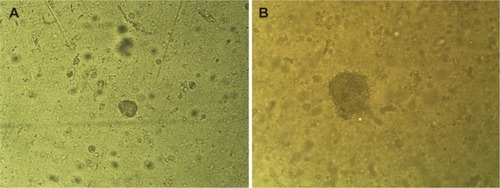
We evaluated the ability of these cells to grow in an anchorage-independent fashion using soft agar colony assays. The cells readily formed colonies (>50 µm diameter) within 6 days (), with a mean diameter of 93.85 µm. After 14 days, the colonies were continuing to expand, reaching a mean diameter of 282.75 µm ().
Figure 4 AMJ13 cells growing in an anchorage-independent fashion using soft agar colony assays.
Analysis of karyotype
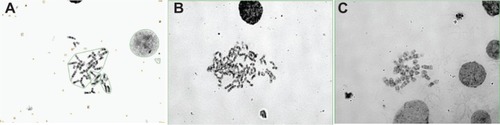
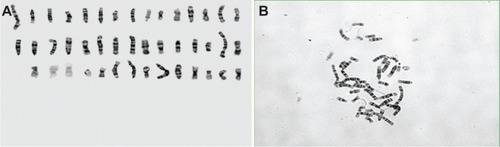
Cytogenetic analysis of the AMJ13 cell line showed a complex karyotype with many abnormal structural and numerical changes, with the main chromosomal aberrations resulting from rearrangement, breaks, and translocations (). The G banding of the chromosomes of the passage 39 cells used to estimate the karyotyping of the new established breast cancer cell line, because it has the most dominant clones which were examined.
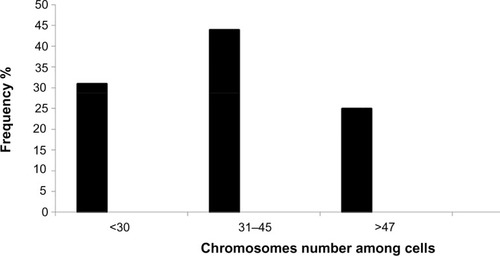
The chromosomal aberrations of the new established breast cancer cell line were both numerical and structural. Chromosomal numbers differed between the cells () that ranged from 31 to 45 were 44%, this represented the majority of cells in the population on the slide, second group that revealed 31% percentage of cells with chromosome number (<30) and 25% been cells with chromosome number more than 47 ( and ).
Figure 5 Analysis of Karyotype.
Immunocytochemistry
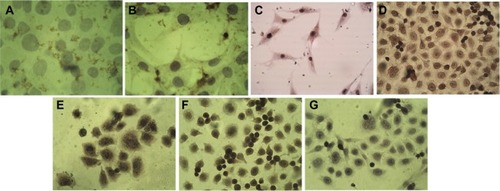
ER, PR, and Her-neu2 gene expression in the AMJ13 breast cancer cell line was detected using an anti-mouse IgG immunocytochemistry kit (). The results showed that the cells were negative for ER and PR, and weakly positive for Her-neu2 gene expression when compared with a negative control. Rabbit anti-mouse BRCA1 and BRCA2 genes and vimentin were used to detect these important markers. AMJ13 cells were positive for BRCA1 nuclear staining, but to a lesser degree than BRCA2, which was positive for both nuclear and cytoplasmic staining (). Vimentin was strongly positive ().
Figure 8 Immunocytochemistry analysis of AMJ13 cell line.
Discussion
Here we describe the development and characterization of the first continuous breast cancer cell line from an Iraqi patient, known as the AMJ13 cell line. There are presently more than 70 breast cancer cell lines in the American Type Culture Collection, but few of them are derived from Asians.Citation4 The AMJ13 cell line is the first Arabian and Middle Eastern breast cancer cell line and was derived from an Iraqi patient with histologically diagnosed poorly differentiated infiltrative ductal carcinoma. It was graded 3/3 according to the Nottingham modification of the Bloom–Richardson system and T2, N0, Mx on TNM staging. Cell lines from primary breast carcinoma are among the most difficult to establish in culture.Citation14
Established human cancer cell lines are a valuable resource for studying cancer cell biology, and these cultured cells are considered to be a model for the physiological processes occurring in vivo and for developing new strategies to combat cancer cell growth and progression of the disease.Citation4 Cell cultures established directly from human tumors serve as unique models for studying and manipulating potentially relevant molecular and cellular processes underlying malignant disease and identification of novel biological therapeutic targets. The majority of breast cancer-derived cell lines are derived from secondary tumors or pleural effusions in patients with advanced breast cancer.Citation3
The newly established AMJ13 cell line grew as an adherent monolayer with characteristic elongated epithelial-like cell morphology. Scanning electron microscopy confirmed that these cells having a characteristic polygonal epithelial squamous shape with many short and thin processes, and that they grow upon each other, confirming loss of contact inhibition, which is one of the features of transformation. The cultured cells maintained a morphology consistent with that of the primary culture in subsequent subculture passages, as occurs in other breast cancer cell lines with the same morphological characteristics.Citation4
Measurements taken by scanning electron microscopy showed that these cancer cells had a mean length of 73.8 µm, a mean width of 33 µm, a mean nucleus diameter of 11.1 µm, and microvilli ranging from 0.792 µm to 5.98 µm in length. These measurements will be useful for future studies attempting to characterize these cells after a long time in culture. Cytoplasmic protrusions and morphological features similar to those in our AMJ13 cells have been demonstrated in other cell lines, including those for human breast cancer epithelial cells.Citation15
Our cells have been grown continuously for over 2 years and have undergone more than 70 passages. The AMJ13 cell line appears to be immortal, given that growth has continued after recovery from cryopreservation.
The AMJ13 cell population doubling time is 22 hours, which is more rapid than for many other breast cancer cell lines. The BC-021 cell line, established from invasive ductal carcinoma in Chinese patients, has a doubling time of 46 hours,Citation4 as does the HMA-1 cell line.Citation16 However, the population doubling time of our AMJ13 cell line is similar to the 28 hours noted for the ANGM5 cell line established from an Iraqi patient with cerebral glioblastoma.Citation17 The similarity in population doubling time between our new established cell line and that of the ANGM5 cell line, as well as the difference to other non-Iraqi cell line may be refer to external conditions such as environmental or ethnic factors specific to Iraq. Some researchers have reported that environmental risk factors affected the nature of cancers.Citation18 AMJ13 cells have the ability to grow in an anchorage-independent fashion in soft agar colony assays, with formation of colonies within 6 days and continued expansion at 14 days, which confirms the transformed nature of these cells and their potential for metastasis.Citation19
Analysis of the AMJ13 cell karyotype showed abnormal numerical and structural changes in the chromosomes, indicating a complex karyotype resulting from chromosomal rearrangement, breaks, and translocation. These complex aberrations showing chromosomes with abnormal undefined structure that we call it “marker chromosomes”. Marker chromosomes are characterized by their abnormal length and structure in comparison with normal human chromosomes, which can lead to a decrease in the number of chromosomes in the cells to less than 46.
The appearance of marker chromosomes is common in cancer cells, and results from irregular cell cycle division. This phenomenon was reported by Matano et al,Citation16 who identified four marker chromosomes among 41 chromosomes in the karyotype of an HMA-1 breast cancer cell line. Further, Wang et alCitation20 identified 20 marker chromosomes in the karyotype of a HDQ-P1 breast cancer cell line. Similar chromosomal aberrations have been reported in the Iraqi ANGM5 glioblastoma cell line,Citation17 which shows marker chromosomes but no normal chromosomes within the cell.Citation21 The chromosomal instability in general cause aneuploidy cells to be harmful more than the effect from change in nucleotide level, this chromosomal instability effects on wide range of proliferation, apoptosis and differentiation of normal cells converted to tumor cells by loss the function of suppressor gene or loss the inhibition of oncogene or both effectors. All these factors may generate tumor cells that are resistant to antitumor agents.Citation22
The immunocytochemistry analysis showed that most of the AMJ13 cells weakly expressed the HERneu2 protein, with no PR or ER expression. ER, PR, and HER-neu2 gene expression status influences the management of breast cancerCitation23 because it provides both therapeutic and prognostic informationCitation24 and predicts the response to endocrine therapy.Citation25 AMJ13 cells were positive for BRCA1 nuclear staining, but to a lesser degree than for BRCA2, which was positive for both nuclear and cytoplasmic staining. Tulchin et alCitation26 frequently found reduced BRCA1 nuclear staining in breast tumor tissue when compared with normal tissue, and less BRCA1 staining with higher histological tumor grade. Strong positive staining of the nucleus and cytoplasm suggests mutation in BRCA1 because of its role in cell cycle control and DNA repair. BRCA2 mutation causes multiple chromosomal abnormalities, as shown in the cytogenetic analysis reported by Gretarsdottir et al,Citation27 which was consistent with the complex chromosomal abnormalities found in our AMJ13 cell line. Moreover, our AMJ13 breast cancer cell line is strongly positive for vimentin, and Vora et alCitation28 have reported that gain of vimentin expression is an indicator of biologically aggressive breast carcinoma.
Conclusion
We have successfully established an Iraqi breast cancer cell line, which to our knowledge is the first in the Middle East, and is named AMJ13 (for the research team and patients’ initials and the year in which the line was established). The AMJ13 cell line has a very complex and interesting karyotype with many numerical and structural abnormalities as well as loss of expression of two important receptors (ER and PR), weak Her/neu2 protein expression, and positivity for BRCA1/2 and vimentin. This cell line will be a very helpful tool in breast cancer research and could assist in developing new therapies for this disease.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This work was supported by the Iraqi Center for Cancer and Medical Genetic Research, Mustansiriya University, the Iraqi Ministry of Higher Education and Scientific Research.
References
- ColemanMPQuaresmaMBerrinoFCancer survival in five continents: a worldwide population-based study (CONCORD)Lancet Oncol20089873075618639491
- Iraqi Cancer BoardResults of the Iraqi Cancer Registry 2009BaghdadIraqi Cancer Registry Center, Ministry of Health2010
- PandrangiSLRaju BagadiSASinhaNKEstablishment and characterization of two primary breast cancer cell lines from young Indian breast cancer patients: mutation analysisCancer Cell Int20141411424502646
- ShenCGuMLiangDEstablishment and characterisation of three new human breast cancer cell line derived from Chinese breast cancer tissuesCancer Cell Int20099219121212
- BrentonJDCareyLAAhmedAACaldasCMolecular classification and molecular forecasting of breast cancer: ready for clinical application?J Clin Oncol200523297350736016145060
- AströmAKJinDImamuraTChromosomal localization of three human genes encoding bone morphogenetic protein receptorsMamm Genome199910329930210051328
- TrentJMCytogenetic and molecular biologic alterations in human breast cancer: a reviewBreast Cancer Res Treat1985532212293896352
- GebhartEBrüderleinSAugustusMSiebertEFeldnerJSchmidtWCytogenetic studies on human breast carcinomasBreast Cancer Res Treat1986821251383469005
- CervenkaJKoulischerLGorlinRChromosomes in Human CancerSpringfield, IL, USACharles C Thomas1973
- GioanniJLe FrançoisDZanghelliniEEstablishment and characterisation of a new tumorigenic cell line with a normal karyotype derived from a human breast adenocarcinomaBr J Cancer19906218132390488
- DoyleAGriffithsBCell and Tissue Culture for Medical ResearchNew York, NY, USAWiley Interscience2000
- VoisinLJulienCDuhamelSMEK2 is sufficient but not necessary for proliferation and anchorage-independent growth of SK-MEL-28 melanoma cellsPLoS One20088337
- YaseenNCytogenetic study of human colorectal cancer cellPhD thesisSheffield, UKUniversity of Sheffield2000
- HeoCKBahkYYChoEWTumor-associated autoantibodies as diagnostic and prognostic biomarkersBMB Rep2012451267768523261052
- HassRBertramCCharacterization of human breast cancer epithelial cells (HBCEC) derived from long term cultured biopsiesJ Exp Clin Cancer Res20092812719751512
- MatanoSOhuchiNHirakawaHEstablishment of an estro-gen receptor-positive cell line (HMA-1) derived from human breast carcinomaTohoku J Exp Med199116421691821750037
- Al-ShammariAMAl-JubooryAAsmaaAAliAMAl-HiliZAYaseenNYEstablishment and characterization of a chemoresistant glioblastoma cell line from an Iraqi patientAbstract presented at the 105th Annual Meeting of the American Association for Cancer ResearchApril 5–9, 2014San Diego, CA, USA
- KingJBRobinsMWCancer Biology2nd edUpper Saddle River, NJ, USAPearson Prentice Hall Inc2000
- MoriSChangJTAndrechekERAnchorage-independent cell growth identifies tumors with metastatic potentialOncogene200928312796280519483725
- WangCSGouletFLavoieJEstablishment and characterization of a new cell line derived from a human primary breast carcinomaCancer Genet Cytogenet20001201587210913678
- AlmukhtarAAStudying chromosomal aberration and some gene abnormalities in local glioblastoma cell lineMSc thesisBaghdad, IraqCouncil of Science for Women, University of Baghdad
- TresnasariKTakakuwaTHamMFRahadianiNNakajimaHAozasaKTelomere dysfunction and inactivation of the P16(INK4a)/Rb pathway in pyothorax-associated lymphomaCancer Sci200798797898417428253
- Azizun-NisaBhurgriYRazaFKayaniNComparison of ER, PR and HER-2/neu (C-erb B 2) reactivity pattern with histologic grade, tumor size and lymph node status in breast cancerAsian Pac J Cancer Prev20089455355619256737
- OnitiloAAEngelJMGreenleeRTMukeshBNBreast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survivalClin Med Res2009741319574486
- CuiXSchiffRArpinoGOsborneCKLeeAVBiology of progesterone receptor loss in breast cancer and its implications for endocrine therapyJ Clin Oncol200523307721773516234531
- TulchinNOrnsteinLDikmanSLocalization of BRCA1 protein in breast cancer tissue and cell lines with mutationsCancer Cell Int20131317023855721
- GretarsdottirSThorlaciusSValgardsdottirRBRCA2 and p53 mutations in primary breast cancer in relation to genetic instabilityCancer Res19985858598629500438
- VoraHHPatelNARajvikKNCytokeratin and vimentin expression in breast cancerInt J Biol Markers2009241384619404921