Abstract
Since its discovery in 1958, Burkitt lymphoma (BL) has been extensively studied and has become a model for tumorigenesis, but its pathogenesis has not been completely explained and understood yet. The aim of this review was to summarize the current knowledge about BL and, in particular, to discuss the role of miRNAs in its pathogenesis and their possible use as diagnostic and prognostic indicators. The impact of viral-encoded miRNAs is also discussed, with the Epstein–Barr infection being almost invariably detected in the endemic variant of this tumor.
Keywords:
Introduction
Discovery of Burkitt lymphoma (BL)
BL was first described by Denis Burkitt in 1958Citation1 during a field trip to sub-Saharan Africa and was subsequently named after him. The British surgeon observed that this particular tumor had a very high incidence in this geographic region, referred to as the “lymphoma belt” of Africa, in which other infectious diseases are also very common, such as malaria and arboviral infections.Citation2,Citation3 Nevertheless, even though ~60 years have passed since the first description, it is not clear yet what impact, if any, these infectious diseases may have in driving BL in endemic areas.Citation4
BL has been referred to as the “Rosetta stone” of cancer because it is the first tumor for which a viral association has been described (with the Epstein–Barr virus [EBV]),Citation5 the first tumor in which a specific chromosomal translocation has been identified (involving the MYC proto-oncogene)Citation6,Citation7 and the first tumor successfully treated by chemotherapy.Citation8 For this reason, BL has a very important “historic” role, as it has improved our understanding of the molecular mechanisms happening in cancer, and it is still considered a model for tumorigenesis.
The EBV was isolated by Sir Anthony Epstein in 1964 from a BL-derived cell line,Citation5 and now clear evidence highlight that this virus is not simply a bystander, but it actively promotes transformation through its encoded genome products.Citation9
Classification of BL
According to the World Health Organization classification, BL can be defined as a “single morphological and clinical entity, with variations in clinical presentation”.Citation10 Three subtypes of this tumor have been described, namely, the endemic (eBL), the sporadic (sBL) and the immunodeficiency-associated form (ID-BL or human immunodeficiency virus [HIV]-BL) that differ in geographic distribution and the extent of association with viruses, with EBV being the most relevant.Citation10 Despite this distinction, these variants can still be considered the same entity as they all share the same clinical presentation and the same molecular profile,Citation11,Citation12 with some differences in their miRNA profile.Citation13 The hallmark of BL is the constitutive activation of the MYC proto-oncogene, which leads to deregulated and increased expression of the Myc protein. In the vast majority of cases, this imbalance is achieved through a chromosomal translocation, which puts the MYC gene, mapping on chromosome 8, under the transcriptional control of immunoglobulin (Ig) gene promoters. When these promoters are very active, following a translocation, there is a strong and sustained expression of the MYC gene, with a resulting upregulation of its protein product.Citation6,Citation7 Three different translocations have been described involving different Ig loci (t[8;14], t[8;2] and t[8;22]), of which the t(8;14) is the most frequently observed in BL (~80% of BL cases).Citation6,Citation7 However, in the past 2 decades, it has been observed that BL cases overexpressing Myc, but lacking an identifiable translocation do exist.Citation14 These cases are indistinguishable from translocated BLs with respect to clinical presentation and share the same gene expression profile,Citation11 though they present with differences in their miRNA signature, as explained later in this review.Citation15 This suggests that alternative pathogenetic mechanisms responsible for increased levels of the Myc protein exist, besides the chromosomal translocation. Interestingly, no differences regarding the presence of MYC translocation are observed among the different clinical variants of BL. BL immunophenotype shows the expression of B-cell-associated antigens (eg, CD19, CD20 and CD22) and additional proteins such as CD10, BCL6, CD38, CD77 and CD43, which suggests its derivation from late germinal center (GC) cells.Citation10 However, it has been postulated that sBL and eBL derive from GC cells at different stages of differentiation, as they show a different pattern of Ig hypermutation and signs of antigen selection.Citation16 Based on this observation, it is possible that sBL derives from early GC cells (centroblasts), whereas eBL derives from late GC cells (centrocytes), as the latter show a higher number of somatic hypermutations in the Ig genes.Citation16
Clinical variants of BL: endemic, sporadic and immunodeficiency associated
Despite BL showing a very homogeneous molecular profile,Citation11,Citation12 differences can be pinpointed regarding the geographic distribution of this tumor and association with EBV. The endemic form has a very high incidence in Equatorial Africa, where other climatic conditions and infectious agents may possibly act as cofactors, though it is still debated at which extent and through which mechanisms.Citation4 The endemic form of BL is preferentially observed in young children (especially males), with a peak incidence of 4–7 years. It most commonly presents in extranodal sites, with the jaw and other facial bones being very frequently affected. Most importantly, the degree of association of this clinical variant with EBV is extremely high, the virus being detected in ~100% of eBL cases.Citation10 Such a strong association suggests an active involvement of EBV in Burkitt pathogenesis, and recent literature proves an important role for its encoded products,Citation9 although there is still a lot to uncover. Based on the simple association with EBV, 90–95% of the world population test positive for EBV, but BL incidence is much lower. This suggests that other factors may be required for BL pathogenesis.
The sporadic form occurs anywhere in the world and is histologically identical to eBL. It is still a pediatric disease, accounting for ~30% of childhood lymphomas (and 1%–2% of all lymphomas), and yet has a higher incidence in males, though with a higher median age of incidence (12 years). Another difference is that, despite sBL also showing an extranodal presentation, jaw tumors are less frequent, with the gastrointestinal tract being the most common site of involvement. Very interestingly, EBV is less commonly associated with sBL, being detected only in ~20%–30% of cases.Citation10
As far as the immunodeficiency-related form is concerned, this clinical variant is particularly frequent in HIV-positive individuals, accounting for one-third of HIV-associated lymphomas, and it is therefore also referred to as HIV-related BL. This tumor is mostly observed in adults; it mainly shows a nodal presentation, with a generalized lymph node involvement and the bone marrow and central nervous system involvement also being common. Extranodal disease is possible, but it is much less frequent than in endemic and sporadic forms. The immunodeficiency-related variant shows a variable extent of association with EBV, ranging from 30% to 90% of all cases.Citation10 Noteworthy, this form is not only observed in HIV-infected patients but also in other types of immunodeficiency (ie, posttransplant), although BL may be the first disease to manifest in HIV-infected individuals, as it occurs in patients with a still high CD4+ count and not having overt acquired immunodeficiency syndrome yet. This evidence suggests that, despite its role as an oncogenic virus still being debated, HIV may actively contribute to BL pathogenesis either indirectly, through a continuous antigenic stimulation, or directly, through its encoded genome products.Citation17
MYC deregulation in BL
The MYC proto-oncogene is a powerful transcription factor and plays very important physiological functions, being involved in the control of proliferation, cell growth, metabolism, apoptosis and differentiation.Citation18 It belongs to the Myc family of transcription factors (comprising MYC, MYCN and MYCL), of which MYC is the best characterized. It was originally identified because of its homology with v-MYC, the transforming gene of the MC29 avian leukemia virus,Citation19 and subsequently, its deregulation has been reported in a wide range of human tumors, though its activation may be achieved through different pathogenetic mechanisms.Citation20 MYC may either induce or repress transcriptional activation, thus regulating the expression of many downstream targets and consequent biological pathways.Citation21 To carry out transcriptional control, the Myc protein binds to other transcriptional regulators such as Max or Mnt, Mxd1-4 (Mad1, Mxi1, Mad3 and Mad4) and Mga, which influence Myc transcriptional regulation pushing toward either activation or repression of downstream targets. Proliferative stimuli induce the expression of MYC and lead to the formation of Myc:Max heterodimers and concomitant activation of target gene expression, thus resulting in transcriptional activation.Citation21 Other transcription factors may compete with Max for binding to Myc (Mnt, Mxd1-4 and Mga), thus resulting in transcriptional repression.Citation22 In addition, Myc can bind to transcription factors Sp1 and Miz1 and may interfere with their transcriptional activator capability. The complex Myc–Miz1 recruits DNA methyl transferase 3a and histone deacetylase 3 to gene promoters, leading to DNA cytosine methylation and histone deacetylation, therefore causing gene expression silencing. The Myc–Miz1 complex can, therefore, induce the formation of heterochromatin on its target sites and function as a transcriptional repressor complex.Citation22 Due to its key involvement in transcription regulation, Myc expression and function must be tightly controlled.
Pathological activation of MYC associated with gain-of-function mutations has been commonly described in cancer. It can be due to chromosomal translocations leading to promoter rearrangements (as observed in most BL cases), gene amplifications (commonly reported in breast cancer), virus-mediated insertional mutagenesis (less commonly observed, due to random insertions of viruses within the genome) and Myc protein stabilization, mainly due to genetic mutations.Citation21 Though all these mechanisms are possible in BL, the most commonly observed cause of Myc deregulation is usually the presence of a balanced translocation involving chromosome 8, where MYC maps, and different partners. Mutations of the MYC coding sequence have also been described in BL, as well as variations in its copy number, but they occur in a minority of casesCitation23 and do not seem to account for the main reason of MYC upregulation. Nevertheless, in the last few years, a few cases of BL in which none of the abovementioned mechanisms could possibly explain Myc hyperexpression have been described, and alternative pathogenetic mechanisms were investigated. No matter what is leading to MYC deregulation, the consequence is an increased expression of its protein product which is invariably associated with genomic instability, uncontrolled cell proliferation, escape from immune surveillance and malignant transformation.Citation20
Additional genetic lesions in BL
Despite MYC deregulation being absolutely crucial for BL pathogenesis, MYC imbalance is not the only genetic lesion identified in BL and other recurrent or sporadic lesions have also been described. Several genes have been reported to be mutated in BL, such as the tumor suppressors ID3Citation24,Citation25 or TCF3,Citation26 whose mutations seem to be quite common in BL, especially in its sporadic variant. Additional mutations have been detected in genes belonging to the PI3K pathway,Citation27 in the SWI/SNF family members and in ARID1A and SMARCA4A among others, which suggest functional alterations of the nucleosome remodeling complex.Citation28 In addition, genes whose mutations have already been described in other B-cell lymphomas, such as MYC itself, DDX3X, CCND3 and FBXO11, among others, have also been reported to be mutated in BL.Citation25,Citation26 Recently, mutations of particular genes have been reported to occur at a different frequency in eBL and sBL, and a correlation between a distinct mutation pattern and the existence of some viral infections has been suggested.Citation29 This observation may indicate that different pathogenetic mechanisms may exist in eBL and sBL and again suggests that viruses may play an active role in contributing to BL development.
Genetic lesions other than point mutations have also been described in BL. In particular, copy number alterations including gains of 1q, 9q, 12q, 13q, 20q, 22q and Xq and losses of 4q, 13q and 17p have been reported.Citation25,Citation30–Citation34 Additionally, trisomy 1qCitation31 and tetrasomy 1q have also been described.Citation32 Interestingly, gains of 11q have been frequently reported in a subset of tumors resembling BL but lacking MYC translocation,Citation35 although it is debated whether such cases should be diagnosed as BLs or rather as different aggressive B-cell tumors with a Burkitt-like presentation.Citation35 In addition, uniparental disomy, whose role has been recently highlighted in cancer,Citation36 does not seem to play a major role in the pathogenesis of BL.Citation37
Myc upregulation due to impairment of post-transcriptional regulation: the role of miRNAs
Regulation of gene expression must be finely tuned and may be controlled at different levels. Transcriptional regulation can be achieved by epigenetic changes, which regulate the accessibility of specific DNA sequences through methylation of histones and DNA, thus determining a conformational change in the chromatin and preventing the expression of genes when their function is not required. Regulation of gene expression is further controlled at the post-transcriptional level, when a certain mRNA has already been transcribed, but translation into the correspondent protein product is impaired. Post-transcriptional regulation is achieved by small non-coding RNAs, of which miRNAs have been intensively studied in the last few years.
miRNAs were isolated for the first time from Caenorhabditis elegans in 1998,Citation38 and since the first observation, they have been described in a wide range of organisms, including humans. They are small sequences of non-coding RNA that, in their mature form, have sizes of 18–24 bp, though they are processed from longer precursors during a maturation process that also shuttles them from the nucleus to the cytoplasm.Citation38 After maturation, the miRNAs bind to complementary mRNA sequences and prevent their translation into the corresponding proteins. Depending on their degree of complementarity with the target mRNAs, they can either lead to mRNA degradation, when there is a perfect pairing, or simply to translation impairment, if there are mismatches in the pairing. However, no matter whether the mRNA is degraded or not, the consequence of miRNA–mRNA binding is that production of the protein coded by that particular mRNA is prevented. This further level of regulation allows shutting down the expression of specific genes even when their transcription into an mRNA has already taken place. More importantly, it is worth mentioning that a single miRNA can target hundreds of mRNAs based on a short sequence complementarity. Deregulation of a single miRNA may, therefore, result in deregulated expression of many genes, thus affecting several distinct pathways and biological functions within the cell. With the function of miRNA being so delicate in tuning the gene expression, their function must be strictly controlled as its imbalance might lead to disturbance of gene expression. Deregulation of miRNA expression and function has, therefore, been reported in a plethora of human diseases, including cancer.Citation39
MYC and miRNAs control each other’s expression: the existence of a feedback regulatory loop
We have already mentioned the importance of MYC as a transcription regulator. With its capability to bind to target sequences on DNA, Myc can control the expression of coding as well as non-coding regions in the DNA, including genes and miRNAs. Myc is known to regulate the expression of ~60 miRNAs,Citation40,Citation41 either positively or negatively influencing their expression (). As a transcriptional activator, Myc can induce the expression of selected miRNAs, of which the miR-17-92 cluster is the prototypical example,Citation42 thus influencing the expression of miRNA downstream target genes and eventually influencing the related biological processes (). Induction of miR-17-92 by MYC has been previously reported not only in BL and other B-cell tumorsCitation42 but also in various different tumors including breast, lung, colon, stomach and prostate (for a review, see Bui and MendellCitation43). Inhibition of downstream target genes of the miR-17-92 cluster enhances tumorigenicity by boosting cell proliferation, tumor cell survival and angiogenesis, along with metabolic reprogramming.Citation43 Very interestingly, among the downstream targets of this cluster, there are tumor suppressors such as PTEN and BIM, the first gene being an antagonist of PI3K activity and the latter having a proapoptotic function.Citation43–Citation46 Overexpression of miR-17-92 induced by Myc, therefore, results in loss of regulatory control on cell growth mediated by these tumor suppressors. A recent study investigated the expression of each member of this cluster in BL and analyzed whether there was a correlation between their expression and prognosis of BL.Citation47 The results of this study indicated that miR-17 and miR-20a were highly expressed in BL and determined lack of expression of the Bim protein.Citation47 In addition, a significant correlation between high levels of miR-17 and poor overall survival was also recorded, thus indicating the influence of miRNA expression as a prognostic value in BL.Citation47
Figure 1 An overview of MYC–miRNA regulatory loop and related pathways.
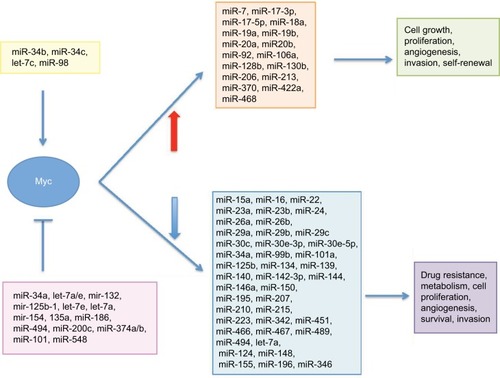
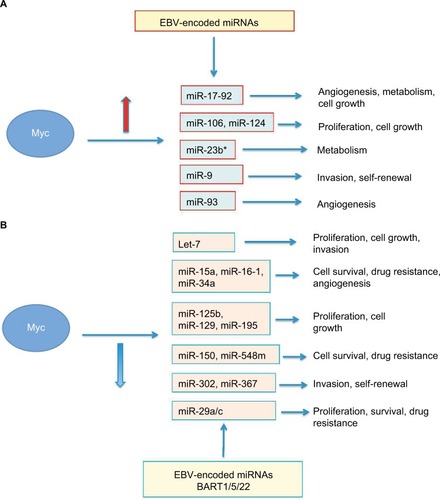
Figure 2 An overview of the pathways affected by Myc-regulated miRNAs.
Notes: (A) Myc-induced miRNAs and their regulated pathways. EBV-encoded miRNAs may compete with the miR-17-92 cluster for regulation of the same target genes. (B) Myc-repressed miRNAs and their regulated pathways. EBV-encoded BARTs may compete with cellular miR-29 family members.
At the same time, Myc can also repress the expression of specific miRNAs (ie, the miR-29 family), thus leading to increased expression of miRNA target genes and imbalance of cellular pathways (). However, MYC itself is a gene and, therefore, its expression is also controlled at the post-transcriptional level by miRNAs, of which probably the best studied is the let-7 family.Citation48 MYC expression, both at the transcriptional and post-transcriptional levels, is therefore controlled through different mechanisms to ensure that this protein will be produced only when its function in the cell is needed. Thus, miRNAs–MYC form a feedback regulatory loop controlling each other’s expression in an inverse and reciprocal manner (). When this miRNAs–MYC autoregulation fails, the expression of MYC and miRNAs is no longer regulated and may result in diseases and cancer.
A recent study reported the upregulation of the YY1 gene, which is an oncogenic transcription factor able to induce MYC expression, in BL as a consequence of downregulation of specific miRNAs.Citation49 This transcription factor was previously found to be upregulated in other non-Hodgkin’s lymphomas (NHLs)Citation50,Citation51 and plays a role in resistance to chemotherapy and immunotherapy in NHL cell lines.Citation52 YY1 can also act as a transcriptional repressor of tumor suppressors such as p16, p27, p73 and p53.Citation53–Citation55 In particular, its inhibitory effect on p53 is related to evasion from apoptosis,Citation56 which may be crucial for transformed cells, pointing at YY1 as an indicator of aggressiveness in NHLs. Very interestingly, upregulation of YY1 reported in this study is a consequence of repression of specific miRNAs, some of which, such as has-miR-363 and hsa-miR-200a, are among the top 20 miRNAs repressed in BL, thus reinforcing its functional role in the pathogenesis of BL.Citation49
Exploring the MYC–miRNA interaction in BL: sustaining Myc hyperexpression in the absence of a translocation
Approximately 10% of BL cases lack an identifiable MYC translocation, but do express the Myc protein at a level comparable to MYC-translocated BL cases.Citation57 This observation prompted many scientists worldwide to explore alternative pathogenetic mechanisms that could explain a higher expression of Myc in the absence of genetic lesions, either trans-location or copy number alterations. Given the functional relationship between MYC and miRNAs, one possible scenario to explore was to investigate whether there was an imbalance in MYC-regulating miRNA expression that could eventually explain increased Myc protein levels. A pioneer study published in 2008 compared the expression of six miRNAs predicted to target MYC (hsa-miR-155, has-miR-30a-3p, hsa-miR-34b, hsa-let-7c, hsa-let-7a and hsa-miR-98) between BL cases carrying or not an MYC translocation.Citation57 The results of this study highlighted a diminished expression of two of them (hsa-let-7a and hsa-miR-34b), thus suggesting that increased expression of Myc may be a consequence of downregulation of specific miRNAs.Citation57 However, of even greater interest was the observation that hsa-miR-34b was downregulated only in BL cases lacking the translocation, whereas reduced expression of hsa-let-7a was observed in all BL cases, irrespective of the translocation status. This observation suggested the alteration of hsa-miR-34b as potentially responsible for Myc hyperexpression in the absence of any genetic lesions.Citation57 A later study of the same group identified hsa-miR-9* as a second miRNA specifically downregulated only in BL cases lacking MYC translocation.Citation58 This observation was of particular interest because hsa-miR-9* does not directly target MYC, but may indirectly regulate its expression through E2F1, whose expression is induced by Myc,Citation59,Citation60 and that in turn activates MYC expression through a feedback autoregulatory loop that also involves the miR-17-92 cluster.Citation61–Citation63 Hsa-miR-9* downregulation observed in BL cases lacking MYC translocation could determine the upregulation of E2F1, which then increases and sustains MYC expression. The complete miRNA expression profile was then investigated in BL cases with or without MYC translocation and differential expression of four miRNAs (hsa-miR-29a, hsa-miR-29b, hsa-miR-513a-5p and hsa-miR-628-3p) was identified.Citation15 A single miRNA is able to control the expression of many target genes. Therefore, we investigated the impact of the 4 dysregulated miRNAs so-identified on the global gene expression and identified 64 putative target genes of such miRNAs were identified by bioinformatics.Citation15 The 64 predicted target genes are involved in important biological processes such as gene expression regulation, proliferation and DNA modification. Very interestingly, among differentially expressed genes, some, such as MYCN and the DNMT family of proteins, were of particular interest, with MYCN being a homologue of MYC and the DNMT proteins being reported to be altered in cancer very frequently.Citation64 DNMTs were reported to be specifically upregulated in BL cases lacking the translocation, suggesting that an aberrant epigenetic control occurs in this set of BL cases. As discussed earlier, interaction of Myc with DNMT family members can influence chromatin conformation and subsequent accessibility to RNA polymerase for transcription.Citation22 Deregulation of DNMTs by the miR-29 family has also been recently described in another study, which highlights the importance of epigenetic regulation in BL.Citation65 In particular, this study shows hypermethylation of p16 following overexpression of DNMTs, which might favor cell proliferation due to lack of control on cell cycle.Citation65 Of great interest is the finding that MYCN overexpression occurs only in cases lacking MYC translocation. MYCN expression is usually not detected in BL cases, but its deregulation is frequently observed in other cancers, such as neuroblastoma, where there is a different genetic mechanism (amplification of the MYC gene) which is responsible for over-expression of the Myc protein.Citation66 High expression of MYCN in cases lacking MYC translocation may indicate the existence of an alternative cooperative mechanism ensuring high expression of members of the MYC family in the absence of genetic lesions involving MYC. Very interestingly, deregulation of two of the differentially expressed miRNAs (miR-513a-5p and miR-628-3p) has been recently described in human neuroblastoma,Citation67,Citation68 with the miR-628-3p expression correlating with the prognosis of this tumor.Citation69
MYC pathway in BL: downregulated miRNAs target genes belonging to the MYC pathway
Several genes are transcriptionally controlled by Myc, and the existence of an MYC pathway has been identified in cells. As Myc is highly expressed in BL, it was investigated to what extent the Myc-regulated pathway was affected in BL and through which mechanisms. Results from a previous research study report that MYC target genes are upregulated in BL or gamma-irradiated mice tumors.Citation70 In this study, 41 miRNAs were found to be downregulated in gamma-irradiated mice lymphomas and 17 miRNAs in BL, resulting in upregulation of miRNA target genes. Interestingly, an enrichment of the MYC pathway was observed among upregulated genes, thus suggesting that upregulation of MYC pathway may be a consequence of transcriptional repression of specific miRNAs.Citation70
miRNA expression profile: a BL signature for differential diagnosis
BL is a very homogeneous entity in terms of gene expression and has a distinctive, unique pattern, which distinguishes it from any other B-cell lymphomas.Citation11,Citation12 This observation is extremely useful for diagnostic purposes, as we can classify borderline cases or cases with a histologic “Burkitt-like” presentation based on their distinctive molecular profile.Citation11,Citation12 Despite showing some subtle differences in their miRNA profile, only a few differences can be identified at the gene expression level in cases with or without MYC translocation after enrichment, yet indicating a very high homogeneity of these tumors. The three clinical forms of BL share the same molecular signature, although differences in gene expression can be observed between EBV+ and EBV− BL cases.Citation71 However, such variations may be attributed to the presence of the virus rather than to differences between clinical variants.Citation71 The miRNA profile of BL was analyzed and compared to that of diffuse large B-cell lymphoma (DLBCL); a signature of 38 miRNAs was identified, which comprises MYC-regulated and nuclear factor-kB-associated miRNAs.Citation13 The same study also reported that only six miRNAs were differentially expressed between eBL and sBL, thus reinforcing the notion that BL is a very homogeneous entity and its molecular uniqueness can be used for differential diagnosis with other B-cell lymphomas.Citation13
More recently, additional studies have confirmed the efficacy of miRNA profile for differential diagnosis and have proved that its reliability can be comparable to gene expression profile results, so far considered the “gold standard” for molecular analyses. A recent study identified a 27-miRNA signature able to distinguish BL from DLBCL, which could also be validated in formalin-fixed paraffin-embedded cases, thus even reinforcing its possible diagnostic application.Citation72 Another research identified by deep sequencing the existence of a 22-miRNA signature, which could be used to discriminate BL from DLBCL and follicular lymphomas, again highlighting the importance of molecular profiles for differential diagnosis.Citation73
Noteworthy, low or no expression of hsa-miR-155 (or its precursor BIC) was detected in BL, as reported by several studies,Citation74–Citation76 despite this miRNA being one of the most commonly upregulated in B-cell lymphomas.Citation77–Citation79 It has been recently demonstrated that low levels of this miRNA determine an increased expression of AICDA, which increases the frequency of MYC translocation.Citation80 Intriguingly, a study shows that higher expression of miR-155 can be found in a subset of EBV-positive BL cell lines expressing a viral latency III program, which is usually not detected in primary BLs, whereas low or no expression of this miRNA was detected in EBV-positive BL cases expressing a latency I program, which represent the vast majority of BLs.Citation75 It has sometimes been speculated that EBV infection may promote MYC translocation in BL. This observation may suggest a possible mechanism leading to MYC translocation in EBV-positive cases by maintaining low miR-155 and consequently inducing AICDA expression. Interestingly, downregulation of this miRNA can also be used for differential diagnosis,Citation81 as high expression of miR-155 is normally reported in other B-cell tumors, where it seems to have a clinical significance as it is associated with chemotherapy failure in DLBCL.Citation72
and list the miRNAs whose expression has been reported to be unbalanced in BL.
Table 1 List of miRNAs reported to be downregulated in BL
Table 2 List of miRNAs reported to be upregulated in BL
Virus-encoded miRNAs and deregulation of host cell gene expression: contribution of EBV
EBV is very often associated with BL, especially in its endemic form. Despite an extensive discussion about this virus, with its latency programs and pathogenetic mechanisms being beyond the aim of this review, it is worth mentioning that EBV may contribute to the pathogenesis of BL through its encoded proteins (genes and viral miRNAs). In BL cells, EBV expresses a latency I program, in which EBNA1 is the only viral protein expressed. Previous studies have demonstrated the role of this protein in the pathogenesis of BL.Citation82,Citation83 EBV also encodes for 44 mature viral miRNAs (viRNAs) belonging to two families (BART and BHRF).Citation84 Expression of these viral-encoded miRNAs is also latency regulated, and only a few viRNAs belonging to the BART family are detected in BL.Citation85 It has also been demonstrated that three members of the BART family (BART-1-3p, BART-5-5p and BART-22-3p) exhibit high similarity with cellular miRNAs, including the miR-29 family ().Citation86–Citation91 The expression of viRNAs should be carefully monitored as they compete with cellular miRNAs for the same target genes in the host cell. An interesting study has shown that in a BL-derived cell line expressing a latency III program, EBV-encoded miRNAs target the same genes as the miR-17-92 cluster ().Citation92 Despite the BL primary tumors mostly expressing a latency I program, this observation is of interest as it highlights how EBV infection may contribute to deregulate key cellular pathways such as transcription, apoptosis and cell cycle.Citation92 Expression of viRNAs could, therefore, result in an aberrant post-transcriptional regulation in infected cells. The presence of EBV can impact on cellular miRNA signature in BLCitation85,Citation93 and on cellular gene expression profile.Citation93 An important role for EBV-Bart6 has been suggested, as this miRNA is capable of regulating the expression of PTEN and interleukin-6 receptor complex in infected cells, thus influencing survival and interleukin-6 downstream pathways.Citation85 A later study confirmed this finding and highlighted the existence of a synergistic effect between Bart-6 and miR-142, which was previously reported to be upregulated in BL,Citation85 to repress PTEN.Citation94 This latter observation is of interest as it highlights the active role of EBV in BL pathogenesis and its interplay with the cellular machinery. It has also been demonstrated that BARTs target Casp3 in BL and may therefore result in an antiapoptotic effect, thus resulting in a growth advantage for the infected cells.Citation95
It is worth mentioning that EBV infection contributes to lymphomagenesis also through mechanisms other than miRNA regulation. We had previously mentioned that a different mutational profile can be observed between eBL and sBL, with endemic cases showing a lower mutation rate.Citation29 With the degree of association with EBV being the main difference between the endemic and sporadic variants, it is reasonable to postulate that such difference may be due to the existence of additional pathogenetic mechanisms in EBV-positive cases.Citation29 In a recent research paper, the methylome of EBV-positive vs EBV-negative BL-derived cell lines was compared, and the results of this study demonstrated that the presence of the virus is associated with a specific pattern of DNA methylation, suggesting that EBV may contribute to BL pathogenesis through an epigenetic mechanism.Citation96 In particular, this paper has demonstrated a higher level of methylation in EBV-positive samples involving, among others, key genes such as ID3 and TCF3 which are usually mutated in sBL, but whose mutation rate is lower in eBL.Citation29 Diminished expression of these genes in eBL may, therefore, be a consequence of epigenetic regulation rather than deriving from genetic lesions. This finding suggests that BLs have similar gene expression patterns, but underlying mechanisms may be different and may depend on the presence of EBV.Citation96
Although an extensive discussion of other parasites that might act as cofactors in Burkitt lymphomagenesis is beyond the scope of this review, it is worth making a brief comment on another parasite, the protozoon Plasmodium falciparum that causes malaria, as there is a striking overlap in the geographic incidence of eBL and this disease. Even though not much is known about the mechanisms that the parasite uses to contribute to tumor formation, recent literature shows that P. falciparum infection drives EBV-infected cells through GC, and it is capable of deregulating the expression of the AICDA gene (also referred to as AID), which would lead to chromosomal translocations, as we mentioned earlier.Citation97–Citation99 Translocations would mainly occur in EBV-infected cells within the GC that more likely would tolerate it. This observation reinforces the speculation that other cofactors are required for the occurrence of BL in endemic areas and that these parasites play an active role in Burkitt lymphomagenesis.
miRNAs as prognostic indicators of BL
Given the clear involvement of miRNA deregulation in the pathogenesis of BL, their diagnostic and prognostic value has been evaluated. A recent study reports that the identification of three circulating miRNAs (miRNA-21, miRNA-23a and miRNA-125b) in the plasma of BL patients may be used as a diagnostic indicator and could be related to clinicopathologic parameters.Citation100 Increased expression was observed for miR-21 and miR-23, and it was also correlated with some clinicopathologic parameters (tumor staging, increased white blood cells, increased serum lactate dehydrogenase level, CD10 expression and size of the tumor >6 cm).Citation100 Very interestingly, the expression level of these miRNAs decreased significantly following chemotherapy, suggesting that these miRNAs could be used to monitor therapy effi-cacy.Citation100 Also, an inverse correlation between the level of these miRNAs and patients’ outcome was established, indicating these miRNAs act as prognostic indicators as well.Citation100 Very recently, the expression of another miRNA (hsa-miR-10a-5p) has been linked to the prognosis of BL patients, with this being downregulated in non-survivors.Citation101 Interestingly, genes targeted by this miRNA are involved in control of apoptosis and their overexpression could favor cell growth. Additionally, high expression of CD59 as a result of hsa-miR-10a-5p imbalance may determine reduced sensitivity to chemo- and immunotherapy and explain treatment failure and reduced overall survival in BL.Citation101
miRNAs as potential targets in novel treatments for BL
BL is classically treated by a combination of chemotherapy and immunotherapy.Citation102 Nevertheless, due to the aggressiveness of this tumor, it is imperative to explore more effective therapeutic alternatives. One such possibility would be to target miRNAs to either suppress or induce the expression of target genes, which might be relevant for a better prognostic outcome. Recent literature is providing useful information about new drugs or possible new therapeutic targets, including miRNAs. An obvious target for BL treatment would be MYC and its related network. A recent study reports that the use of INZ(c), a second generation of Inauhzin, is able to suppress Myc expression and it results in inhibition of cell growth in lymphoma cells.Citation103 Suppression of MYC is achieved through the miRNA pathway, as the expression of MYC-targeting miRNAs, such as miR-24 and miR-34a, is induced upon treatment to reduce Myc levels.Citation103 Noteworthy, this small molecule does not have considerable side effects and could be used in combination with doxorubicin to reduce Myc expression, allowing the administration of a lower dose of doxorubicin with consequent reduction of side effects.Citation103 However, due to the pleiotropic activities regulated by MYC, it is very difficult to design therapeutic approaches to inhibit its expression in human tumors without interfering with its physiological functions, and other potential targets should be explored.
Treatment with the combination of histone deacetylase inhibitor and chemotherapy results in induction of apoptosis in BL cells through the proapoptotic BCL2-related family member Bim protein.Citation104 A recent study of the same group describes that the use of combination of histone deacetylase inhibitor and chemotherapy could prevent cell growth in BL by regulating PI3K/Akt, suggesting that other targets, such as the PI3K/Akt signaling network, in addition to MYC should be further explored.Citation105 The combination of demethylating agents and chemotherapy could be used to revert the expression of aberrantly silenced genes and miRNAs, such as p16 and miR-101, miR-143 and miR-145, in BL tumor models.Citation105 Of these, miR-145 directly targets MYC and is expressed through the PI3K pathway, which is deregulated in BL.Citation106 Re-expression of miR-145 by this combinatorial approach may, therefore, result in reduction of MYC expression levels.Citation105
Regulation of cell proliferation and induction of a more differentiated phenotype could be another possible approach as BL is the fastest growing tumor. It has been recently reported that the re-expression of miR-150 could be used as a possible promising therapeutic target because of its capability of reducing cell proliferation by targeting B-Myb.Citation107,Citation108 In addition, this would result in the acquisition of a more differentiated phenotype as BL results from an impairment during differentiation toward plasma cells.Citation109,Citation110
Conclusion
MYC overexpression is the hallmark of BL and it can be consequent to several pathogenetic mechanisms. Recent literature highlights the key role of miRNAs in the pathogenesis of BL that can imbalance Myc and its associated pathways. Detection of miRNA expression can be used for diagnostic and therapeutic purposes. Re-expression of endogenous miRNAs through the administration of demethylating drugs to revert their silencing, or ectopic introduction of exogenous small RNAs that target deregulated genes could represent an exciting alternative to current therapies to improve the overall survival and reduce the side effects in BL patients. However, despite encouraging results, there is still much to uncover before such innovative therapeutic approaches may enter daily practice, and research in the field should be pursued to better clarify their role in the pathogenesis of BL.
Disclosure
The authors report no conflicts of interest in this work.
References
- BurkittDPA sarcoma involving the jaws in African childrenBr J Surg19584621813628987
- MoormannAMChelimoKSumbaOPExposure to holoendemic malaria results in elevated Epstein–Barr virus loads in childrenJ Infect Dis20051911233123815776368
- van den BoschCLloydGChikungunya fever as a risk factor for Endemic Burkitt’s lymphoma in MalawiTrans R Soc Trop Med Hyg200094670470511198662
- MoormannAMBaileyJAMalaria – how this parasitic infection aids and abets EBV-associated Burkitt lymphomagenesisCurr Opin Virol201620788427689909
- EpsteinMABarrYMCultivation in vitro of human lymphoblasts fromBurkitt’s malignant lymphomaLancet1964125225314090852
- Dalla-FaveraRBregniMEriksonJPattersonDCroceCMHuman c-myc oncgene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cellsProc Natl Acad Sci USA198279782478276961453
- TaubRKirschIMortonCLenoirGAaronsonSLederPTranslocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cellsProc Natl Acad Sci USA198279783778416818551
- OettgenHFCliffordPBurkittDMalignant lymphoma involving the jaw in African children: treatment with alkylating agents and actinomycinCancer Chemother Rep196328253413939520
- Shannon-LoweCRickinsonABBellAEpstein–Barr virus-associated lymphomasPhilos Trans R Soc Lond B Biol Sci201737217322016027128893938
- LeonciniLRaphaelMSteinHHarrisNLJaffeESKluinPMBurkitt lymphomaWHO Classification of Tumours of Haematopoietic and Lymphoid Tissues4th edInternational Agency for Research on CancerLyon, FranceIARC Press2008262264 Revised edition published in 2016
- HummelMBentinkSBergerHMolecular Mechanisms in Malignant Lymphomas Network Project of the Deutsche KrebshilfeA biologic definition of Burkitt’s lymphoma from transcriptional and genomic profilingN Engl J Med20063542419243016760442
- DaveSSFuKWrightGWLymphoma/Leukemia Molecular Profiling ProjectMolecular diagnosis of Burkitt’s lymphomaN Engl J Med20063542431244216760443
- LenzeDLeonciniLHummelMThe different epidemiologic subtypes of Burkitt lymphoma share a homogenous micro RNA profile distinct from diffuse large B-cell lymphomaLeukemia2011251869187621701491
- van RijkAMasonDJonesMTranslocation detection in lymphoma diagnosis by split8 signal FISH: a standardised approachJ Hematop2008111912619669210
- De FalcoGAmbrosioMRFuligniFBurkitt lymphoma beyond MYC translocation: N-MYC and DNA methyltransferases dysregulationBMC Cancer20151566868026453442
- BellanCLazziSHummelMImmunoglobulin gene analysis reveals 2 distinct cells of origin for EBV-positive and EBV-negative Burkitt lymphomasBlood20051061031103615840698
- LuzziAMorettiniFGazaneoSHIV-1 Tat induces DNMT over-expression through microRNA dysregulation in HIV-related non Hodgkin lymphomasInfect Agent Cancer20149415725705251
- EilersMEisenmanRNMyc’s broad reachGenes Dev200822202755276618923074
- HaywardWSNeelBGAstrinSMActivation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosisNature198129058064754806261142
- KalkatMDe MeloJHickmanKAMYC deregulation in primary human cancers Genes (Basel)201786E15128587062
- PelengarisSKhanMEvanGc-MYC: more than just a matter of life and deathNat Rev Cancer200221076477612360279
- HerkertBEilersMTranscriptional repression: the dark side of mycGenes Cancer20101658058621779459
- GreenoughaADaveSSNew clues to the molecular pathogenesis of Burkitt lymphoma revealed through next-generation sequencingCurr Opin Hematol20142132633224867287
- LoveCSunZJimaDThe genetic landscape of mutations in Burkitt lymphomaNat Genet2012441321132523143597
- RichterJSchlesnerMHoffmannSRecurrent mutation of the ID3 gene in Burkitt lymphoma identified by integrated genome, exome and transcriptome sequencingNat Genet2012441316132023143595
- SchmitzRYoungRMCeribelliMBurkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomicsNature201249011612022885699
- SanderSCaladoDPSrinivasanLSynergy between PI3K signaling and MYC in Burkitt lymphomagenesisCancer Cell20122216717922897848
- ShainAHPollackJRThe spectrum of SWI/SNF mutations, ubiquitous in human cancersPLoS One20138e5511923355908
- AbateFAmbrosioMRMundoLDistinct viral and mutational spectrum of Endemic Burkitt lymphomaPloS Pathog20151110e100515826468873
- GarciaJLHernandezJMGutierrezNCAbnormalities on 1q and 7q are associated with poor outcome in sporadic Burkitt’s lymphoma. A cytogenetic and comparative genomic hybridization studyLeukemia2003172016202414513052
- SalaverriaIZettlABeàSLeukemia and Lymphoma Molecular Profiling Project (LLMPP)Chromosomal alterations detected by comparative genomic hybridization in subgroups of gene expression-defined Burkitt’s lymphomaHaematologica2008931327133418698080
- RougASWendtlandPBendixKKjeldsenESupernumerary isochromosome 1, idic(1)(p12), leading to tetrasomy 1q in Burkitt lymphomaCytogenet Genome Res201414271324217199
- SchiffmanJDLorimerPDRodicVGenome wide copy number analysis of paediatric Burkitt lymphoma using formalin-fixed tissues reveals a subset with gain of chromosome 13q and corresponding miRNA over expressionBr J Haematol201115547748621981616
- HavelangeVAmeyeGThéateIGFCH (Groupe Francophone de Cytogénétique Hématologique)Patterns of genomic aberrations suggest that Burkitt lymphomas with complex karyotype are distinct from other aggressive B-cell lymphomas with MYC rearrangementGenes Chromosomes Cancer201352819223012230
- SalaverriaIMartin-GuerreroIWagenerRA recurrent 11q aberration pattern characterizes a subset of MYC-negative high-grade B-cell lymphomas resembling Burkitt lymphomaBlood20141231187119824398325
- TunaMKnuutilaSMillsGBUniparental disomy in cancerTrends Mol Med200915312012819246245
- ScholtysikRKreuzMKlapperWMolecular Mechanisms in Malignant Lymphomas Network Project of Deutsche KrebshilfeDetection of genomic aberrations in molecularly defined Burkitt’s lymphoma by array-based, high resolution, single nucleotide polymorphism analysisHaematologica201095122047205520823134
- FireAXuSMontgomeryMKKostasSADriverSEMelloCCPotent and specific genetic interference by double-stranded RNA in Caenorhabditis elegansNature19983918068119486653
- CalinGASevignaniCDumitruCDHuman microRNA genes are frequently located at fragile sites and genomic regions involved in cancersProc Natl Acad Sci USA20041012999300414973191
- ChangTCYuDLeeYSWidespread microRNA repression by Myc contributes to tumorigenesisNat Genet2008401435018066065
- RobertusJLKluiverJWeggemansCMiRNA profiling in B non-Hodgkin lymphoma: a MYC-related miRNA profile characterizes Burkitt lymphomaBr J Haematol201014989691820331457
- O’DonnellKAWentzelEAZellerKIDangCVMendellJTc-Myc-regulated microRNAs modulate E2F1 expressionNature2005435704383984315944709
- BuiTVMendellJTMyc: Maestro of MicroRNAsGenes Cancer20101656857520882107
- XiaoCSrinivasanLCaladoDPLymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytesNat Immunol20089440541418327259
- JinHYOdaHLaiMMicroRNA-17-92 plays a causative role in lymphomagenesis by coordinating multiple oncogenic pathwaysEMBO J201332172377239123921550
- VenturaAYoungAGWinslowMMTargeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clustersCell2008132587588618329372
- RobainaMCFaccionRSMazzoccoliLmiR-17-92 cluster components analysis in Burkitt lymphoma: overexpression of miR-17 is associated with poor prognosisAnn Hematol20169588189127044389
- SampsonVBRongNHHanJMicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cellsCancer Res200767209762977017942906
- HafsiSCandidoSMaestroRCorrelation between the over-expression of Yin Yang 1 and the expression levels of miRNAs in Burkitt’s lymphoma: a computational studyOncol Lett2016111021102526893685
- CastellanoGTorrisiELigrestiGThe involvement of the transcription factor Yin Yang 1 in cancer development and progressionCell Cycle200981367137219342874
- BonavidaBHuerta-YepezSBaritakiSOverexpression of Yin Yang 1 in the pathogenesis of human hematopoietic malignanciesCrit Rev Oncog20111626126722248059
- VegaMIJazirehiARHuerta-YepezSBonavidaBRituximab-induced inhibition of YY1 and Bcl-xL expression in Ramos non-Hodgkin’s lymphoma cell line via inhibition of NF-kappa B activity: role of YY1 and Bcl-xL in Fas resistance and chemoresistance, respectivelyJ Immunol20051752174218316081784
- WangJStovallDBRussellGBYin Yang 1 plays an essential role in breast cancer and negatively regulates p27Am J Pathol20121802120213322440256
- WuSMuraiSKataokaKMiyagishiMCooperative regulation of p73 promoter by Yin Yang 1 and E2F1Nucleic Acids Symp Ser (Oxf)200751347348
- GrönroosETerentievAAPungaTEricssonJYY1 inhibits the activation of the p53 tumor suppressor in response to genotoxic stressProc Natl Acad Sci USA2004101121651217015295102
- LibraMTorrisiECastellanoGComputational evaluation of Yin Yang 1 transcript levels in the spectrum of B-cellNeoplasia Immunopathol Dis Therap20101115125
- LeucciECoccoMOnnisAAltered expression of c-mycregulating miRNAs in MYC negative endemic Burkitt lymphoma casesJ Pathol200821644045018802929
- OnnisADe FalcoGAntonicelliGAlteration of microRNAs regulated by c-Myc in Burkitt lymphomaPLoS One201059e1296020930934
- LeoneGDeGregoriJSearsRJakoiLNevinsJRMyc and Ras collaborate in inducing accumulation of active cyclin E/Cdk2 and E2FNature19973874224269163430
- CollerHAFormanJJLegesse-MillerA“Myc’ed messages”: myc induces transcription of E2F1 while inhibiting its translation via a microRNA polycistronPLoS Genet200738e14617784791
- HossainAKuoMTSaundersGFMir-17-5p regulates breast cancer cell proliferation by inhibiting translation of AIB1 mRNAMol Cell Biol200626218191920116940181
- SylvestreYDe GuireVQueridoEAn E2F/miR- 20a autoregula-tory feedback loopJ Biol Chem20072822135214317135249
- BuenoMJGomez de CedronMLaresgoitiUFernandez-PiquerasJZubiagaAMMalumbresMMultiple E2F-induced microRNAs prevent replicative stress in response to mitogenic signalingMolCell Biol2010301229832995
- ZhangWXuJDNA methyltransferases and their roles in tumorigenesisBiomark Res20175128127428
- RobainaMCMazzoccoliLArrudaVODeregulation of DNMT1, DNMT3B and miR-29s in Burkitt lymphoma suggests novel contribution for disease pathogenesisExp Mol Pathol20159820020725746661
- StriederVLutzWRegulation of N-myc expression in development and diseaseCancer Lett200218010711912175541
- MuthuMCheriyanVTMunieSMechanisms of neuroblastoma cell growth inhibition by CARP-1 functional mimeticsPLoS One201497e10256725033461
- SchulteJHSchoweBMestdaghPAccurate prediction of neuroblastoma outcome based on miRNA expression profilesInt J Cancer2010127102374238520473924
- SchulteJHMarschallTMartinMDeep sequencing reveals differential expression of microRNAs in favorable versus unfavorable neuroblastomaNucleic Acids Res201038175919592820466808
- BuenoMJGómez de CedrónMGómez-LópezGCombinatorial effects of microRNAs to suppress the Myc oncogenic pathwayBlood20111176255626621478429
- PiccalugaPPDe FalcoGKustagiMGene expression analysis uncovers similarity and differences among Burkitt lymphoma subtypesBlood20111173596360821245480
- IqbalJShenYHuangXGlobal microRNA expression profiling uncovers molecular markers for classification and prognosis in aggressive B-cell lymphomaBlood20151251137114525498913
- HezavehKKloetgenABernhartSHAlterations of microRNA and microRNA-regulated messenger RNA expression in germinal center B-cell lymphomas determined by integrative sequencing analysisHaematologica2016101111380138927390358
- MetzlerMStrisselPLStrickREmergence of translocation t(9;11)-positive leukemia during treatment of childhood acute lym-phoblastic leukemiaGenes Chromosomes Cancer20043916716914695998
- KluiverJHaralambievaEde JongDLack of BIC and MicroRNA miR-155 expression in primary cases of Burkitt lymphomaGenes Chromosomes Cancer20064514715316235244
- KluiverJvan den BergAde JongDRegulation of pri-microRNA BIC transcription and processing in Burkitt lymphomaOncogene2007263769377617173072
- van den BergAKroesenBJKooistraKHigh expression of B-cell receptor inducible gene BIC in all subtypes of Hodgkin lymphomaGenes Chromosomes Cancer2003371202812661002
- EisPSTamWSunLAccumulation of miR-155 and BIC RNA in human B cell lymphomasProc Natl Acad Sci USA2005102103627363215738415
- KluiverJPoppemaSde JongDBIC and miR-155 are highly expressed in Hodgkin, primary mediastinal and diffuse large B cell lymphomasJ Pathol2005207224324916041695
- Slezak-ProchazkaIKluiverJde JongDInhibition of the miR-155 target NIAM phenocopies the growth promoting effect of miR-155 in B-cell lymphomaOncotarget20157323912400
- ZajdelMRymkiewiczGChechlinskaMmiR expression in MYC-negative DLBCL/BL with partial trisomy 11 is similar to classical Burkitt lymphoma and different from diffuse large B-cell lymphomaTumor Biol20153653775388
- LeucciEOnnisACoccoMB-cell differentiation in EBV-positive Burkitt lymphoma is impaired at posttranscriptional level by miRNA-altered expressionInt J Cancer20101261316132619530237
- OnnisANavariMAntonicelliGEpstein–Barr nuclear antigen 1 induces expression of the cellular microRNA hsa-miR-127 and impairing B-cell differentiation in EBV-infected memory B cells. New insights into the pathogenesis of Burkitt lymphomaBlood Cancer J20122e8422941339
- De FalcoGAntonicelliGOnnisALazziSBellanCLeonciniLRole of EBV in microRNA dysregulation in Burkitt lymphomaSemin Cancer Biol20091940140619619656
- AmbrosioMRNavariMDi LisioLThe Epstein–Barr encoded BART-6-3p microRNA affects regulation of cell growth and immuno response in Burkitt lymphomaInfect Agent Cancer201491224731550
- ParkSYLeeJHHaMNamJWKimVNmiR-29 miRNAs activate p53 by targeting p85 alpha and CDC42Nat Struct Mol Biol200916232919079265
- GottweinECullenBRViral and cellular microRNAs as determinants of viral pathogenesis and immunityCell Host Microbe2008337538718541214
- LungRWTongJHSungYMModulation of LMP2A expression by a newly identified Epstein–Barr virus-encoded microRNA miRBART22Neoplasia2009111174118419881953
- ZhuJYPfuhlTMotschNIdentification of novel Epstein–Barr virus microRNA genes from nasopharyngeal carcinomasJ Virol2009833333334119144710
- BentwichIAvnielAKarovYIdentification of hundreds of conserved and nonconserved human microRNAsNat Genet20053776677015965474
- LandgrafPRusuMSheridanRA mammalian microRNA expression atlas based on small RNA library sequencingCell20071291401141417604727
- RileyKJRabinowitzGSYarioTALunaJMDarnellRBSteitzJAEBV and human microRNAs co-target oncogenic and apoptotic viral and human genes during latencyEMBO20123122072221
- PiccalugaPPNavariMDe FalcoGVirus-encoded microRNA contributes to the molecular profile of EBV-positive Burkitt lymphomasOncotarget201571224240
- ZhouLBuYLiangYZhangFZhangHLiSEpstein–Barr virus (EBV)-BamHI-A Rightward Transcript (BART)-6 and cellular microRNA-142 synergistically compromise immune defense of host cells in EBV-positive Burkitt lymphomaMed Sci Monit2016224114412027796281
- VereideDavid TSetoEriChiuYa-FangEpstein–Barr virus maintains lymphomas via its miRNAsOncogene201433101258126423503461
- Hernandez-VargasHGruffatHCrosMPViral driven epigen-etic events alter the expression of cancer related genes in Epstein-Barrvirus naturally infected Burkitt lymphoma cell linesSci Rep201775852586828724958
- Thorley-LawsonDDeitschKWDucaKATorgborCThe link between Plasmodium falciparum Malaria and Endemic Burkitt’s lymphoma-new insight into a 50-year-old EnigmaPLOS Pathog2016121e1005331e1005331526794909
- TorgborCAwuahPDeitschKKalantariPDucaKAThorley-LawsonDAA multifactorial role for P. falciparum malaria in endemic Burkitt’s lymphoma pathogenesisPLoS Pathog201410e100417024874410
- RobbianiDFDeroubaixSFeldhahnNPlasmodium infection promotes genomic instability and AID-Dependent B cell lymphomaCell201516272773726276629
- LiJZhaiX-WWangH-SQianX-WMiaoHZhuX-HCirculating MicroRNA-21, MicroRNA-23a, and MicroRNA-125b as biomarkers for diagnosis and prognosis of Burkitt lymphoma in childrenMed Sci Monit2016224992500227991481
- OduorCIMovassagMKaymazYHuman and Epstein–Barr virus miRNA profiling as predictive biomarkers for Endemic Burkitt lymphomaFront Microbiol2017850151328400759
- DozzoMCarobolanteFDonisiPMBurkitt lymphoma in adolescents and young adults: management challengesAdolesc Health Med Ther20168112928096698
- JungJHLiaoJ-MZhangQInauhzin(c) Inactivates c-Myc Independently of p53Cancer Biol Ther201516341241925692307
- Dos Santos FerreiraACFernandesRAKweeJKKlumbCEHistone deacetylase inhibitor potentiates chemotherapy-induced apoptosis through Bim upregulation in Burkitt’s lymphoma cellsJ Cancer Res Clin Oncol2012138231732522131152
- FerreiraACRobainaMCRezendeLMSeverinoPKlumbCEHis-tone deacetylase inhibitor prevents cell growth in Burkitt’s lymphoma by regulating PI3K/Akt pathways and leads to upregulation of miR-143, miR-145, and miR-101Ann Hematol20149398399324577510
- SachdevaMZhuSWuFp53 represses c-Myc through induction of the tumor suppressor miR-145Proc Natl Acad Sci USA200910693207321219202062
- ChenSWangZDaiXRe-expression of microRNA-150 induces EBV-positive Burkitt lymphoma differentiation by modulating c-Myb in vitroCancer Sci2013104782683423521217
- WangMYangWLiMLiYLow expression of miR-150 in pediatric intestinal Burkitt lymphomaExp Mol Pathol20149626126624613688
- StaudtLMDaveSThe biology of human lymphoid malignancies revealed by gene expression profilingAdv Immunol20058716320816102574
- LinKITunyaplinCCalameKTranscriptional regulatory cascades controlling plasma cell differentiationImmunol Rev2003194192812846804
