?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Spondias mombin has been used in traditional medicine for the management of several diseases, including memory loss. This study aimed to evaluate the cholinesterase inhibitory activity of the methanol extract of the leaves and its derived fractions, as well as carry out detailed phytochemical investigations leading to the isolation and characterization of bioactive compounds from the plant. The acetyl cholinesterase (AChE) and butyryl cholinesterase (BUChE) inhibitory activities were evaluated by colorimetric and thin-layer chromatography bioautographic assay techniques. The ethyl acetate fraction was most active against both enzymes, with percentage inhibition of 58.10 ± 1.08% and 52.66 ± 1.34% against AChE and BUChE, respectively. Three compounds, namely, botulin, campesterol and phytol, with IC50 of 0.88 μg/mL (AChE), 4.67 μg/mL (BuChE); 1.89 μg/mL (AChE), 4.08 μg/mL (BuChE) and 12.51 μg/mL (AChE), 23.89 μg/mL (BuChE), respectively, were isolated from the supernatant of the ethyl acetate fraction. The isolated cholinesterase inhibitory compounds correlate with the known memory-enhancing property of the plant and thus support one of its uses in ethnomedicine.
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by low levels of acetylcholine (ACh) in the brain, with associated cognitive deficit and memory impairment.Citation1 Acetylcholinesterase (AChE) and butyrylcholinesterase (BUChE) are enzymes that catalyze hydrolysis of ACh and butyrylcholine, respectively. The activities of these enzymes increase progressively in AD patients as the severity of dementia progresses,Citation2 and their inhibition results in an increase in the levels of ACh and butyrylcholine in the brain, as well as a corresponding increase in cholinergic functions in AD patients.Citation3 Thus, most drugs available today for the management of AD are cholinesterase inhibitors, such as galantamine, rivastigmine and donepezilCitation4 even though their effectiveness in long-term treatment is debatable.Citation5 Moreover, the primary targets recommended for AD treatment are both AChE and BuChE, but some inhibitors are more selective than others.Citation6
There is still the need to search for newer anticholinesterase treatment for AD due to the serious side effects associated with available drugs. Natural products, especially medicinal plants, have been considered valuable sources of drugs for many conditions, including AD, especially since plants have been used to enhance memory traditionally.Citation7
Spondias mombin, a medium-sized, occasionally large deciduous tree with long compound leaves, large panicles of small white flowers and yellow plumb-like fruits,Citation8 is a common inclusion in remedies used in ethnomedicine in southwestern Nigeria as a memory enhancer and/or an antiaging agent.Citation9 All parts of the S. mombin tree are of medicinal importance in traditional medicine. The leaves have been used as an oxytocic agent,Citation10 particularly for the expulsion of placenta in goats and women when normal delivery of such is delayed or impossible and as an ingredient in postpartum medication.Citation11,Citation12 It is useful as an antidiarrheal agent for the treatment of wounds and as an astringent.Citation13–Citation15 It is also used in treating inflammatory and arthritic conditions.Citation16 In Nigeria, it is used in treating intestinal disorders, particularly those associated with typhoid, diarrhea and dysentery.Citation17 It is also a component of traditional antituberculosis recipes.Citation15 The fruit decoction is used as a diuretic and febrifuge. The bark and leaves are used as an emetic and for hemorrhoids, gonorrhea and leucorrhea.Citation18 A decoction of the leaves and flower is taken as a relief for stomach ache, various inflammatory conditions and wound healings.Citation19 In southwestern Nigeria, the leaves are used traditionally for the treatment of psychiatric disorders.Citation20
Several biological activities of the plant have been reported, including antiviral,Citation21–Citation23 antibacterial and mollus-cicidal,Citation15,Citation23 β-lactamase inhibitory,Citation24 anti-inflammatory,Citation16 wound healing,Citation19 antipsychotic, anticonvulsant and sedative,Citation18,Citation20 abortifacient,Citation11 oxytocic,Citation25 antimicrobial,Citation26 anti-fertility,Citation27 antigonadotrophic,Citation28 hematinic,Citation29 antioxidant,Citation30 antidiabeticCitation31 and anticholinesterase activities.Citation32
The compounds isolated from this plant include caryophyllene, myrcene, hexanal, 3-hexenol and (e)-2-hexenal from the fruits,Citation33 cinnamic acid, 4-hydroxycinnamic acid, 3-methoxy-4-hydrocinnamic acid, 3-methoxy-4-hydroxycinnamic acid, benzaldehyde, linalool, hexanoic acid, alpha-terpineol, palmitic acid and octanoic acid,Citation34 as well as anacardic acid.Citation24 The leaves and stems contain two ellagitannins galloylgeraniin and geraniin and two caffeoyl esters 5-O-caffeoylquinic acid and 2-O- caffeoyl hyroxycitric acid, which have also been reported.Citation21,Citation22 Pelandjuaic acid and 6-(8Citation1Z,11Citation1Z-heptadecadienyl) salicyclic acid have been reported from the ethanolic extract of the leaves and stems of S. mombin.Citation23 Two new phytosterols (mombintanes I and II),Citation35 one new coumarin and three new flavonoids (mombinrin, mombincone, mombinoate and mombinol, respectively) have also been reported.Citation36 The plant also contains estra-17-propoxy, 3,4–dimethoxy–3yl benzoate.Citation37
This study assessed the cholinesterase inhibitory activity of S. mombin leaves, and isolated and characterized its anticholinesterase compounds.
Materials and methods
Chemicals
The chemicals used were as follows: acetylthiocholine iodide (ATChI), butyrylcholine chloride (BuChCl), 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB), physostigmine (eserine) salicylate (Sigma-Aldrich, St Louis, MO, USA); and electric eel AChE (EC 3.1.1.7, type VI-s) and horse butyxylcholinesterase (EC 3.1.1.8) (Fluka Co, Germany). The other reagents and buffers, which include disodium hydrogen orthophosphate dihydrate (Na2HPO4∙2H2O) and sodium dihydrogen phosphate (NaH2PO4∙12H2O), were of analytical grade. Silica gel for vacuum liquid chromatography (VLC) (American Society for Testing and Materials [ASTM]) and precoated thin-layer chromatography (TLC) plates with silica gel G60 PF254 (EMD Millipore, Billerica, MA, USA).
Plant material collection and authentication
S. mombin was identified by Mr Oladele of the Department of Pharmacognosy, Faculty of Pharmacy, and was authenticated by Dr H Illoh of the Botany Department, Obafemi Awolowo University, Ile Ife, where herbarium specimen with herbarium number IFE 9572 was deposited. The leaves were collected from the Medicinal farm of the Obafemi Awolowo University Campus in August 2005.
Preparation of extract and fractions
The powdered leaves were extracted with 80% methanol by maceration for 72 hours, and the extract was concentrated to dryness at 40°C on a rotary evaporator. The crude extract was partitioned into n-hexane, ethyl acetate and water. Both the extract and the fractions were screened for their AChE and BuChE inhibitory activities.
Bulk extraction with ethyl acetate and precipitation studies
The powdered leaves of the plant were extracted with 100% ethyl acetate. Nonpolar lipid components were precipitated out by gradual addition of methanol. Both the filtered precipitate and the supernatants were then assessed for their cholinesterase inhibitory activities.
Phytochemical and cholinesterase analyses
TLC of both precipitates and supernatant was done with chloroform–hexane (7:3, v/v) as the solvent system. Some of the developed plates were sprayed with different phytochemical screening reagents, such as vanillin/sulfuric acid, antimony trichloride, Dragendorff’s reagent and anisaldehyde spray reagents. The other plates were used for the TLC bioautographic enzyme assay.
Cholinesterase inhibition assay
Cholinesterase inhibitory activities of the crude extract, fractions, precipitate, supernatant and isolated compounds were analyzed in a 96-well microplate reader according to the modified method of Ellman.Citation38,Citation39
The reaction mixture was made up of 2000 mL 100 mM phosphate buffer (pH 8.0), 100 mL of test sample stock solution in methanol (at 42.5 μg/mL final concentration), 100 mL enzyme, either AChE or BuChE at a final concentration of 0.003 μ/mL or 0.001 μ/mL, respectively, and 100 μL of DTNB (0.3 mM) prepared in 100 M phosphate buffer pH 7.0 containing 120 mM sodium bicarbonate. Preincubation of the assay mixture was done on a water bath at 37°C for 30 minutes following proper mixing, and the reaction started by the addition of 100 μL of ATChI or butyrylthiocholine chloride (BTChCI) at a final concentration of 0.5 mM. Methanol was used as the negative control, while eserin ((–) physostigmine) was used as the positive control. Change in absorbance at λmax 412 was recorded at ambient temperature every 30 seconds for 5 minutes. All determinations were done in triplicate, and percentage inhibition was calculated as follows:
where a is the ΔA/min of control, b is the ΔA/min of test sample and ΔA is the change in absorbance.
TLC bioautographic assay method was also used to monitor active spots.Citation40 The various samples were spotted on precoated aluminum TLC plates (G60 PF254) and developed in appropriate solvent systems. The developed plates were air-dried, sprayed with 2.55 × 10–3 units/mL of the cholinesterase enzyme till saturation and then incubated at 37°C for at least 20 minutes before spraying with 0.5 mM of the substrate (ATChI or BTChCI, respectively) and DTNB. Positive result was indicated by white spots on a yellow background.
Isolation of bioactive components
VLC of S. mombin supernatant (19.20 g) was done on silica gel 60 (Sigma-Aldrich), using n-hexane, dichloromethane and methanol as solvents. Fractions were monitored using TLC on precoated G60 PF254 (0.25 mm) plates with vanillin/sulfuric acid reagent and heating at 100°C for a few minutes. A total of 103 subfractions collected were bulked into six based on their TLC patterns. The six bulked samples were tested for their AChE inhibitory activity using TLC bioautographic method. Active subfractions were bulked together and purified further using VLC, leading to the isolation of three bioactive compounds through preparative TLC (PTLC).
Analysis of bioactive compounds
The isolated compounds were subjected to a number of analyses, including different spectroscopic analyses such as 1H-nuclear magnetic resonance (NMR) and 13C-NMR (CDCl3, 300 Hz), TLC analysis in different solvent systems, solubility in water and determination of IC50.
Results
Medicinal plants are known to contain different classes of chemical compounds called secondary metabolites, which are responsible for their various biological activities. Phytochemical analysis of S. mombin was carried out on developed TLC plates. Partial purification of the methanol extract was done by precipitation. Thus, spraying the developed TLC plates of precipitate and supernatant of S. mombin with different phytochemical reagents is shown in for vanillin/H2SO4, Dragendorff’s reagent, antimony trichloride and anisaldehyde spray, respectively. Various colors were observed for the spots with the different reagents, indicating the possible nature of these chemical constituents. Organic compounds generally show color reactions to concentrated sulfuric acidCitation41 and could be indicative for detecting steroidal and terpenoidal compounds.Citation42 gave colors with vanillin/H2SO4, which are more prominent in the supernatant than in the precipitate. Alkaloids are detected with Dragendorff’s reagent as an orange–brown zone against a yellow background.Citation42 This seems to be absent in the spotted samples, as seen in . Cardiac glycosides, saponins, terpenoids and flavonoids give colored spots with antimony trichloride, and this can be seen in this plant (), while terpenoids can also be detected with anisaldehyde spray giving purple, blue or red spots.Citation42 Again, more colors were detected in the supernatant with anisaldehyde when compared with the precipitate (). Several bioactive constituents belonging to various classes have been previously reported in S. mombin. These include coumarins, flavonoids,Citation36 sterols,Citation35 phenolsCitation33,Citation34 and tannins.Citation21,Citation43
Figure 1 Spray analysis of the TLC plates of precipitate and supernatant with phytochemical reagents.
Abbreviation: TLC, thin-layer chromatography.
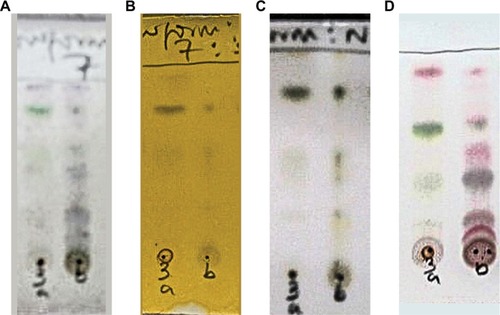
The precipitate and the supernatant were subjected to cholinesterase inhibitory analysis. Qualitative TLC bioautographic () as well as quantitative EllmanCitation39 colorimetric assays () showed that cholinesterase inhibitory activity of the supernatant was better than that of the precipitate. The phytochemical investigations, which showed more constituents in the supernatant when compared with the precipitate, corroborate the observed better cholinesterase inhibitory activity of the supernatant. Thus, activity-directed fractionation using VLC with TLC bioautography of the supernatant was carried out. shows the spots of the various bulked fractions after VLC, while shows the AChE assay results, from which it was observed that subfractions A–C were active. These were subjected to a combination of repetitive VLC and PTLC to isolate the active compounds, which were then identified through spectroscopic analysis.
Figure 2 TLC bioautographic assay of precipitate and supernatant.
Abbreviations: AChE, acetyl cholinesterase; BUChE, butyryl cholinesterase; TLC, thin layer chromatography.
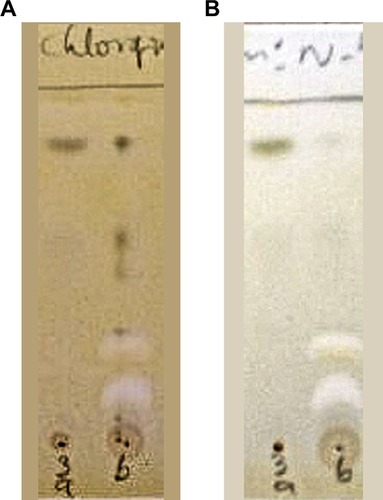
Figure 3 Bulked samples from the VLC of Spondias mombin supernatant.
Abbreviations: ATChI, acetylthiocholine iodide; DTNB, 5,5′-dithio-bis- (2-nitrobenzoic acid); VLC, vacuum liquid chromatography.
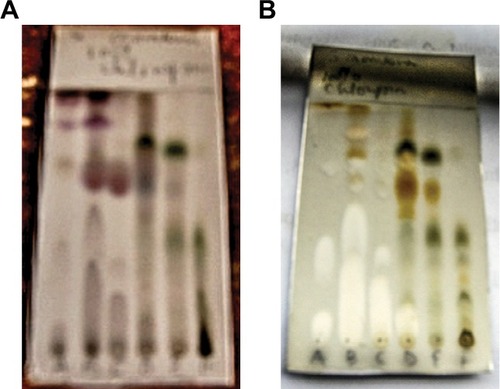
Table 1 Percentage cholinesterase inhibitory activity of Spondias mombin samples
Compound 1
Spectral data
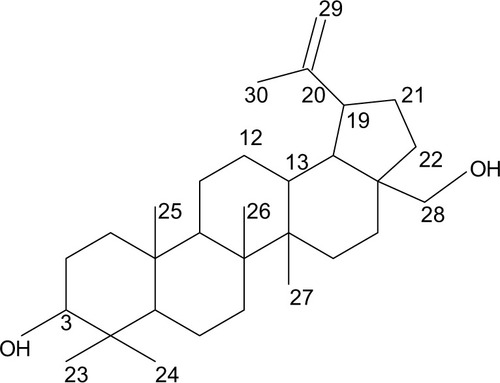
The 1H-NMR spectrum (CDCl3, 300 Hz) showed signals at δ7.8 (m), δ7.75 (m), δ5.45 (t), δ4.6 (s) and δ4.5 (d). The 13C-NMR spectrum (CDCl3, 300 Hz) showed signals at 38.71 (C-1), 20.90 (C-2), 78.83 (C-3), 35.57 (C-4), 55.24 (C-5), 18.30 (C-6), 34.06 (C-7), 39.35 (C-8), 54.96 (C-9), 37.34 (C-10), 27.22 (C-11), 24.92 (C-12), 37.83 (C-13), 39.99 (C-14), 27.19 (C-15), 29.48 (C-16), 47.08 (C-17), 50.22 (C-18), 48.97 (C-19), 150.8 (C-20), 29.66 (C-21), 36.65 (C-22), 27.92 (C-23), 15.96 (C-24), 15.46 (C-25), 16.64 (C-26), 14.33 (C-27), 59.41 (C-28), 109.40 (C-29) and 19.70 (C-30).
Structure elucidation
Compound 1 (35 mg) was isolated as a white powder with retardation factor (Rf) 0.46 in hexane:chloroform 3:7 and Rf 0.35 in 100% chloroform (). It gave purple color with both vanillin/H2SO4 and anisaldehyde spray reagent, indicating its steroidal nature,Citation44 and had a melting point range of 256°C–258°C ().
Table 2 TLC profiles of the isolated compounds
Table 3 Analysis of isolated compounds
The 13C-NMR spectrum in the distortionless enhancement by polarization transfer experiment showed that there were 6CH3, 11CH2, 6CH and 7C. Thus, Compound 1 is a C-30 carbon compound.
The 1H-NMR showed a proton at δ4.5 (d) germinal to the hydroxyl group and had a corresponding carbon chemical shift at δ59.41. There was also an olefinic proton at δ4.6, which resided on the carbon at δ109.40. This proton was assigned to C-22, which is a terminal CH2 ().
Compound 2
Spectral data
The 13C-NMR data are as follows: 36.92 (C-1), 34.35 (C-2), 72.22 (C-3), 42.73 (C-4), 141.17 (C-5), 122.14 (C-6), 28.67 (C-7), 32.80 (C-8), 50.53 (C-9), 32.33 (C-10), 21.50 (C-11), 37.66 (C-12), 40.18 (C-13), 57.17 (C-14), 23.42 (C-15), 26.45 (C-16), 56.45 (C-17), 12.26 (C-18), 19.82 (C-19), 36.56 (C-20), 19.44 (C-21), 32.31 (C-22), 24.72 (C-23), 46.23 (C-24), 29.54 (C-25), 20.25 (C-26), 19.20 (C-27) and 12.40 (C-28).
Structure elucidation
Compound 2 (20 mg) had Rf values of 0.2 and 0.27 in hexane:chloroform 2:8 and 100% chloroform, respectively, with purple color in both vanillin/H2SO4 and anisaldehyde spray reagent ().
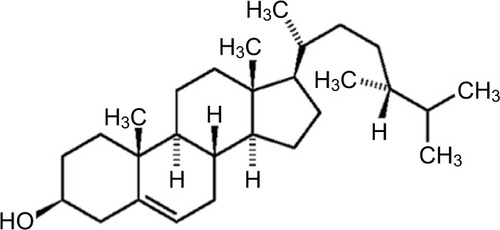
The 13C-NMR spectrum of Compound 2 showed that it is a C-28 compound. The attached proton test (APT) revealed 3 quaternary (3 C), 10 methylene (10 CH2), 6 methyl (6 CH3) and 9 methine (9 CH) carbons. The 1H-NMR showed one olefinic proton at δ5.40, with a corresponding carbon chemical shift of δ121.14 in the heteronuclear multiple quantum coherence spectrum. It also revealed the presence of one oxygenated methylene proton at δ3.5, as confirmed by the downfield chemical shift at δ72.22. In the heteronuclear multiple bond coherence spectrum, the diagnostic olefinic proton and the proton germinal to the OH showed connectivity with the quaternary carbon resonating at 141.17. From the combined 1H-NMR, 13C-NMR and APT experiments, as well as comparison with literature data, Compound 2 was identified to be campesterol ().Citation45,Citation46
Compound 3
Spectral data
13C-NMR spectra of Compound 3 showed signals at 59.85 (C-1), 123.48 (C-2), 130.92 (C-3), 40.29 (C-4), 25.55 (C-5) 33.21 (C-6) 30.13 (C-7), 37.78 (C-8), 24.89 (C-9), 37.08 (C-10), 33.11 (C-11), 37.70 (C-12), 25.22 (C-13), 39.79 (C-14), 28.40 (C-15), 23.15 (C-16), 23.05 (C-17), 20.17 (C-18), 20.14 (C-19) and 16.86 (C-20).
Structure elucidation
Compound 3 (19 mg) was isolated as a yellowish liquid with Rf of 0.64 in hexane:chloroform 1:1 and 0.51 in 100% chloroform. It gave a purple color with vanillin/H2SO4 and a pink color with anisaldehyde spray reagent ().
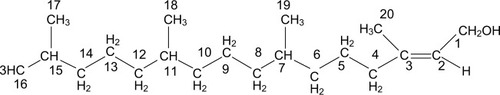
The 13C-NMR spectrum revealed 5CH3, 10CH2, 4CH and 1C=C, indicating a C-20 compound. The 1H-NMR spectrum showed a signal at δ 5.4 (t), representing an olefinic proton, assigned to C-2. The signal at δ 4.1 (d) is an alcoholic proton assigned to the proton residing on C-1. A triplet at δ1.98 was assigned to the proton on C-4, while the multiplets at δ 1.44 and δ1.35 are the methine protons on C-7 and C-11. The other methine proton on C-15 had its signal at δ1.52. In addition, the multiplets at δ1.30–δ1.03 were assigned to the protons on C-6, C-8, C-9, C-10, C-12 and C-13, while the signal at δ1.65 (s) was assigned to the methyl proton on C-20. The OH group had a signal at δ1.66. Analysis of the spectra and comparison with literature values showed that Compound 3 is phytol ().Citation47
Discussion
Three compounds with cholinesterase inhibitory activity were successfully isolated from S. mombin and identified in this study. S. mombin exhibited potent cholinesterase inhibitory activity attributable to the presence of the various isolated compounds and of significance in the management of neurodegenerative disorders such as Alzheimer’s disease. This plant has been used for enhancement of memory in traditional medicine,Citation9 and plants with such history have been previously reported by various researchers to possess cholinesterase inhibitors.Citation48–Citation50
On comparison with data in literature,Citation51–Citation53 Compound 1 was identified as betulin. Betulin has been previously reported in several plants for its different biological activities.Citation51 However, it is being linked with the cholinesterase inhibitory activity for the first time, with an IC50 of 0.88 μg/mL against AChE and 4.67 μg/mL against BuChE.
Compound 3, which is phytol, has been previously reported by us for its cholinesterase inhibitory activity.Citation54 Phytol is a diterpene alcohol, while betulin is a lupane-type triterpene. Several reports have implicated terpenoids as good cholinesterase inhibitors.Citation55–Citation57 In 2004, dihydrotanshinone, cryptotanshinone, tanshinone I and tanshinone IIA were identified as the first example of diterpenoids that inhibit AChE.Citation58 Several others have since been reported. Triterpenes have also been documented as potent cholinesterase inhibitors.Citation59–Citation61 Some other oleanane triterpene saponin compounds have also been implicated in the treatment of dementia and mild cognitive impairment by previous researchers who are already seeking patency in the USA.Citation62
Compound 2 identified as campesterol has been previously reported in several plant species, including rapeseed oil (Brassica napa),Citation63 soybean oil (Glycine max)Citation64 and wheat germ oil (Triticum spp.).Citation65 Campesterol, though not new, is being reported for cholinesterase inhibitory activity for the first time, with an IC50 of 1.89 μg/mL (AChE) and 4.08 μg/mL (BuChE). It is a phytosterol, and several phytosterols have been reported to possess cholinesterase inhibitory activity to varying extents.Citation66–Citation68 However, others such as stigmasterol and β stigmasterol were reported in a molecular docking experiment to have weak bonding with AChE proteins when compared with Aricept®–AChE complex, even though they had comparable Glide score.Citation69
Conclusion
Studies relating to the identification of naturally occurring secondary metabolites from medicinal plants have allowed the discovery of important drugs, including inhibitors of several enzymes such as AChE. This has been useful in the development of new drugs for clinical use. We isolated and identified three compounds from S. mombin with good cholinesterase inhibitory ability. These compounds could be candidates for further studies in the development of new drugs for the treatment of disorders such as AD.
Disclosure
The authors report no conflicts of interest in this work.
References
- MurrayaPAFaraoniaMBCastroaMJAlzaaNPCavallaroVNatural AChE inhibitors from plants and their contribution to Alzheimer’s disease therapyCurr Neuropharmacol20131138841324381530
- KhaledNZRAnaCCSPauloMPFChistianeMFPhytoconstituents and evaluation of acetylcholinesterase inhibition by methanol extract of Liquidambar styraciflua (L.) aerial partsJ Appl Pharm201426143152
- ČolovićMBKrstićDZLazarević-PaštiTDBondžićAMVasićVMAcetylcholinesterase inhibitors: pharmacology and toxicologyCurr Neuropharmacol201311331524179466
- ChopraKMisraSKuhadACurrent perspectives on pharmacotherapy of Alzheimer’s diseaseExpert Opin Pharmacother201112333535021222549
- SangnoiYSakulkeoOYuenyongsawadSAcetylcholinesterase-inhibiting activity of pyrrole derivatives from a novel marine gliding bacterium, Rapidithrix thailandicaMar Drugs20086457858619172195
- MehtaNAdemASabbaghMNew acetylcholinesterase inhibitors for Alzheimer’s diseaseInt J Alzheimers Dis2012201218
- HoughtonPJHowesMJNatural products and derivatives affecting neurotransmission relevant to Alzheimer’s and Parkinson’s diseaseNeurosignals200514222
- LawrenceGHMTaxonomy of Vascular PlantsIndianapolis, INMacmillan1971545574
- ElufioyeTOOladeleATCyril-OlutayoCMAgbedahunsiJMAdesanyaSAEthnomedicinal study and screening of plants used for memory enhancement and antiaging in Sagamu, NigeriaEur J Med Plants201223262275
- AyensuESMechanical Plants of West AfricaAlgonac, MIReference Publications Inc1978282
- OffiahVNAnyanwuIIAbortifacient activity of an aqueous extract of Spondias mombin leavesJournal of Ethnopharmacol198926317320
- KramerAMosqueraERuizJRodrinquezEEthnobotany and biological activity of plants utilized during pregnancy and childbirth in the Peruvian AmazonHerforthAWGorospeKKheelJFraissinetPRosaneDRodriguezEEmanations from the Rainforests and the Carribean4Ithaca, NYCornell University200210
- Oliver-BeverBMedicinal Plants in NigeriaIbadanThe Nigerian College of Arts, Science and Technology1960760 Being a Course of Four Lectures Delivered in April 1959 in the Pharmacy Department
- KokwaroJOMedicinal Plants of East AfricaNairobi, KanpalaEast Africa Literature Bureau1976384
- AboKAOgunleyeVOAshidiJSAntimicrobial potential of Spondias mombin, Croton zambesicus and Zygotritonia croceaPhytother Res199913649449710479760
- AbadMJBermejoACarreteroEMartinez-AcitoresCNogueraBVillarAAnti-inflammatory activity of some medicinal plant extracts from VenezuelaJ Ethnopharmacol199655163689121169
- BurkilHMThe Useful Plants of West Tropical Africa32nd edKew, LondonRoyal Botanic Gardens1995857
- AyokaAOAkomolafeROIwalewaOEUpkonmwanEOStudies on the anxiolytic effect of Spondias mombin L. (Anacardiaceae) extractsAfr J Tradit Complement Altern Med200522153165
- VillegasLFFernandezIDMaldonadoHEvaluation of the wound-healing activity of selected traditional medicinal plants from PeruJ Ethmopharmacol1997553193200
- AyokaAOAkomolafeROIwalewaOEAkanmuAMUpkonmwanEOSedative, anti-epileptic and anti-psychotic effects of Spondias mombin L (Anacardiaceae) in mice and ratsJ Ethnopharmacol2005103216617516188408
- CorthoutJPietersLAClaeysMVanden BergheDAVlietinckJAAntiviral ellagitanins from Spondias mombinPhytochemistry199130411291130
- CorthoutJPietersLAClaeysMVanden BergheDAVlietinckJAAntiviral caffeoyl esters from Spondias mombinPhytochemistry199231619791981
- CorthoutJPietersLAClaeysMGeertsSVanden BergheDAVlietinckJAAntibacterial and molluscicidal phenolic acids from Spondias mombinPlanta Med19946054604637997478
- CoatesNJGilpinMLGwynnMNSB-202742, a novel b-lactamase inhibitor isolated from Spondias mombinJ Nat Prod19945756546578064298
- NworuCSAkahPAOkoliCOOkoyeTCOxytocic activity of leaf extract of Spondias mombinPharm Biol2007455366371
- AmadiESOyekaAOnyeagbaRAOkoliIUgboguOCStudies on the antimicrobial effects of Spondias mombin and Baphia nittida on dental caries organismPak J Biol Sci200710339339719069507
- UchenduCNIsekTAntifertility activity of aqueous ethanolic leaf extract of Spondias mombin (Anacardiaceae) in ratsAfr Health Sci20088316316719357744
- AsuquoOREkanemTBUdohPBEluwaMAMesembeOEAnti-gonadotrophic effect of Spondias mombin leaf extract in male Wistar ratsJ Biol Agric Healthc2012271417
- AsuquoROEkanemBTUdohBPMesembeEOEbongEPHaematinic potential of Spondias mombin leaf extract in Wistar ratsAdv Biores2013425356
- MadukaHCCOkpogbaANUgwuCEPhytochemical, antioxidant and microbial inhibitory effects of Spondias mombin leaf and stem bark extractsJ Pharm Biol Sci2014921417
- MokeEGIlodigweEEOkontaJMAntidiabetic activity and toxicity evaluation of aqueous extracts of Spondias mombin and Costus afer on Wistar ratsBr J Pharm Res201565333342
- ElufioyeTOObuotorEMSennugaATAgbedahunsiJMAdesanyaSAAcetylcholinesterase and butyrylcholinesterase inhibitory activity of some selected Nigerian medicinal plantsBraz J Pharm2010204472477
- Ceva-AntunesPMBizzoHRAlvesSMAntunesOAAnalysis of volatile compounds of tapereba (Spondias mombin L.) and caga (Spondias mombin L) by simultaneous distillation and extraction (SDE) and solid phase micro extraction (SPME)J Agric Food Chem200357513871392
- AdedejiJHartmanGRosinRTChi TangHFree and glycosidically bound aroma compounds in hog plum (Spondias mombin L.)J Agric Food Chem199139814941497
- OlugbuyiroJAOMoodyJOHamannMTPhytosterols from Spondias mombin Linn with antimycobacterial activitiesAfr J Biomed Res2013161192427818608
- OlugbuyiroJAOMoodyJOAnti-tubercular compounds from Spondias mombinInt J Pure Appl Sci Technol20131927687
- EchemeJOAhuchoguAAUchegbuRIIsolation and characterization of estra-17-propoxy, 3/, 4/–dimethoxy – 3yl benzoate from the leaves of Spondias mombin LinnJ Nat Sci Res2014419172177
- HoughtonPJAgbedahunsiJMAdegbulugbeACholine esterase inhibitory properties of alkaloids from two Nigerian crinum speciesPhytochemistry200465212893289615501257
- EllmanGLCourtneyKDAndresVJRFeather-stoneRMA new and rapid colorimetic determination of acetylcholinesterase activityBiochem Pharmacol19617889513726518
- RheeIKvan de MeentMIngkaninanKVerpoorteRScreening for acetylcholinesterase inhibitors from Amaryllidaceae using silica gel thin-layer chromatography in combination with bioactivity stainingJ Chromatogr A200191521722311358251
- HarborneJBPhytochemical Methods A Guide to Modern Techniques of Plant AnalysisLondonChapman & Hall197311
- PothierJNatural Products: Thin Layer (Planar) ChromatographyAcademic PressUK200034593475
- NjokuPCAkumefulaMIPhytochemical and nutrient evaluation of Spondias mombin leavesPak J Nutr200766613615
- OsmanSMKhalekSMAKoheilMAEl-HaddadAEWinkMA new steroidal compound (β-sitosterol-3-O-butyl) isolated from Caesalpinia gilliesii flowersInt J Appl Res Nat Prod2015821419
- JajuSBIndurwadeNHSakarkarDMFuloriaNKAliMDBasuSPIsolation of sitosterol diglucosyl caprate from Alpinia galangaPharmacognosy Res2010226426621808579
- JainPSBariSBIsolation of lupeol, stigmasterol and campesterol from petroleum ether extract of woody stem of Wrightia tinctoriaAsian J Plant Sci20109163167
- ArigoniDWEisenreichCLatzelSDimethylallyl pyrophosphate is not the committed precursor of isopentenyl pyrophosphate during terpenoid biosynthesis from 1-deoxyxylulose in higher plantsProc Natl Acad Sci U S A1999964130913149990020
- HowesMJPerryNSHoughtonPJPlants with traditional uses and activities, relevant to the management of Alzheimer’s disease and other cognitive disordersPhytother Res20031711812557240
- KennedyDOScholeyABThe psychopharmacology of European herbs with cognition-enhancing propertiesCurr Pharm Des200612354613462317168769
- BaradaranARabieiZRafieianMShirzadHA review study on medicinal plants affecting amnesia through cholinergic systemJ Herbmed Pharmacol20121139
- TolstikovGAFlekhterOBShultzEEBaltinaLATolstikovAGBetulin and its derivatives: chemistry and biological activityChem Sustain Dev200513129
- SharmaPPRoyRKAnuragBGuptaDPentacyclic triterpinoids from Betula utilis and Hyptis suaveolensInt J PharmTech Res20102215321558
- UddinGWaliullahBSSiddiquiMAlamASadatAUddinAAChemical constituents and phytotoxicity of solvent extracted fractions of stem bark of Grewia optiva Drummond ex BurretMiddle East J Sci Res20111818591
- ElufioyeTOObuotorEMAgbedahunsiJMAdesanyaSAAcetyl and butyrylcholinesterase inhibiting constituent from Morinda lucida Benth (Rubiaceae)Br J Pharm Res201565358365
- PerryNSHoughtonPJTheobaldAJennerPPerryEKIn-vitro inhibition of human erythrocyte acetylcholinesterase by Salvia lavan-dulaefolia essential oil and constituent terpenesJ Pharm Pharmacol200052789590210933142
- MiyazawaMYamafujiCInhibition of acetylcholinesterase activity by bicyclic monoterpenoidsJ Agric Food Chem20055351765176815740071
- ÖztürkMAnticholinesterase and antioxidant activities of Savoury (Satureja thymbra L.) with identified major terpenes of the essential oilFood Chem201213414854
- RenYHoughtonPJHiderRCHowesMJNovel diterpenoid acetylcholinesterase inhibitors from Salvia miltiorhizaPlanta Med200470320120415114495
- Barbosa FilhoJMMedeirosKCDinizMDNatural products inhibitors of the enzyme acetylcholinesteraseRevista Brasileira de Farmacognosia2006162258285
- MukherjeePKKumarVHoughtonPJScreening of Indian medicinal plants for acetylcholinesterase inhibitory activityPhytother Res200721121142114517639556
- ErcetinTSenolFSOrhanIETokerGComparative assessment of antioxidant and cholinesterase inhibitory properties of the marigold extracts from Calendula arvensis L. and Calendula officinalis LInd Crops Prod2012361203208
- KimBChoiWLeeSUnited States Patient Application Number 201001909682006
- AmarSEckeWBeckerHCMöllersCQTL for phytosterol and sinapate ester content in Brassica napus L. collocate with the two erucic acid genesTheor Appl Genet200811681051106118335203
- ShiHNamPKMaYComprehensive profiling of isoflavones, phy-tosterols, tocopherols, minerals, crude protein, lipid, and sugar during soybean (Glycine max) germinationJ Agric Food Chem20105884970497620349962
- Ruibal-MendietaNLRozenbergRDelacroixDLSpelt (Triticum spelta L.) and winter wheat (Triticum aestivum L.) whole meals have similar sterol profiles, as determined by quantitative liquid chromatography and mass spectrometry analysisJ Agric Food Chem200452154802480715264918
- OliveiraAPValentãoPPereiraJASilvaBMTavaresFAndradePBFicus carica L.: metabolic and biological screeningFood Chem Toxicol200947112841284619747518
- ParkSJKimDHJungJMThe ameliorating effects of stigmasterol on scopolamine-induced memory impairments in miceEur J Pharmacol20126761647022173129
- PereiraVVSilvaRRDuarteLPTakahashiJAChemical constituents of Jacaranda oxyphylla and their acetylcholinesterase inhibitory and antimicrobial activitiesRec Nat Prod2016103392
- RohitMAshokTVijaykumarRKashniyalKMolecular docking study of Cassia tora, Brassica campestris and Calotropis procera as acetylcholinesterase inhibitorIndian J Pharm Educ Res2016501116122