Abstract
Breast cancer is the most common cancer diagnosed in women. Each year, thousands die either because of disease progression or failure of treatment. Breast cancer is classified into different subtypes based on the molecular expression of estrogen receptor (ER), progesterone receptor, and/or human epidermal growth factor receptor 2 (HER2). These receptors represent important therapeutic targets either through monoclonal antibodies or through small-molecule inhibitors directed toward them. However, up to 40% of patients develop either a primary or a secondary resistance to the current treatments. Therefore, there is an urgent need for investigating new targets in order to overcome the resistance and/or enhance the current therapies. Cell cycle is altered in many human cancers, especially in breast cancer. Cyclin-dependent kinases (CDKs), especially CDK4 and CDK6, play a pivotal role in cell cycle progression that makes them potential targets for new promising therapies. CDK inhibition has shown strong antitumor activities, ranging from cytostatic antiproliferative effects to synergistic effects in combination with other antitumor drugs. In order to overcome the drawbacks of the first-generation CDK inhibitors, recently, new CDK inhibitors have emerged that are more selective to CDK4 and CDK6 such as palbociclib, which is the most advanced CDK4/6 inhibitor in trials. In preclinical studies, palbociclib has shown a very promising antitumor activity, especially against ERα+ breast cancer subtype. Palbociclib has gained world attention, and US the Food and Drug Administration has accelerated its approval for first-line treatment in combination with letrozole for the first-line systematic treatment of postmenopausal women with ERα+/HER2− locally advanced or metastatic breast cancer. In this review, we discuss the potential role of CDK inhibition in breast cancer treatment, and focus on palbociclib progress from preclinical studies to clinical trials with mentioning the most recent ongoing as well as planned Phase II and Phase III trials of palbociclib in advanced breast cancer.
Introduction
A million and a half new cases of breast cancer are reported annually around the world.Citation1 In the US, breast cancer is the most common cancer diagnosed in women, with an estimated number of 231,840 new cases in 2015.Citation2 It accounts for 29% of the total cancers among women with 40,290 estimated deaths, ranking second in the cancer mortality list in women.Citation2 Breast cancer is a heterogeneous disease with different clinical and biological behaviors. Although it affects the same anatomical organ, it has different clinical manifestations, etiology, prognosis, clinical outcomes, and responds differently to treatment.Citation3 The therapeutic options vary from primary surgery to targeted therapy, hormonal therapy, and/or chemotherapy; however, more studies and investigations need to be done for better and more effective treatment of breast cancer.Citation4
Expression of hormonal receptors (HR), including estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), is used to classify breast cancers and aid in selecting appropriate treatment therapies.Citation5 Furthermore, breast cancers can be classified into luminal breast cancers expressing HR (such as ER and PR) and HER2 and basal-like cancers that lack expression of both HR and HER2.Citation6 Based on the previous classification, breast cancers can also be subdivided into five subtypes: Luminal A (HR+/HER2−), luminal B (HR+/HER2+), HER2-enriched (HR-/HER2+), basal-like (80%–90% are triple negative), and normal breast-like/unclassified breast cancers.Citation7,Citation8
ERα+ breast cancer accounts for ~70% of breast cancers, and most of ERα+ subtypes show a good response to hormonal therapy, including selective ER modulators and aromatase inhibitors.Citation9 Whereas the therapy of HER2+ cancers is based on targeting the overexpressed receptor with monoclonal antibodies.Citation5 On the other hand, triple-negative breast cancer (TNBC) (with negative expression of ER, PR, and HER2) is mostly treated with cytotoxic chemotherapeutic drugs.Citation10
Metastatic breast cancer
Advanced breast cancer, also known as stage IV breast cancer, is a type of breast cancer that is usually referred to the metastatic status of the tumor, which means that the tumor cells have spread from their local site to the surrounding and then to other distant sites. Breast cancer metastasis is the most common cause of cancer-related death owing to the incurable nature of these metastases.Citation11,Citation12 Metastatic disease develops in >20% of patients with early-stage breast cancer.Citation13 Approximately 75% of patients with metastatic breast cancers (MBCs) are HR+ with ERα+ and/or PR+.Citation14 In general, HR+ breast cancer subtypes usually develop late bone metastasis, whereas early visceral metastasis is usually due to TNBC.Citation15 Patients with MBC usually have median overall survival of 2–3 years from the time of their first diagnosis.Citation13 Generally, metastasis accounts for >90% of all cancer mortalities.Citation16
Tamoxifen, anastrazole, and letrozole are the first-line hormonal therapies approved for HR+ MBC due to their high efficacy and tolerability.Citation9,Citation17 Although hormonal therapy is effective in most patients with HR+ breast cancers, due to primary and secondary resistance, some patients will not respond to first-line hormonal treatment.Citation18 Therefore, a complete resistance to hormonal therapy is usually developed in ~40% of initial responder patients, and eventually they will depend totally on chemotherapy.Citation19,Citation20 Furthermore, up to 30% of patients with breast cancer will relapse with metastasis.Citation21
Hence, new targets have to be discovered, and new treatment therapies have to be studied in order to optimize the current therapies and improve patient outcomes. Studying the mechanisms of resistance to the current therapies has identified new possible targets to circumvent the resistance problem; such targets are cyclin-dependent kinase 4 and 6 (CDK4/6), phosphatidylinositol 3-kinase/mammalian target of rapamycin (PI3K/mTOR) pathway, and poly adenosine diphosphate ribose polymerase.Citation22
Alterations of PI3K/mTOR genes occur in >80% of patients with breast cancer (28%–47% of ER+ breast cancers), and this pathway regulates many cellular processes that promote cell proliferation and metastasis.Citation22 Cell proliferation by PI3K might be promoted through the activation of ER by phosphorylation.Citation23 Interestingly, PI3K/mTOR pathway was reported to be upregulated in HR+ tumors that were resistant to endocrine therapy.Citation22
Herein, we review the newly developed drug palbociclib and its impact on breast cancer, especially the metastatic disease.
CDK role in cell cycle
Cyclin D–CDKs–retinoblastoma (Rb) pathway drives cells through checkpoint in the cell cycle initiating the DNA replication and, hence, starting cell division.Citation24 DNA synthesis (S), mitotic (M), and gaps (G1 and G2) phases are the four phases of cell cycle. During G1 phase, cells reach a checkpoint when they decide whether to transit to (S) phase or quit the current cell cycle.Citation25 Alteration in cell cycle is one of the hallmarks of cancer.Citation26 The activation of CDKs by cyclin D proteins plays an important role in G1/S cell cycle transition by phosphorylation of Rb protein. Rb protein acts as a tumor suppressor controlling G1/S transition of cell cycle.Citation27 When Rb is phosphorylated, it loses its negative control on G1/S transition.Citation28 Hence, activating E2F family of transcription factors that allows DNA replication to start.Citation29 Cyclin D proteins provide a link between mitogenic signals and the machinery of cell cycle.Citation30 Interestingly, the enforced overexpression of cyclin D in cultured cells can shorten the G1 interval.Citation31 Therefore, increasing the replication capacity which might result in tumorigenesis.
The major role played by CDKs in cell cycle regulation was discovered for the first time in fission yeasts.Citation32 CDKs are serine/threonine-based kinases comprising numerous subtypes, divided into two main groups according to their functions. Mainly CDK2, CDK4, and CDK6 are involved in cell cycle G1/S transition, while CDKs 7–11 function as transcriptional regulators.Citation24,Citation33
CDK4 and CDK6 have ~71% sequence homology, and both of them interact with the D-type cyclins (D1, D2, and D3).Citation34 INK4 proteins, such as p16, are endogenous CDK4/6 inhibitors; they inhibit cyclin D–CDK4/6 function through binding to CDK4/6 and reduce the affinity to cyclin D proteins.Citation35
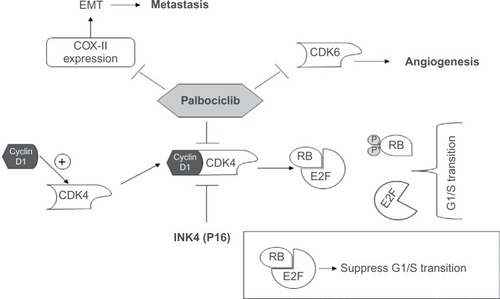
Recently, CDK6 was reported to promote the angiogenesis process. Moreover, CDK6 plays an important role in activating hemopoietic stem cells; thus, CDK6 inhibition may have an antiangiogenesis activity; however, myelosuppression is suspected to be a common side effect associated with selective CDK4/6 inhibitor therapy.Citation36,Citation37
Around 90% of human solid cancers were reported to have alterations in cyclin D–CDK–Rb pathway.Citation38 Alteration of such pathways can occur by different mechanisms, such as inactivation of p16 (happens in 50% of invasive breast cancers) and CDK4 mutations. Additionally, cyclin D1 was reported to be overexpressed in ~70% of breast cancer, and also overexpression of cyclin D1-encoding gene (CCND1) and CDK4 were characterized for breast cancer.Citation14,Citation39
Loss of Rb expression has been reported in ~20%–30% of breast cancers especially in TNBC. In addition, loss of Rb facilitates epithelial-to-mesenchymal transition process, thus, increasing the metastatic and invasive potential of breast cancer cells.Citation40,Citation41
CDK inhibitors
In the 1990s, first-generation CDK inhibitors were introduced in preclinical and clinical trials.Citation42 Flavopiridol (administrated as intravenous infusion), a nonselective first-generation CDK inhibitor, was the first to enter clinical trials, and it was found to have different strong antitumor effects such as enhancing cell cycle arrest, in addition to its antiangiogenic, proapoptotic, and synergetic potential with antitumor chemotherapeutic agents.Citation43,Citation44 However, due to their low therapeutic index, low sensitivity, and multitargets, first-generation CDK inhibitors have been discontinued.Citation45 Therefore, there is an urgent need for developing more selective CDK inhibitors. Palbociclib (PD0332991), abemaciclib (LY2835219), and ribociclib (LEE011) were then introduced as a new generation of CDK inhibitors with high selective inhibition to CDK4 and CDK6.Citation25
Palbociclib
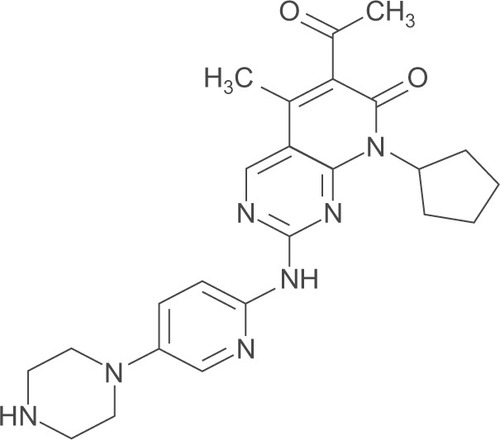
Palbociclib or PD0332991 was first introduced as a potent CDK4/6 inhibitor that has strong antitumor activity by Fry et al.Citation46 Palbociclib (brand name: Ibrance®) is an oral potent selective CDK4/6 inhibitor developed by Pfizer (NY, USA) ().Citation47 PD0332991 blocks ATP binding to the CDK4/6 enzymes with half-maximal inhibitory concentration (IC50) 0.011 µmol/L for CDK4/cyclin-D1, 0.009 µmol/L for CDK4/cyclin-D3, and 0.015 µmol/L for CDK6/cyclin-D2 complexes.Citation38
As we mentioned before, CDK4/6 inhibition can result in many pharmacological activities based on the different roles of these enzymes (). It was expected that palbociclib would show strong antiproliferative and antiangiogenic activities. Currently, palbociclib is the most advanced selective CDK4/6 inhibitor in clinical development.Citation25
Figure 2 Palbociclib mechanism of action.
Abbreviations: CDK, cyclin-dependent kinase; COX-II, cyclooxygenase-II; EMT, epithelial-to-mesenchymal transition; RB, retinoblastoma protein.
Other than breast cancer, preclinical studies of palbociclib on different types of cancers showed a promising antiproliferative activity. Palbociclib successfully inhibited tumor proliferation in different cancer cell lines and xenograft mouse models including neuroblastoma,Citation48 glioblastoma,Citation49 colorectal carcinoma,Citation50 advanced Rb+ bladder cancerCitation51 gastric cancer,Citation52 and sarcomas.Citation53
Preclinical trials
Among 47 human cell lines of different subtypes of breast cancer, ERα+ subtypes were found to be most sensitive to palbociclib growth inhibition.Citation54 ERα+ subtypes’ sensitivity to CDK4/6 inhibitors may be due to the hyperactivation of cyclin D1 and CDK4/6 that was reported in those subtypes.Citation20,Citation21 While in Rb-deficient MDA-MB-468 (ERα−) human breast cancer cell lines, palbociclib showed no antiproliferative effect.Citation46 Loss of Rb expression was reported to render the cancer cells resistant to CDK4/6 inhibition therapy.Citation49,Citation55 p16 overexpression in Rb-deficient breast cancer cells might account for the resistance to palbociclib, as CDK4/6 enzymes were already inhibited by the overexpressed p16.Citation56 Interestingly, cyclin D1 overexpression and Rb phosphorylation in ERα+ cancers were reported to contribute to the resistance to the hormonal therapy.Citation42 Additionally, cyclin D1 is one of the ER transcriptional targets, thus, rationalizing the use of CDK4/6 inhibitors in ERα+ breast cancer.Citation57
Besides antiproliferative activity, in MDA-MB-231 (ERα−) and T47D (ERα+) breast cancer cells, palbociclib has shown strong antimetastatic activity in a dose-dependent manner through reducing cyclooxygenase-II expression.Citation58 Cyclooxygenase-II has been reported to be associated with the activation of epithelial-to-mesenchymal transition process, which helps the epithelial cells to lose their epithelial characteristics and gain mesenchymal characteristics, therefore, increasing their invasive and metastatic potentials.Citation59,Citation60 Moreover, CDK4/6 inhibition by palbociclib induced senescence in melanoma cell lines by promoting Forkhead Box M1 degradation.Citation61
Furthermore, in two in vivo animal studies, palbociclib was found to be a substrate to efflux transporters P-glycated protein and breast cancer resistance protein. These transporters limit palbociclib delivery to the brain.Citation62,Citation63 When adding elacridal (dual P-glycated protein and breast cancer resistance protein inhibitor) to palbociclib, the brain levels of palbociclib were increased significantly, therefore enhancing its efficacy in treating brain metastasis.Citation62 Breast cancer is highly metastatic to the brain, making it the second most common cause of brain metastasis; therefore, there is always a crucial need to enhance the crossing of antitumor drugs to the blood–brain barrier.Citation62 On the other hand, abemaciclib (LY2835219) reached brain at low doses, suggesting that it may offer a better activity in treating breast cancer brain metastasis.Citation64
Clinical trials of hormonal therapy combination with CDK4/6 inhibitors were triggered when hormonal therapy resistance was observed to be linked with genes that are regulated through cyclin D–CDK–Rb pathway.Citation44 Moreover, apoptosis was observed to be increased, when cells were therapeutically arrested and then treated with hormonal therapy.Citation41 Therefore, controlling cell cycle by controlling cyclin D–CDK–Rb pathway using CDK4/6 inhibitors may have a synergistic activity when combined with hormonal therapies, as well as, reduce the resistance acquired to that class of treatment..
Clinical trials in breast cancer
Phase I trials
Schwartz et alCitation65 conducted the first Phase I study of palbociclib in humans to determine maximum tolerated dose (MTD) and dose-limiting toxicities (DLTs) of the 2/1 schedule of palbociclib treatment (2 weeks once daily and 1 week off treatment). Patients with non-Hodgkin’s lymphoma and/or Rb+ advanced solid tumor were eligible for this study. Six patients, out of the 33 enrolled patients, experienced DLT. Myelosuppression was the common adverse effect including neutropenia (grade 3) that occurred in 24% of patients, whereas nonhematological toxicity was of mild-to-moderate intensity including fatigue, diarrhea, and constipation. Moreover, the MTD was found to be 200 mg once daily.Citation65
Another Phase I study by Flaherty et alCitation66 identified the DLT and MTD for the 3/1 schedule of palbociclib treatment (3 weeks once daily and 1 week off treatment). The enrolled 41 patients had Rb+ advanced solid tumors (five patients had breast cancer). The MTD was found to be 125 mg once daily. Only five patients had DLTs; neutropenia (grade 3, 12%) was the most common, while nonhematological adverse events were fatigue, diarrhea, and nausea. These results were consistent with the results of the Phase I study of Schwartz et alCitation65, and both of the studies showed that palbociclib was well tolerated and neutropenia was the only significant DLT.Citation66 Besides the MTD and DLTs, Schwartz et alCitation65 and Flaherty et alCitation66 determined the pharmacokinetic parameters of palbociclib for the 2/1 schedule and 3/1 schedule ().
Table 1 Palbociclib pharmacokinetic parameters for 2/1 schedule and 3/1 schedule
Phase II trials
DeMichele et alCitation67 studied palbociclib in a Phase II trial as a single agent in advanced breast cancer. Eligible patients were confirmed to have Rb-positive MBC, and of the 37 enrolled patients, 33 patients were HR+ (7% ERα+, 4% PR+, and 22% ERα+/PR+).Citation67 Clinical benefit ratio was 21% for patients with HR+ and 29% for patients with HR+/HER2− who were exposed to least two prior lines of hormonal therapy. Progression-free survival (PFS) was significantly longer for patients with HR+ rather than HR− (P=0.03). On the other hand, patients exposed to chemotherapy with fewer than two prior therapies had greater clinical benefit ratio than those exposed to heavy treatment.Citation67 Most adverse events were mostly cytopenias due to myelosuppression. Neutropenia (grade 3/4) was the most frequently occurring cytopenia with 51% of patients, and it was the reason for 46% of all dose modifications. Although neutropenia was common, neutropenic fever/infection was rare, suggesting that bone marrow progenitors were only suppressed during treatment but still functioning in infections.Citation67 According to the presented results, palbociclib could be effective as a single agent, but more trials have to be conducted with larger sample size to confirm the applicability and efficacy of palbociclib as a single agent in MBC. Similarly, the other selective CDK4/6 inhibitors, abemaciclib and ribociclib, as well, showed a significant clinical activity in ER+ breast cancer in early clinical studies.Citation68
Finn et alCitation1,Citation69 conducted the PALOMA-1/TRIO-18 trial; PALOMA-1 was a Phase I/II study. In Phase I, nine postmenopausal women with ERα+/HER2− breast cancer were enrolled to assess the safety, tolerability, and drug–drug interactions of combining palbociclib with letrozole. The combination was well tolerated and encouraged the Phase II of the study. Phase II was a randomized, open-label trial conducted on 165 eligible patients who were postmenopausal with advanced ERα+/HER2− breast cancer and never had any systematic treatment. This Phase II study assessed the efficacy of combination of palbociclib with letrozole versus letrozole alone.
The combination therapy showed a significant improvement in the PFS (primary end point) of 10 months longer in combination group (20.2 months) than letrozole alone group (10.2 months) (hazard ratio 0.488, 95% confidence interval [CI] 0.319–0.748; one-sided P=0.0004).Citation1 Neutropenia (54% for grade 3/4), leukopenia (43% all grades), and fatigue (40% all grades) were the most common adverse events reported for the combination group (n=83). Febrile neutropenia had not occurred in any of the patients in the combination group.Citation1 In the same study, amplification of cyclin D1 and loss of p16 did not show any benefit as biomarkers for CDK4/6 inhibition treatment patients’ selection.Citation1
PALOMA-1 showed a significant improvement in the PFS when palbociclib was combined with letrozole. Thus, after the results were published, palbociclib received breakthrough therapy designation from US Food and Drug Administration (FDA) in April 2013.Citation70 In February 2015, palbociclib received FDA accelerated approval for use in combination with letrozole for the first-line treatment of postmenopausal women with ERα+/HER2− locally advanced breast cancer or MBC.Citation17,Citation70 Apart from breast cancer, a Phase II trial conducted on liposarcoma showed an improved PFS when patients were treated with palbociclib.Citation71
Phase III trials
PALOMA-3, a randomized, double-blinded, Phase III study, involving 521 patients with advanced breast cancer, was conducted by Turner et al.Citation72 The efficacy of palbociclib in combination with fulvestrant versus fulvestrant alone was assessed, and PFS was the primary end point. Eligible patients had advanced HR+/HER2− breast cancer and had relapsed or progressed disease during prior hormonal therapy. The PFS was improved in palbociclib/fulvestrant group (9.2 months) more than the median PFS of fulvestrant alone group (3.8 months) (hazard ratio for disease progression or death, 0.42; 95% CI, 0.32–0.56; P<0.001).Citation72
The adverse events were consistent with the previous studies with neutropenia (occurred in 62% of patients) as the major adverse event, and only 0.6% of patients experienced febrile neutropenia. Finally, the ongoing and planned Phase II and Phase III clinical trials of palbociclib in MBC are presented in .
Table 2 Ongoing and future palbociclib trials
Palbociclib in combination with chemotherapy
Since TNBC has no target and its treatment depends on chemotherapy, the combination of palbociclib to cytotoxic chemotherapy has to be assessed as to whether it will have additive or antagonistic effects to the cancer chemotherapy.
In a preclinical study using Rb-proficient TNBC (MDA-MB-231 and Hs578T) cells, palbociclib when combined with anthracycline “doxorubicin” was found to antagonize doxorubcin cytotoxic effects. Additionally, in mice with Rb-proficient MDA-MB-231 xenografts, the cytotoxic effects of doxorubicin were inhibited upon co-administration of palbociclib. Moreover, the authors assessed the dependence of palbociclib on Rb expression status; they repeated the same in vivo experiment but used Rb-deficient MDA-MB-231 xenografts and found that palbociclib had no effect on cytotoxic effects of doxorubicin efficacy, suggesting that Rb expression is essential for palbociclib cytostatic activity.Citation10
In another study, the impact of combining palbociclib with taxane and anthracycline chemotherapies was assessed using TNBC Rb-proficient MDA-MB-231 and Hs578T breast cancer cell models. The results of the combination were consistent with the previous study; palbociclib was found to antagonize their cytotoxic effects, suggesting that palbociclib altered DNA repair, blocked the chemotherapy-induced cell damage, and prevented cell death.Citation73 In another in vivo study on genetically engineered murine models of Rb-proficient (MMTV-c-neu) and Rb-deficient (C3-TAg) mice, it was found that combining palbociclib with carboplatin resulted in decreased cytotoxic activity versus carboplatin alone in the Rb-proficient MMTV-c-neu mice. On the other hand, Rb-deficient (C3-TAg) mice were resistant to palbociclib and the combination therapy showed no antagonistic effect to carboplatin cytotoxicity.Citation74
Interestingly and in contrast, paclitaxel cytotoxicity was found to be increased when cells were synchronized after short exposure to palbociclib (24 hours) prior to paclitaxel treatment. These observations suggest that the duration and the time of administration of palbociclib are important and should be considered carefully when we combine palbociclib with chemotherapies.Citation73 Based on the last observation, a Phase I trial of palbociclib and paxlitaxel combination was conducted, and the combination was found to be safe and well tolerated.Citation75 Overall, we should wait for the results of the upcoming trials to determine the efficacy of these combinations in clinical practice.
Conclusion and future directions
Breast cancer is not a single disease; it is a set of different heterogeneous diseases that affect one anatomical organ. Major progress and discoveries have been made in breast cancer field; but unfortunately, breast cancer still has high incidence and limited therapeutic options. Current therapies are well tolerated and of high efficacy, but unfortunately, some patients develop resistance and relapse to more advanced and metastatic disease. Thus, the urge for developing new treatments is crucial. The extensive analysis of the resistance and its mechanisms are needed to discover new promising targets. CDKs are one of the most promising new therapeutic targets. First-generation CDK inhibitors showed good antitumor activities in preclinical studies; however, they show a serious high toxicity due to their lack of specific targets. Therefore, new selective CDK4/6 inhibitors have emerged to overcome this drawback. Palbociclib is the most advanced member in this new generation of CDK4/6 inhibitors.
Preclinical data confirmed palbociclib antitumor activity in multiple tumors. In addition, Phase I studies showed its efficacy tolerability and safety, which encouraged going to the next step in clinical trials. Reversible neutropenia is the only significant adverse event for palbociclib, and fortunately, it can be managed easily by dose adjusting without affecting the efficacy. Palbociclib in combination with hormonal therapy (letrozole) gained FDA accelerated approval after PALOMA-1 results were published. This combination therapy showed a significant and remarkable improvement in PFS in postmenopausal women with ERα+/HER2− locally advanced breast cancer or MBC.
Until now, Rb protein is the only reliable indicator for palbociclib activity; in Rb-proficient cells, palbociclib showed antitumor activities, while in Rb-deficient/mutant cells, palbociclib lost its activity. We propose that Rb as an indicator is sufficient for now, in this early stage, for palbociclib applications, but in the future, we may have to find more indicators and biomarkers to assess palbociclib efficacy and monitor its activities. Additionally, more preclinical studies have to be conducted on combining palbociclib with cytotoxic chemotherapeutic agents and cautiously assess the safety and efficacy of these combinations. Overall, palbociclib seems to be a very promising drug and carries a hope for millions of patients with breast cancer, especially those who suffer from late-stage metastasis.
Author contributions
Conception and design, data acquisition and analysis, and writing the manuscript: Moataz Ehab and Mohamad Elbaz. Study supervision: Mohamad Elbaz.
Disclosure
The authors report no conflicts of interest in this work.
References
- FinnRSCrownJPLangIThe cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 studyLancet Oncol2014161253525524798
- SiegelRLMillerKDJemalAAmerican Cancer SocietyCancer Facts & Figures 2015AtlantaAmerican Cancer Society2015651529
- WeigeltBGeyerFCReis-FilhoJSHistological types of breast cancer: how special are they?Mol Oncol20104319220820452298
- de RuijterTCVeeckJde HoonJPJvan EngelandMTjan-HeijnenVCCharacteristics of triple-negative breast cancerJ Cancer Res Clin Oncol2011137218319221069385
- MatsumotoAJinnoHAndoTBiological markers of invasive breast cancerJpn J Clin Oncol20154hyv153
- Darb-EsfahaniSLoiblSMüllerBMIdentification of biology-based breast cancer types with distinct predictive and prognostic features: role of steroid hormone and HER2 receptor expression in patients treated with neoadjuvant anthracycline/taxane-based chemotherapyBreast Cancer Res2009115R6919758440
- MasoodSBreast cancer subtypes: morphologic and biologic characterizationWomens Health (Lond Engl)201612110311926756229
- American Cancer SocietyBreast Cancer Facts & Figures 2015–2016AtlantaAmerican Cancer Society Inc2015
- AlmstedtKSchmidtMTargeted therapies overcoming endocrine resistance in hormone receptor-positive breast cancerBreast Care201510316817226557821
- McClendonAKDeanJLRivadeneiraDBCDK4/6 inhibition antagonizes the cytotoxic response to anthracycline therapyCell Cycle201211142747275522751436
- LuJSteegPSPriceJEBreast cancer metastasis: challenges and opportunitiesCancer Res200969124951495319470768
- ElbazMNasserMWRaviJModulation of the tumor microenvironment and inhibition of EGF/EGFR pathway: novel anti-tumor mechanisms of cannabidiol in breast cancerMol Oncol20159490691925660577
- ManginiNSWesolowskiRRamaswamyBLustbergMBBergerMJPalbociclib: a novel cyclin-dependent kinase inhibitor for hormone receptor-positive advanced breast cancerAnn Pharmacother201549111252126026324355
- O’SullivanCCOvercoming Endocrine Resistance in Hormone-Receptor Positive Advanced Breast Cancer-The Emerging Role of CDK4/6 InhibitorsInt J cancer Clin Res201524
- HudisCAGianniLTriple-negative breast cancer: an unmet medical needOncologist201116suppl 111121278435
- LawsonDABhaktaNRKessenbrockKSingle-cell analysis reveals a stem-cell program in human metastatic breast cancer cellsNature2015526757113113526416748
- BeaverJAAmiri-KordestaniLCharlabRFDA approval: palbociclib for the treatment of postmenopausal patients with estrogen receptor-positive, HER2-negative metastatic breast cancerClin Cancer Res201521214760476626324739
- RingADowsettMMechanisms of tamoxifen resistanceEndocr Relat Cancer200411464365815613444
- Yamamoto-ibusukiMArnedosMAndréFTargeted therapies for ER +/HER2- metastatic breast cancerBMC Med20151313726059247
- MayerELTargeting breast cancer with CDK inhibitorsCurr Oncol Rep201517544325716100
- NagarajGMaCRevisiting the estrogen receptor pathway and its role in endocrine therapy for postmenopausal women with estrogen receptor-positive metastatic breast cancerBreast Cancer Res Treat2015150223124225762475
- HosfordSRMillerTWClinical potential of novel therapeutic targets in breast cancer: CDK4/6, Src, JAK/STAT, PARP, HDAC, and PI3K/AKT/mTOR pathwaysPharmgenomics Pers Med2014720321525206307
- HayashiSKimuraMMechanisms of hormonal therapy resistance in breast cancerInt J Clin Oncol201520226226725652907
- MalumbresMBarbacidMTo cycle or not to cycle: a critical decision in cancerNat Rev Cancer20011322223111902577
- MurphyCGDicklerMNThe role of CDK4/6 inhibition in breast cancerOncologist201520548349025876993
- HanahanDWeinbergRAThe hallmarks of cancerCell20001001577010647931
- RoesleySNASuryadinataRMorrishECyclin-dependent kinase-mediated phosphorylation of breast cancer metastasis suppressor 1 (BRMS1) affects cell migrationCell Cycle201615113715126771717
- LundbergASWeinbergRAFunctional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexesMol Cell Biol19981827537619447971
- HarbourJWDeanDCThe Rb/E2F pathway: expanding roles and emerging paradigmsGenes Dev200014192393240911018009
- EkholmSVReedSIRegulation of G1 cyclin-dependent kinases in the mammalian cell cycleCurr Opin Cell Biol200012667668411063931
- SherrCJRobertsJMLiving with or without cyclins and cyclin-dependent kinasesGenes Dev200418222699271115545627
- NursePMasuiYHartwellLUnderstanding the cell cycleNat Med1998410110311069771732
- CasimiroMCVelasco-VelázquezMAguirre-AlvaradoCPestellRGOverview of cyclins D1 function in cancer and the CDK inhibitor landscape: past and presentExpert Opin Investig Drugs2014233295304
- LiaoYFengYShenJHornicekFJDuanZThe roles and therapeutic potential of cyclin-dependent kinases (CDKs) in sarcomaCancer Metastasis Rev2015 Epub 2015 Dec 15
- DukelowTKishanDKhasrawMMurphyCGCDK4/6 inhibitors in breast cancerAnticancer Drugs201526879780626053278
- KollmannKHellerGSchneckenleithnerCA kinase-independent function of CDK6 links the cell cycle to tumor angiogenesisCancer Cell201324216718123948297
- ScheicherRHoelbl-KovacicABelluttiFCDK6 as a key regulator of hematopoietic and leukemic stem cell activationBlood201512519010125342715
- AltenburgJDFaragSSThe potential role of PD0332991 (palbociclib) in the treatment of multiple myelomaExpert Opin Investig Drugs2015242261271
- PeuralaEKoivunenPHaapasaariK-MBloiguRJukkola-VuorinenAThe prognostic significance and value of cyclin D1, CDK4 and p16 in human breast cancerBreast Cancer Res2013151R523336272
- KonecnyGECyclin-dependent kinase pathways as targets for women’s cancer treatmentCurr Opin Obstet Gynecol2016281424826642065
- CadooKAGucalpATrainaTAPalbociclib : an evidence-based review of its potential in the treatment of breast cancerBreast Cancer (Dove Med Press)20144612313325177151
- TamuraKDevelopment of cell-cycle checkpoint therapy for solid tumorsJpn J Clin Oncol201545121097110226486823
- TanARYangXBermanAPhase I trial of the cyclin-dependent kinase inhibitor flavopiridol in combination with docetaxel in patients with metastatic breast cancerClin Cancer Res200410155038504715297405
- AsgharUWitkiewiczAKTurnerNCKnudsenESThe history and future of targeting cyclin-dependent kinases in cancer therapyNat Rev Drug Discov201514213014625633797
- CriscitielloCVialeGEspositoACuriglianoGDinaciclib for the treatment of breast cancerExpert Opin Investig Drugs201423913051312
- FryDWHarveyPJKellerPRSpecific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenograftsMol Cancer Ther20043111427143815542782
- PfizerIBRANCE, FDA drug sheet Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207103s000lbl.pdfAccessed April 23, 2016
- RihaniAVandesompeleJSpelemanFVan MaerkenTInhibition of CDK4/6 as a novel therapeutic option for neuroblastomaCancer Cell Int20151517626225123
- SchröderLBWMcDonaldKLCDK4/6 inhibitor PD0332991 in glioblastoma treatment: does it have a future?Front Oncol2015525926649278
- LiCQiLBellailACHaoCLiuTPD-0332991 induces G1 arrest of colorectal carcinoma cells through inhibition of the cyclin-dependent kinase-6 and retinoblastoma protein axisOncol Lett2014751673167824765199
- SatheAKoshyNSchmidSCCDK4/6 inhibition controls proliferation of bladder cancer and transcription of RB1J Urol2016195377177926318986
- HuangSYeHGuoWCDK4/6 inhibitor suppresses gastric cancer with CDKN2A mutationInt J Clin Exp Med201587116921170026380006
- PerezMMuñoz-GalvánSJiménez-GarcíaMPMarínJJCarneroAEfficacy of CDK4 inhibition against sarcomas depends on their levels of CDK4 and p16ink4 mRNAOncotarget2015638405574057426528855
- FinnRSDeringJConklinDPD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitroBreast Cancer Res2009115R7719874578
- BoscoEEKnudsenESRB in breast cancer: at the crossroads of tumorigenesis and treatmentCell Cycle20076666767117361100
- DeanJLThangavelCMcClendonAKReedCAKnudsenESTherapeutic CDK4/6 inhibition in breast cancer: key mechanisms of response and failureOncogene201029284018403220473330
- MorikawaAHenryNLPalbociclib for the treatment of estrogen receptor-positive, HER2-negative metastatic breast cancerClin Cancer Res201521163591359626100274
- QinGXuFQinTPalbociclib inhibits epithelial-mesenchymal transition and metastasis in breast cancer via c-Jun/COX-2 signaling pathwayOncotarget2015639417944180826540629
- BoccaCIevolellaMAutelliRExpression of Cox-2 in human breast cancer cells as a critical determinant of epithelial-to-mesenchymal transition and invasivenessExpert Opin Ther Targets201418212113524325753
- TaubeJHHerschkowitzJIKomurovKCore epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypesProc Natl Acad Sci U S A201010735154491545420713713
- AndersLKeNHydbringPA systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cellsCancer Cell201120562063422094256
- ParrishKEPokornyJMittapalliRKBakkenKSarkariaJNElmquistWFEfflux transporters at the blood-brain barrier limit delivery and efficacy of cyclin-dependent kinase 4/6 inhibitor palbociclib (PD-0332991) in an orthotopic brain tumor modelJ Pharmacol Exp Ther2015355226427126354993
- de GooijerMCZhangPThotaNP-glycoprotein and breast cancer resistance protein restrict the brain penetration of the CDK4/6 inhibitor palbociclibInvest New Drugs20153351012101926123925
- StegerGGGnantMBartschRPalbociclib for the treatment of postmenopausal breast cancer – an updateExpert Opin Pharmacother201617225526326679057
- SchwartzGKLoRussoPMDicksonMAPhase I study of PD 0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (schedule 2/1)Br J Cancer2011104121862186821610706
- FlahertyKTLoRussoPMDeMicheleAPhase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancerClin Cancer Res201218256857622090362
- DeMicheleAClarkASTanKSCDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessmentClin Cancer Res2015215995100125501126
- VanArsdaleTBoshoffCArndtKTAbrahamRTMolecular pathways: targeting the cyclin D-CDK4/6 axis for cancer treatmentClin Cancer Res201521132905291025941111
- FinnRHurvitzSAllisonMPhase I Study of PD 0332991, a Novel, Oral, Cyclin-D Kinase (CDK) 4/6 Inhibitor in Combination with Letrozole, for First-Line Treatment of Metastatic Post-Menopausal, Estrogen Receptor-Positive (ER+), Human Epidermal Growth Factor Receptor 2 (HER2)-NegatiCancer Res20096924 Supplement50695069
- DhillonSPalbociclib: first global approvalDrugs201575554355125792301
- DicksonMATapWDKeohanMLPhase II trial of the CDK4 inhibitor PD0332991 in patients with advanced CDK4-amplified well-differentiated or dedifferentiated liposarcomaJ Clin Oncol201331162024202823569312
- TurnerNCRoJAndréFPalbociclib in hormone-receptor-positive advanced breast cancerN Engl J Med2015373320921926030518
- DeanJLMcClendonAKKnudsenESModification of the DNA damage response by therapeutic CDK4/6 inhibitionJ Biol Chem201228734290752908722733811
- RobertsPJBisiJEStrumJCMultiple roles of cyclin-dependent kinase 4/6 inhibitors in cancer therapyJ Natl Cancer Inst2012104647648722302033
- ClarkASO’DwyerPJHeitjanDA phase I trial of palbociclib and paclitaxel in metastatic breast cancerJ Clin Oncol201432suppl abstr 5275s