Abstract
Background
Diastolic left ventricular (LV) dysfunction appears more prevalent in ankylosing spondylitis (AS). The effects of tumor necrosis factor alpha (TNF-α) blocking therapy, a strong and effective anti-inflammatory drug, on diastolic LV function in AS are unknown. The objective of the study was to find the effects of 1-year treatment with golimumab 50 mg subcutaneously once per month on systolic and diastolic LV dysfunction in AS patients.
Methods
Forty consecutive AS patients were treated with TNF-α blocking therapy for 1 year. Transthoracic echocardiography was performed in all patients at baseline and after 1 year of treatment.
Results
Diastolic LV function improved after treatment in four out of six (67%) AS patients who completed follow-up (P=0.125), and did not develop or worsen in any of the other patients. Treatment with TNF-α blocking therapy had no effect on systolic LV function.
Conclusion
These findings give support to the hypothesis that diastolic LV dysfunction improves during treatment with TNF-α blocking therapy.
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory rheumatic disease that affects the spinal column causing pain and decreased spinal flexibility.Citation1,Citation2 Extra-spinal manifestations including cardiac pathology are common in AS.Citation3–Citation5 The cardiac pathology linked to AS includes valvular dysfunction, in particular of the aortic valve, conduction disorders and heart failure (HF).Citation3,Citation6–Citation9
It is conceivable that these cardiac abnormalities originate due to the systemic inflammatory process inherent to AS, as inflammation may affect the aortic root, aortic and mitral valve cups, the atrioventricular node and the proximal septum.Citation10,Citation11 Also, inflammation accelerates the process of atherosclerosis, increasing the chance of developing ischemic heart disease and HF.Citation12–Citation14 Finally, inflammation might affect the myocardium and endocardium itself, leading to an abnormal filling pattern termed diastolic left ventricular (LV) dysfunction.Citation15 Diastolic LV dysfunction is caused by impaired relaxation of the left ventricle and may eventually lead to HF with preserved ejection fraction.Citation16 Failing pump function of the heart is termed systolic LV dysfunction and may lead to HF with reduced ejection fraction.Citation16
Recently, we performed a review that suggested a higher prevalence of diastolic LV dysfunction in AS patients, an important precursor to chronic HF that may contribute to the enhanced morbidity and mortality in AS.Citation8 Considering this, in this study we investigated the precise magnitude of LV dysfunction, particularly diastolic LF dysfunction, in AS patients and we examined the effect of tumor necrosis factor alpha (TNF-α) blocking therapy on LV function during a treatment period of 1 year.
Methods
Study population
Consecutive AS patients were included at the rheumatology departments of the VU University Medical Center (VUmc) and Reade, Amsterdam, The Netherlands, from November 2012 to May 2014. All patients fulfilled the 1984 Modified New York criteria for AS.Citation17 Patients were included when they were eligible for treatment with TNF-α blocking therapy and were treated for 1 year with golimumab (Simponi®; Merck Sharp & Dohme B.V., Haarlem, The Netherlands) 50 mg subcutaneously once a month. Switching to another TNF-α blocker during the study was allowed. Cardiac function was assessed with transthoracic echocardiography (TTE) at baseline and after 1 year of treatment. Approval was obtained from the local ethics committee (Ethics Committee of the Slotervaart Hospital and Reade, Amsterdam, The Netherlands) and all participating patients gave written informed consent.
Patient and disease characteristics
Medical history included AS history, medication use, hypertension, diabetes mellitus type 2 and CV events. Patients with a history of CV events were subsequently excluded. Physical examination included height, weight and blood pressure measurements. Body mass index was calculated. Hypertension was defined as present if a patient was treated with antihypertensive medication or had an indication for treatment. Blood sample measurements included standard hematological assessment, C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). Disease activity was measured with the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI) and the Ankylosing Spondylitis Disease Activity Score – CRP (ASDAS).
Transthoracic echocardiography
TTE was performed by experienced echo technicians at the VUmc. To exclude inter-observer variability, all recordings of echocardiography data from both AS patients and controls were stored digitally and were afterwards analyzed offline by a single investigator (T.K.). TTE was performed according to the following protocol based on the guidelines provided by American Society of Echocardiography.Citation18 Evaluation of cardiac function consisted of 2D, spectral and color flow Doppler recordings. The 2D recordings were performed in parasternal long- and short-axis views, and apical four-, three-and two-chamber views. Left atrial and ventricular diastolic and systolic diameters, posterior wall thicknesses (PWT) and interventricular septum thicknesses (IVS) were measured during systole and diastole by 2D imaging. Left ventricular mass was calculated with the following formula: 0.8 (1.04) (end diastolic diameter [EDD]+IVS+PWT) 3 – (EDD3)+0.6 (in grams). The relative wall thickness was calculated as following: (IVS+PWT)/EDD. Left ventricular systolic and diastolic volumes and ejection fraction (EF) were calculated from the apical four chamber view using 3D echocardiography or modified Simpson’s method.Citation18 Left atrial volume was measured using modified biplane Simpson’s rule. Aortic and mitral valve function was evaluated using color Doppler flow. Pulsed-Doppler spectral recordings of the mitral inflow were obtained with the sample volume placed at the tips of the mitral leaflets. From the transmitral pulsed-Doppler recordings, peak E and A velocities, the E/A ratio and the E wave deceleration times (DT) were obtained. Pulse wave tissue Doppler imaging was performed in the apical views to acquire mitral annular velocities. The sample volume was positioned at, or within 1 cm of the septal (e’ sept) and lateral (e’ lat) insertion sites of the mitral leaflets.
Definitions
Systolic LV dysfunction was defined as an EF <50%. Expert opinion defined the presence of systolic LV dysfunction if the EF could not be determined due to image quality or other. Diastolic LV dysfunction was graded into three categories: mild (grade I), pseudonormal (grade II) and restrictive (grade III), using criteria based on the recommendations by Nagueh et al.Citation19 Diastolic LV dysfunction was present when at least two out of three measurements (ie, e’ sept, e’ lat, left atrial volume) were abnormal. The specific grade of diastolic LV dysfunction (normal, mild, pseudonormal or restrictive) was defined when at least two of the three measurements (ie, E/A ratio, DT, E/e’ ratio) met that specific grade. Valvular function and aortic diameters were evaluated according to the most recent echocardiographic guidelines.Citation18,Citation20
Statistical analysis
For data analysis, SPSS Version 19.0 (IBM Corp., Armonk, NY, USA) was used. Demographic and disease characteristics were summarized using descriptive statistics. Distribution of data was analyzed with histograms. Values are expressed as mean ± SD, median (interquartile range) or numbers (percentages, %) where appropriate. Independent samples t-tests were used for comparisons of normally distributed continuous variables and Mann–Whitney U-test for non-normally distributed continuous variables. Fisher’s exact test was performed on dichotomous variables. Echocardiographic data of all patients completing the follow-up at 1 year were analyzed using the paired samples t-test, Wilcoxon signed-rank test or McNemar test, where appropriate. A level of P<0.05 was considered statistically significant.
Results
Patient, control and disease characteristics
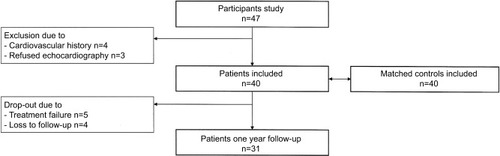
In total, 47 consecutive AS patients were included. Three patients refused echocardiography, and four patients had a history of CV events (myocardial infarction n=2, cerebrovascular accident n=2) and were subsequently excluded (). Baseline data are shown in . Of all AS patients, 33 (83%) were HLA-B27 positive. Before initiation of TNF-α blocking therapy, the mean BASDAI score was 5.6±1.7 and the mean ASDAS score was 3.2±1.2. Six (15%) AS patients had a history of hypertension compared to none in the control group (P=0.011).
Table 1 Baseline characteristics
Baseline echocardiography
In total, 13 (33%) AS patients had cardiac pathology (ie, one or more of the following: systolic and/or diastolic LV dysfunction, aortic valve dysfunction and/or aortic dilatation; one AS patient had two disorders), see .
Table 2 Echocardiographic data
Nine (23%) AS patients had diastolic LV dysfunction at baseline. The mean age of patients with diastolic LV dysfunction was 52.2±7.2 years compared to 38.7±9.8 years in patients with a normal diastolic LV function (P<0.001), and the prevalence of hypertension was 44% in patients with diastolic LV dysfunction compared to 6% in patients with a normal diastolic LV function (P=0.005) (). Levels of CRP: 4 (1–12) mg/L vs 9 (6–20) mg/L, and ESR: 6 (5–25) mm/h vs 14 (6–46) mm/h were higher in the group of patients with diastolic LV dysfunction, however, these differences did not reach statistical significance.
Table 3 Differences in characteristics of study population between AS patients with and without diastolic LV dysfunction at baseline
Follow-up echocardiography after 1 year of treatment
Results are shown in . Of all 40 patients, 31 (78%) completed the study. Two patients switched to adalimumab during follow-up up due to treatment failure. The reasons for dropping out of the study were treatment failure (n=5) or loss to follow-up (n=4). There were no baseline differences between these two groups regarding age and inflammation levels, except that the BASDAI (6.8±1.6 vs 5.2±1.6, P=0.016) and BASFI (6.4±2.8 vs 4.0±2.0, P=0.016) scores were significantly higher in the group of dropouts compared to those who completed the study. Treatment with TNF-α blocking therapy resulted in a significant decrease in inflammatory and disease activity, with median CRP levels decreasing from 5.0 (2.0–12.0) mg/L to 2.0 (1.9–3.2) mg/L (P<0.001), mean BASDAI decreasing from 5.2±1.6 to 3.7±2.3 (P=0.001) and mean ASDAS decreasing from 3.1±1.2 to 2.0±1.0 (P<0.001).
Table 4 Effects of TNF-α blocking therapy
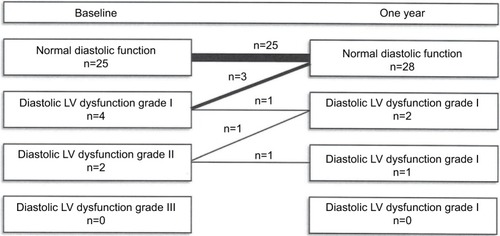
In four out of six AS patients, diastolic LV function improved during treatment (P=0.125), see . In three patients, diastolic LV dysfunction grade I improved to normal function, and one patient with diastolic LV dysfunction grade II had a normal function after 12 months. In none of the other patients, the grade of diastolic LV function worsened, and no new cases of diastolic LV dysfunction were found. Treatment with TNF-α blocking therapy had no effect on LV mass, left atrial volume or systolic LV function.
Discussion
Our observations suggest that TNF-α blocking therapy may favorably influence diastolic LV function in patients with an inflammatory disease. No differences were found in systolic LV function.
Increased diastolic LV dysfunction has previously been reported in AS, comparable to other diseases associated with an increased inflammatory response, such as rheumatoid arthritis, psoriatic arthritis and diabetes mellitus.Citation21–Citation24 There is a growing interest in diastolic LV dysfunction as it has been shown to be an increasing problem with its own morbidity and mortality.Citation25,Citation26 Diastolic LV dysfunction is poorly understood and the pathophysiology of the disease is speculative at best.Citation15,Citation27 Treatment of diastolic LV dysfunction is subject of many studies, all failing to bring improvement.Citation28 Therefore, the present study might attribute to the understanding of this disease and help formulate new points of intervention in order to resolve this growing problem.
Pathogenically, the systemic inflammatory process may cause cardiac fibrosis, subsequently decreasing overall cardiac muscle elasticity and the relaxing abilities.Citation27 In this study, inflammation levels were not significantly different in patients with diastolic LV dysfunction compared to those without. An effect of inflammation can, however, not be disregarded, as it is possible that the cumulative disease and inflammatory burden over several years affects the CV system, which is difficult to establish. Also, the investigated groups might have been too small to detect subtle differences. Patients with diastolic LV dysfunction were older and had a higher blood pressure compared to those without diastolic LV dysfunction, both known risk factors for the development of diastolic LV dysfunction.Citation16 The prevalence of hypertension is often reported higher in AS compared to controls.Citation29–Citation31 The frequent use of NSAIDs, being the cornerstone of AS treatment, might add to this risk through its anti-natriuretic and vasoconstrictor effects.Citation32 Blood pressure should therefore be monitored regularly in all AS patients and properly treated when necessary.
The development of TNF-α blockers has led to great improvements in the treatment of AS, with major reductions in disease and inflammatory activity.Citation33 In this study, we found a potential favorable effect of TNF-α blocking treatment on diastolic LV function, as diastolic LV function normalized in four AS patients during treatment. This potential positive effect may be explained in several ways. First, suppressing inflammation (ie, TNF-α) might lead to improvements in endothelial function through increases in nitric oxide production and a decrease in resting tension in the adjacent cardiomyocytes.Citation27,Citation34–Citation36 Second, lowering of disease activity may lead to improvements in physical capabilities and exercise possibilities resulting in better diastolic cardiac function.Citation37,Citation38 Third, decreased use of NSAIDs might positively affect blood pressure levels and lower the cardiac afterload, improving diastolic LV dysfunction.
This study has several strengths and limitations. First we included a homogeneous group of consecutive AS patients with high disease activity. Second, this is one of the first studies investigating the effects of TNF-α blocking therapy on LV function in AS. A limitation of this study is the relatively low number of included patients, which limits the possibility of detecting associations between cardiac pathology and disease characteristics such as inflammation.
Treatment with TNF-α blocking therapy showed a potential favorable effect on diastolic LV dysfunction, but the precise effect of TNF-α blocking treatment and the exact prevalence of cardiac pathology in AS remain to be determined in future studies.
Data sharing statement
The authors have access to raw data for this study and may be contacted for inquiries. According to national data protection rules, these linked raw data cannot be distributed further.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This is an investigator-initiated study partly financed by an unrestricted grant from Merck Sharp & Dohme, the Netherlands.
Disclosure
The authors report no conflicts of interest in this work.
References
- BraunJSieperJAnkylosing spondylitisLancet20073691379139017448825
- RamiroSvan der HeijdeDvan TubergenAHigher disease activity leads to more structural damage in the spine in ankylosing spondylitis: 12-year longitudinal data from the OASIS cohortAnn Rheum Dis20147381455146124812292
- El MaghraouiAExtra-articular manifestations of ankylosing spondylitis: prevalence, characteristics and therapeutic implicationsEur J Intern Med201122655456022075279
- NurmohamedMTvan der Horst-BruinsmaIMaksymowychWPCardiovascular and cerebrovascular diseases in ankylosing spondylitis: current insightsCurr Rheumatol Rep201214541542122791397
- PetersMJvan der Horst-BruinsmaIEDijkmansBANurmohamedMTCardiovascular risk profile of patients with spondylarthropathies, particularly ankylosing spondylitis and psoriatic arthritisSemin Arthritis Rheum200434358559215609262
- DikVKPetersMJDijkmansPAThe relationship between disease-related characteristics and conduction disturbances in ankylosing spondylitisScand J Rheumatol2010391384120132069
- KlingbergESveälvBGTängMSBech-HanssenOForsblad-D’EliaHBergfeldtLAortic Regurgitation Is Common in Ankylosing Spondylitis: Time for Routine Echocardiography Evaluation?Am J Med2015128111244125026052024
- HeslingaSCvan DongenCJKoningsTCDiastolic left ventricular dysfunction in ankylosing spondylitis–a systematic review and meta-analysisSemin Arthritis Rheum2014441141924655534
- Forsblad-D’EliaHWallbergHKlingbergECarlstenHBergfeldtLCardiac conduction system abnormalities in ankylosing spondylitis: a cross-sectional studyBMC Musculoskelet Disord20131423723937715
- LangeUStapferGDittingTPathologic alterations of the heart and the kidney in patients with ankylosing spondylitisEur J Med Res2007121257358118024267
- BulkleyBHRobertsWCAnkylosing spondylitis and aortic regurgitation. Description of the characteristic cardiovascular lesion from study of eight necropsy patientsCirculation1973485101410274751946
- HaroonNNPatersonJMLiPInmanRDHaroonNPatients With Ankylosing Spondylitis Have Increased Cardiovascular and Cerebrovascular Mortality: A Population-Based StudyAnn Intern Med2015163640941626258401
- SzaboSMLevyARRaoSRIncreased risk of cardiovascular and cerebrovascular diseases in individuals with ankylosing spondylitis: a population-based studyArthritis Rheum201163113294330421834064
- PetersMJvan EijkICSmuldersYMSigns of accelerated preclinical atherosclerosis in patients with ankylosing spondylitisJ Rheumatol201037116116619955053
- ZileMRBrutsaertDLNew concepts in diastolic dysfunction and diastolic heart failure: Part II: causal mechanisms and treatmentCirculation2002105121503150811914262
- ChatterjeeKMassieBSystolic and diastolic heart failure: differences and similaritiesJ Card Fail200713756957617826648
- van der LindenSValkenburgHACatsAEvaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteriaArthritis Rheum19842743613686231933
- LangRMBadanoLPMor-AviVRecommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular ImagingJ Am Soc Echocardiogr201528113925559473
- NaguehSFAppletonCPGillebertTCRecommendations for the evaluation of left ventricular diastolic function by echocardiographyJ Am Soc Echocardiogr200922210713319187853
- VahanianAAlfieriOAndreottiFAntunesMJBaron-EsquiviasGBaumgartnerHGuidelines on the management of valvular heart disease (version 2012)Eur Heart J2012332451249622922415
- AslamFBandealiSJKhanNAAlamMDiastolic dysfunction in rheumatoid arthritis: a meta-analysis and systematic reviewArthritis Care Res2013654534543
- ShangQTamLSYipGWHigh prevalence of subclinical left ventricular dysfunction in patients with psoriatic arthritisJ Rheumatol20113871363137021459943
- LeveltEMahmodMPiechnikSKRelationship Between Left Ventricular Structural and Metabolic Remodeling in Type 2 DiabetesDiabetes2016651445226438611
- SeferovićPMPaulusWJClinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypesEur Heart J201536271718172725888006
- AljaroudiWAlraiesMCHalleyCImpact of progression of diastolic dysfunction on mortality in patients with normal ejection fractionCirculation2012125678278822261198
- HalleyCMHoughtalingPLKhalilMKThomasJDJaberWAMortality rate in patients with diastolic dysfunction and normal systolic functionArch Intern Med2011171121082108721709107
- PaulusWJTschöpeCA novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammationJ Am Coll Cardiol201362426327123684677
- MassieBMCarsonPEMcmurrayJJIrbesartan in patients with heart failure and preserved ejection fractionN Engl J Med2008359232456246719001508
- ChouCHLinMCPengCLA nationwide population-based retrospective cohort study: increased risk of acute coronary syndrome in patients with ankylosing spondylitisScand J Rheumatol201443213213624134400
- PetersMJvan HalmVPVoskuylAEDoes rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A prospective studyArthritis Rheum200961111571157919877093
- HanCRobinsonDWHackettMVParamoreLCFraemanKHBalaMVCardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitisJ Rheumatol200633112167217216981296
- WhiteWBCardiovascular effects of the selective cyclooxygenase-2 inhibitorsSubcell Biochem20074214515817612049
- MaxwellLJZochlingJBoonenATNF-alpha inhibitors for ankylosing spondylitisCochrane Database Syst Rev20154CD005468
- PutkoBNWangZLoJCirculating levels of tumor necrosis factor-alpha receptor 2 are increased in heart failure with preserved ejection fraction relative to heart failure with reduced ejection fraction: evidence for a divergence in pathophysiologyPLoS One201496e9949524923671
- van EijkICPetersMJSernéEHMicrovascular function is impaired in ankylosing spondylitis and improves after tumour necrosis factor alpha blockadeAnn Rheum Dis200968336236618390569
- SariIOkanTAkarSImpaired endothelial function in patients with ankylosing spondylitisRheumatology200645328328616204374
- EdelmannFGelbrichGDüngenHDExercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot studyJ Am Coll Cardiol201158171780179121996391
- BrassardPLegaultSGarneauCBogatyPDumesnilJGPoirierPNormalization of diastolic dysfunction in type 2 diabetics after exercise trainingMed Sci Sports Exerc200739111896190117986895