Abstract
Gastrointestinal metastases from breast cancer are not common. We present a 58-year-old female diagnosed with lobular breast cancer some years before whose relapses were gastric and colonic mucosal. Simultaneous metastases are extremely rare. To our knowledge, no cases of initial dual affectation have been reported. The patient also showed gastritis by Helicobacter pylori. Invasive lobular breast carcinoma is the most frequent special type of breast cancer and carries some specific molecular alterations such as loss of expression of E-cadherin. Although underlying mechanisms of metastasization are not entirely known, chemokines as well as inflammatory events seem to be implicated in this process. Interaction between chemokines and their receptors frequently induces cell migration. We hypothesize that H. pylori, inflammatory cells, and chemokines may create a favorable environment attracting tumor cells.
Introduction
Breast cancer is the most frequent malignancy among women. Approximately 16,000 new cases are diagnosed each year in Spain. The 5-year survival rate is constantly increasing, which implies a growing prevalence in almost all developed countries. Modern adjuvant treatment regimens have yielded a reduction in relapses and higher cure rates.
Relapses have been reported in 30%–80% of patients in spite of surgery, chemotherapy, and radiotherapy, but are rare in early-stage breast cancer.Citation1 The most common sites of distant metastases are in bone, liver, lung, and brain.
Lobular carcinoma is less likely to involve the gastrointestinal tract. The incidence of extrahepatic gastrointestinal tract metastases observed in autopsy studies varies from 4% to 18%, with the most commonly affected organ being the stomach, followed by colon and rectum.Citation2,Citation3 Nevertheless, coexisting solitary metastases to both stomach and colon are extremely rare.
Case report
A 58-year-old female smoker was diagnosed with pulmonary thromboembolism during her first pregnancy, and hypothyroidism and hypertension twenty years ago. She underwent quadran tectomy and lymphadenectomy in May 2006. Pathologic analysis from breast tissue showed a 2.5 cm invasive lobular carcinoma (ILC), grade III, and 20 of 25 axillary nodes were affected. Immunohistochemical results were as follows: estrogen receptor (ER)-positive, progesterone receptor (PR)-positive, Her-2/NEU-negative, p53 and isolated cells stained for Ki67. Body computed tomography and bone scan were negative for advanced disease.
Adjuvant chemotherapy based on doxorubicin 75 mg/m2 and cyclophosphamide 750 mg/m2 every 21 days for four cycles, followed by four courses of docetaxel 75 mg/m2 every 21 days, was administered in July 2006. Radiotherapy was administered to the remaining breast and drainage areas. Exemestane was initiated in February 2007.
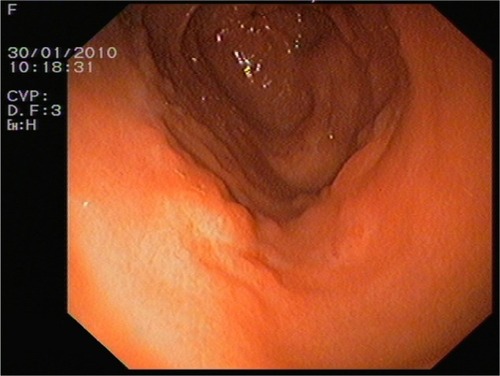
In June 2009, serum markers CEA and CA15.3 began to rise. Chest X-rays, abdominal ultrasound, bilateral mammography, brain magnetic resonance imaging, and positron emission tomography scan did not reveal metastatic disease. A closer follow-up was done. In January 2010, the patient revealed dyspeptic symptoms such as nausea and epigastric pain. She underwent an esophagogastroscopy that demonstrated a granular mucosa with an adenomatous pattern. In the antrum, prepyloric fold did not modify with blowing, and biopsies were taken from all suspected areas (). Pathology revealed mucous gastric metastases from a lobular carcinoma of the breast. Immunohistochemical examination was carried out using primary antibodies manufactured by Dako Denmark A/S (Glostrup, Denmark). Mucous gastric metastases were positive for cytokeratin-19 and hormonal receptors (). Of note, there was a chronic gastritis caused by Helicobacter pylori. A colonoscopy was performed in February 2009, which showed a polyp that was subsequently excised, and a pathology report showed metastases from a lobular breast cancer, also positive for cytokeratin-7 and hormonal receptors ( and ). Both colonic and gastric biopsies were negative for Erb2/NEU protein.
Figure 2 Hematoxylin and eosin staining of the gastric specimen demonstrating strands of invasive cells in the mucosa.
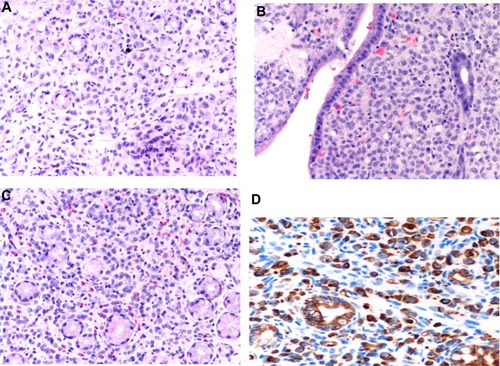
Figure 3 Endoscopic image of colonic mucosa.
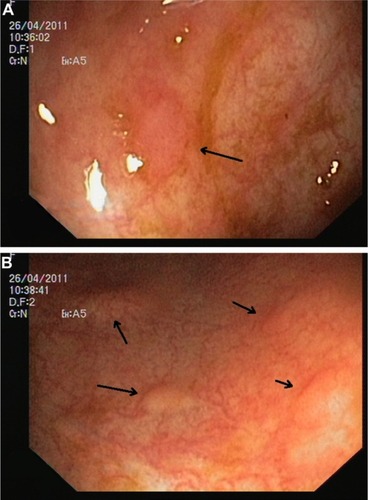
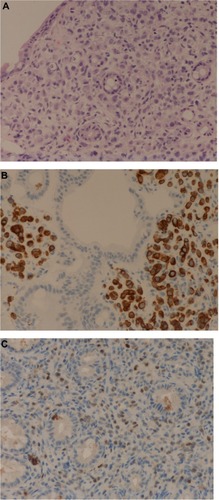
Figure 4 Biopsy of colonic mucosa.
Because of this gastrointestinal progression, capecitabine 1,250 mg/m2 every 12 hours (14 days, every 21) was initiated. Before treatment, CEA was higher than 100 ng/mL (<2.5) and CA15.3 exceeded 200 U/mL (<32.4). After six courses of chemotherapy, CEA decreased to 23 ng/mL, while CA15.3 was elevated near the end of chemotherapy and the patient was asymptomatic. A new esophagogastroscopy showed similar findings. A new positron emission tomography scan revealed a hypermetabolic lesion in the second lumbar vertebra. Pancytopenia developed, so a bone marrow biopsy was performed, revealing metastasis in this location. Therefore, a new line of chemotherapy with paclitaxel–carboplatin–bevacizumab plus zoledronic acid was started. Despite several chemotherapy and hormone therapy lines consisting of cisplatin–gemcitabine, vinorelbine, liposomal doxorubicin, and other aromatase inhibitors, the patient developed brain metastases and finally died in August 2011.
Discussion
Single gastricCitation5 and colonCitation1 metastases and metastases in both locationsCitation6 from breast cancer have been described, but, to our knowledge, this is the first case of a synchronous spread to stomach and colon as the first and unique initial manifestation of metastatic breast cancer.
The incidence of gastric metastases from breast cancer in long-term series and autopsies ranges between 2% and 18%.Citation7 Lobular breast cancer is the predominant histology found in gastrointestinal tract metastases. The affinity of lobular breast cancer cells for this system is well known.Citation3,Citation8 Distant spread may occur many years after the initial treatment for breast cancer.Citation5 Other rare sites are gynecologic organsCitation9 and the peritoneum.Citation1 Ductal tumors tend to metastasize to the liver, the lung, and the brain,Citation5,Citation11 although some cases of intestinal metastases have been reported.Citation8,Citation11
Symptoms from gastrointestinal metastases are inexpressive.Citation1,Citation5,Citation7 Serum marker CA15.3 may be elevated, as in our case.Citation1 The endoscopic pattern usually is confounded with a gastrointestinal cancer. The most frequent pattern of spread is diffuse infiltration.Citation12–Citation14 The median time to development of metastases is approximately 4 or 5 years.Citation13
Metastases to colon could imitate primary colon cancers,Citation1,Citation8 may acquire a similar aspect to linitis,Citation15 or Crohn’s disease,Citation1 and may even be a solitary adenomatous polyp.Citation16 Our patient had a solitary metastatic localization to an adenomatous polyp in the colon as well as stomach infiltration. Although breast cancer metastasis in stomach and colon may coexist,Citation6 it has never been described as the first manifestation of dissemination.
Treatment of gastrointestinal metastases from breast cancer is under discussion. Systemic therapy is the first option, and surgical treatment is considered in cases with obstruction or bleeding.Citation3 There is a low response rate to chemotherapy in ILC patients.Citation17 The median survival rate is 2 years,Citation1,Citation3 which implies a poor prognosis, particularly for gastric metastasis.Citation3 Hormonotherapy and chemotherapy were used to treat our patient. Surgical treatment was not necessary.
Imaging and endoscopic findings are not enough to differentiate primary from secondary gastrointestinal lesions.Citation3,Citation5 Biopsy and pathology are essential for diagnosis. Deep biopsies must be performed with endoscopy,Citation5,Citation7 and histology must be compared with the primary breast cancer.Citation18 Sometimes, microscopy may differ between primary and metastatic disease, but immunohistochemical analysis may be the only consistent method for differentiating these entities.Citation7 Pathologic criteria include infiltration of the serosal, muscular, and submucosal layers by cells, typically in an “Indian file” pattern,Citation11 resulting in a signet ring appearance.Citation19 ER and PR positivity may suggest breast origin, but these are positive in 32% and 12%, respectively, of patients with primary gastric cancer. However, the expression of ultimate-generation antibodies against ERα is reliable and useful in differentiating gastric metastasis from breast cancer.Citation5 Her-2 extracellular domain (ECD) expression may help in the differential diagnosis of primary gastric cancer vs metastatic breast carcinoma. Absence of ECD is related to lobular breast cancer. Both markers, the presence of ERα and the absence of ECD expression, may reliably distinguish these entities.Citation20 In our case, both specimens from stomach and colon were compared with the primary surgical piece removed some years before and were found to match. Breast lesions were characterized by staining for CK7 or CK19 ( and ) and hormone receptors. Cytoplasmic positivity for gross cystic disease fluid protein-15 (GCDFP-15) is useful in identifying breast cancer metastasis to gastric mucosa,Citation5 but, despite its high specificity (90%), its sensitivity is low (50%).Citation3 We did not use this immunohistochemical marker. Positivity for ER, PR, CK7, and CDFP15, as well as negativity for CA19-9, CK20, and CDX2, suggest a breast origin of a gastric lesion,Citation3,Citation8,Citation9,Citation11,Citation19 particularly in a patient with a past history of breast cancer. Staining for p53 and Erb2/NEU are usually negative,Citation1 as in our case.
ILCs are characterized by rather discohesive cells individually dispersed or arranged in a single-cell-file pattern immersed in a fibrous stroma.Citation21 This lack of cohesion could be responsible for the development of distant metastases. Most ILCs are of histological grade I/II, express hormone receptors, and do not overexpress HER2 protein like our patient. The pleomorphic variant of ILCs is reported to have an aggressive clinical behavior.
Beta-catenin plays a central role in the ECD/catenin cell–cell adhesion complex, and it may be involved in cellular signaling pathways. Simultaneously, loss of E-cadherin and alpha-, beta-, and gamma-catenin are important steps in the formation of lobular carcinoma in situ, as a precursor of invasive lobular breast cancer. Inactivation of E-cadherin through CDH1 gene mutation, loss of heterozygosity of chromosomal region 16q22.1, and/or gene promoter methylation is an early event reported in more than 95% of ILCs.Citation22 Moreover, loss or downregulation of CDH1 is associated with tumor dedifferentiation and leads to different events: disruption of the tissue architecture, loss of adhesive properties and proliferation–suppressive function and gain in cellular motility increasing of invasive properties and dissemination of epithelial malignant cells. Of interest, ILC has been reported in families with hereditary diffuse gastric cancer that carry germline inactivation mutations of CDH1.Citation23
From an immunohistochemistry point of view, ILCs are not positive for CK20 and express CK7, CK18, thrombospondin (TSP), and integrin alpha-V.Citation31 ILC cells also express survivin, cathepsin B, TPI1, SPRY1, SCYA14, TFAP2B, osteopontin, HLA-G, and CHC1.
With regard to genomic considerations, there are some genes overexpressed in ILCs that code proteins involved in cell adhesion like VWF, ELN, DPT, and EMCN. There is upregulation of SFRP1, TGFBR2, and IGF1 genes. On the other hand, there are genes overexpressed in classic and pleomorphic ILCs implicated in actin cytoeskeleton remodeling/signaling and cell adhesion networks such as ANKDR28 and AFF1.Citation21
It is worth mentioning the new role of chemokines in mechanisms of metastasization. Chemokines are a superfamily of chemotactic cytokines present in organs that act as specific modulators in leukocyte migration to sites of inflammation, but are also involved in the initiation and promotion of carcinogenesis by providing growth and angiogenic factors. There are two families that represent the bulk of known chemokines: CC (the first two cysteines are adjacent to one another) and CXC (the first two cysteines are separated by one amino acid).Citation24
Chemokines interact with cell-surface receptors, present in tumor cells, which are members of a large superfamily of seven transmembrane G-protein-coupled cell surface receptors (GPCRs). Interaction between chemokines and their receptors induces migration of cells and mediates inflammatory and tumor cell migration. These chemokines stimulate certain cells to express their receptors by autocrine and paracrine mechanisms. More than 40 chemokines and 18 receptors are currently known.Citation24
Chemokine receptors CXCR4 and CXCR7 are highly expressed in breast cancer cells, and they are responsible for the chemotaxis to certain target organs such as lymph nodes. CXCL12 (also named SDF-1alpha) and CCL21 (or 6Ckine) bind CXCR4 and CXCR7.Citation25 The CXCL12/CXCR4 pathway is implicated in the mobilization, trafficking, and homing of cancer stem cells into metastatic sites.Citation26
CXCR4 is implicated in vascularization by initiating and sustaining tumor formation. Signaling through chemokine receptors mediates actin polymerization and pseudopodia formation, favoring invasiveness. CXCR4 also stimulates the production of matrix metalloproteases.Citation27
CXCL12 induces synthesis of metalloprotease MT1-MMP,Citation27 modulates integrin expression, and promotes tumor cell adhesion by attaching cells to extracellular matrix proteins of the basement membrane or to ligands on other cells.Citation28 CXCL12 can also regulate tumor cell apoptosis by activating NF-κB, which in turn inhibits tumor necrosis factor-α (TNF-α) production. Activation of NF-κB can sensitize cancer cells to CXCL12 stimulation through upregulation of CXCR4 expression. Methylation of CXCL12 promoter in the colonic epithelium favors metastases of tumors in the colon, but further studies are necessary to confirm this hypothesis. Breast tumor cells entering vascular or lymphatic circulation may migrate and adhere mainly to areas expressing CXCL12, therefore migrating to different organs.Citation24
Carcinoma cells recruit normal fibroblasts into tumor masses. Fibroblasts produce growth factors, extracellular matrix molecules, and metalloproteases and secrete soluble factors with proinflammatory and suppressant effects. Fibroblasts activate and turn into carcinoma-associated fibroblasts (CAFs). These cells contribute to tumorigenesis and metastasis and are recruited by transforming growth factor beta (TGF-β), platelet-derived growth factor (PDGF), fibroblast growth factor 2 (FGF2), and vascular endothelial growth factor (VEGF) released by tumor cells. In primary tumor and metastatic breast cancer, CAFs produce CXCL12 that promotes tumor growth and enhances angiogenesis through CXCR4 expressed on carcinoma cells and endothelial progenitor cells.Citation29 Cytokines and chemokines derived from CAFs induce immune suppression and facilitate invasion and transendothelial migration. Interestingly, both stroma and cancer cells can produce CXCL12.
Furthermore, hypoxia induces CXCR4 expression and CXCL12 production, sensitizes tumor cells to CXCL12 signals, and promotes tumor metastasis.Citation24,Citation28 CXCL12 attracts vascular endothelial cells and plasmacytoid dendritic cells into the tumor environment, and these cells induce neoangiogenesis through production of IL-8 and TNF-β. CXCL12 expression seems to be regulated by hypoxia and a hormone-triggered signal pathway. The CXCL12/CXCR4-mediated tumor cell proliferation is regulated by estrogen signaling. In our case, the tumor expressed both ER and PR, which makes this pathway feasible.
It is known that interaction between CCL21 and CCR7 plays a crucial role in lymphocytes homing to secondary lymphoid organs through lymphatic vessels. The mechanism by which CCL21 mediates migration of tumor cells to lymph nodes is similar to the mechanism of the lymphocytes’ homing effect. This mechanism may underlie gastric metastasis in H. pylori gastritis by acting as an organ with a great inflammatory property similar to lymph nodes in our patient.
Finally, there is a chemokine subgroup growth-related oncogene (GRO): GRO-2 (CXCL-2), GRO-3 (CXCL-3), and interleukin-8 (CXCL-8). These molecules are overexpressed in colonic adenomas and act as angiogenic factors, attract and activate neutrophils, and stimulate endothelial cell migration. GRO-2 (CXCL-2) and GRO-3 (CXCL-3) bind to the receptor CXCR2, present in neutrophils and endothelial cells, activating them through the NF-κβ pathway. GRO-2 is upregulated in ERα-positive breast cancer patients, and this fact correlates with shorter relapse-free survival.Citation30 Colonic metastasis in our patient was found on a polyp. It is possible that the polyp was an adenoma previously, and breast cancer cells may have homed over a previous adenoma by this chemokine pathway.
Moreover, there is an interesting role of inflammation. Our patient was diagnosed with gastritis related to H. pylori. We hypothesize a possible interaction between the chemokine receptors of circulating breast cancer stem cells and bacterial or inflammatory derived chemokines. It has been reported that there is a relationship between inflammation and tumorigenesis in prostate cancer, where inflammation can induce protumorigenic CXCL16 and CXCR6,Citation31 attracting tumor cells. So in our case, it can be hypothesized that chronic inflammation due to H. pylori infection may have attracted tumor cells to the stomach and colon because of the action of chemokines. Under favorable circumstances, due to the influence of the same or other cytokines and growing/angiogenic factors, these cells may grow and develop metastatic disease. The degree of cellular infiltration by H. pylori seems to be lower in the corpus than in the antrum.50 Our patient had gastric infiltration in the antrum, where H. pylori was also detected.
Infection with H. pylori causes chronic active gastritis, characterized by inflammatory cells infiltrating the gastric epithelial layer and the underlying lamina propria. H. pylori infection is associated with increased rates of expression of CXC chemokines (IL-8, GRO), CC chemokines, normal T-cells, and macrophage inflammatory protein 1-alpha (MIP-1α), as well as increased mucosal levels of IL-8. Some of these upregulations are directly controlled by NF-κB.Citation32 It is possible that all these cytokines induced by H. pylori may attract and activate tumor cells to home to gastric mucosae.
It is feasible that tobacco consumption may enhance inflammation in gastritis caused by H. pylori by inducing GRO and CXCL5 mRNA production. Our patient was a heavy smoker, so this may explain in part the localization of mucosal metastases. Tobacco seems to increase oxygen free radicals from the PMNs and decrease mucus content. Additionally, nicotine may play a role in the induction of CXC chemokine expression, but further studies must be undertaken to confirm this hypothesis.
In colon, metastases may be mediated by receptors of stem cell chemokines in colon bacterial walls. Colon bacteria may produce proteins that mediate inflammation and control metalloprotease production in colonic mucosa in a similar way to H. pylori. Molecules influencing polyp production such as GRO2 and GRO3 may contribute to attract tumor cells. It has been reported that human colon epithelial cells release galectin-3, which can activate NF-κβ in colonic lamina propria fibroblasts and induce the secretion of IL-8 from these cells.Citation33
Finally, proteinase-activated receptor (PAR) mediates the cellular actions of serine proteinases. PAR1 and PAR2 are widely distributed in the gastrointestinal tract, and their activation in gastrointestinal inflammation results in proinflammatory reactions mediated by IL-6, IL-8, and prostaglandins. PAR2 activation induces NF-κβ and AP-1-dependent IL-8 production in association with activation of p38 MAPK and ERK1/2.Citation34 This may be another mechanism favoring tumor growing in inflamed mucus.
Conclusion
Gastrointestinal metastases occur in some cases of lobular breast cancer, but dual infiltration of gastric and colonic regions has not been previously described as the first manifestation of spread. This surprising behavior may involve a mechanism in which chemokines and inflammatory phenomena might be implicated.
The expression of chemokine receptors could determine the type of tumor that metastasizes, whereas the site of dissemination is dictated by expression of the chemokine ligand. Expression of chemokines is passive, and metastatic behavior may be encoded in the primary cancer cell genetic profile.
Further studies are needed to identify the true role of chemokine receptors in metastatic phenomena.
Disclosure
The authors report no conflicts of interest in this work.
References
- SignorelliCPomponi-FormiconiDNelliFPolleraCFSingle colon metastasis from breast cancer: a clinical case reportTumori20059142442716459641
- KidneyCDCohenAJButlerJAbdominal metastases of infiltrating lobular breast carcinoma: CT and fluoroscopic imaging findingsAbdom Imaging1997221561599013524
- CiullaACastronovoGTomaselloGGastric metastases originating from occult breast lobular carcinoma: diagnostic and therapeutic problemsWorld J Surg Oncol200867818652707
- JainSFisherCSmithPMillisRRRubensRDPatterns of metastatic breast cancer in relation to histological typeEur J Cancer199329A215521578297656
- PectasidesDPsyrriAPliarchopoulouKGastric metastases originating from breast cancer: report of 8 cases and review of the literatureAnticancer Res2009294759476420032432
- MalhotraAGuturuPBasimMSRajuGSA rare case of breast cancer metastasis presenting as linitis plastica of the stomach and colon (with videos)Gastrointest Endosc20097055255319699983
- JonesGEStraussDCForshawMJDeereHMahedevaUMasonRCBreast cancer metastasis to the stomach may mimic primary gastric cancer: report of two cases and review of literatureWorld J Surg Oncol200757517620117
- BirlaRMahawarKKOrizuMSiddiquiMSBatraACaecal metastasis from breast cancer presenting as intestinal obstructionWorld J Surg Oncol200864718471290
- ScopaCDAletraCLifschitz-MercerBCzernobilskyBMetastases of breast carcinoma to the uterus. Report of two cases, one harboring a primary endometrioid carcinoma, with review of the literatureGynecol Oncol200596254354715661249
- FondrinierEGuérinOLorimierGA comparative study of metastatic patterns of ductal and lobular carcinoma of the breast from two matched series (376 patients)Bull Cancer19978411011107 French9587361
- TohfeMShamiPAftimosGSaadeMGastrointestinal metastases from breast cancer: a case reportSouth Med J20039662462512938796
- EoWKBreast cancer metastasis to the stomach resembling early gastric cancerCancer Res Treat20084020721019688132
- TaalBGPeterseHBootHClinical presentation, endoscopic features, and treatment of gastric metastases from breast carcinomaCancer2000892214222111147591
- UenoSNakakumaTAramakiNA case of bilateral breast cancer and metastatic gastric cancer with peritonitis carcinomatosa successfully treated with a combination therapy of S-1 and paclitaxelGan To Kagaku Ryoho2009361224712473 Japanese20037459
- TaalBGden Hartog JagerFCSteinmetzRPeterseHThe spectrum of gastrointestinal metastases of breast carcinoma: II. The colon and rectumGastrointest Endosc1992381361411568609
- WiltzOO’TooleKFenoglioCMBreast carcinoma metastatic to a solitary adenomatous polyp in the colonArch Pathol Lab Med19841083183206546672
- KatzASaadEDPorterPPusztaiLPrimary systemic chemotherapy of invasive lobular carcinoma of the breastLancet Oncol20078556217196511
- AlbaMAPiedrafitaEChivite de LeónAAllendeLSáinzSGastric metastasis of breast carcinomaRev Esp Enferm Dig199789647649 Spanish9471208
- López DeograciasMFlores JaimeLArias-CamisónIRectal metastasis from lobular breast carcinoma 15 years after primary diagnosisClin Transl Oncol20101215015320156785
- van VelthuysenMLTaalBGvan der HoevenJJPeterseJLExpression of oestrogen receptor and loss of E-cadherin are diagnostic for gastric metastasis of breast carcinomaHistopathology20054615315715693887
- WeigeltBGeyerFCNatrajanRThe molecular underpinning of lobular histological growth pattern: a genome-wide transcriptomic analysis of invasive lobular carcinomas and grade- and molecular subtype-matched invasive ductal carcinomas of no special typeJ Pathol2010220455719877120
- Cleton-JansenAME-cadherin and loss of heterozygosity at chromosome 16 in breast carcinogenesis: different genetic pathways in ductal and lobular breast cancer?Breast Cancer Res200245811879552
- CarneiroFOliveiraCSurianoGSerucaRMolecular pathology of familial gastric cancer, with an emphasis on hereditary diffuse gastric cancerJ Clin Pathol200861253017513507
- AndreFXiaWConfortiRCXCR4 expression in early breast cancer and risk of distant recurrenceOncologist2009141182118819939894
- ZabelBAWangYLewénSElucidation of CXCR7-mediated signaling events and inhibition of CXCR4-mediated tumor cell transendothelial migration by CXCR7 ligandsJ Immunol20091833204321119641136
- MüllerAHomeyBSotoHInvolvement of chemokine receptors in breast cancer metastasisNature2001410505611242036
- BartoloméRAFerreiroSMiquilena-ColinaMEThe chemokine receptor CXCR4 and the metalloproteinase MT1-MMP are mutually required during melanoma metastasis to lungsAm J Pathol200917460261219147814
- KryczekIWeiSKellerELiuRZouWStroma-derived factor (SDF-1/CXCL12) and human tumor pathogenesisAm J Physiol Cell Physiol2007292C987C99516943240
- OrimoAWeinbergRAStromal fibroblasts in cancer: a novel tumor-promoting cell typeCell Cycle200651597160116880743
- GuptaVYeoGKawakuboHMullerian-inhibiting substance induces Gro-beta expression in breast cancer cells through a nuclear factor-kappaB-dependent and Smad1-dependent mechanismCancer Res2007672747275617363596
- Darash-YahanaMGillespieJWHewittSMThe chemokine CXCL16 and its receptor, CXCR6, as markers and promoters of inflammation-associated cancersPLoS One200948e669519690611
- KidoMTanakaJAokiNHelicobacter pylori promotes the production of thymic stromal lymphopoietin by gastric epithelial cells and induces dendritic cell-mediated inflammatory Th2 responsesInfect Immun20107810811419841072
- LippertEFalkWBatailleFSoluble galectin-3 is a strong, colonic epithelial-cell-derived, lamina propria fibroblast-stimulating factorGut200756435116709662
- YoshidaNYoshikawaTBasic and translational research on proteinase-activated receptors: implication of proteinase/proteinase-activated receptor in gastrointestinal inflammationJ Pharmacol Sci200810841542119098387