Abstract
Glioma remains the most challenging solid organ tumor to treat successfully. Based on the capacity of stem cells to migrate extensively and target invading glioma cells, the transplantation of stem cells as a cell-based delivery system may provide additional tools for the treatment of gliomas. In addition to the use of modified stem cells for the delivery of therapeutic agents, unmodified stem cells have been shown to have growth-suppressing effects on tumors in vitro and in vivo. This review outlines the probable factors involved in tumor tropism and tumor growth suppression, with a specific focus on the use of unmodified stem cells in the treatment of gliomas. Based on these and further future data, clinical trials may be justified.
Introduction
Glioma is the most common type of human primary brain tumor. Malignant gliomas constitute 22%–27% of all brain tumors.Citation1 In spite of many technological advances in neurosurgery, neuroimaging, radiation therapy, and chemotherapy, the prognosis for patients with malignant gliomas is poor. The highly infiltrative nature of glioma cells is the major cause of their dismal prognosis. The glioma cells migrate from the core mass and produce secondary, microsatellite tumors in normal brain parenchyma.Citation2
Removal of microsatellite tumors by surgery is not feasible, and these tumors are the seeds for recurrent tumor growth.Citation3 Invasiveness is regulated by the interplay between secreted proteases (eg, cathepsins) and their endogenous inhibitors (cystatins). Cystatin E/M is a potent inhibitor of cathepsin B, which is frequently overexpressed in gliomas.Citation4
One of the therapeutic strategies to treat glioma is the eradication of invading glioma cell microsatellite tumors before they develop into recurrent tumors.Citation5 Some reports have shown that neural stem cell (NSC) transplantation may be useful in treating several central nervous system (CNS) diseases or injuries. Several groups have used NSCs to treat tumors that affect the CNS.Citation6–Citation9
The presence of tumor signals may influence the behavior of NSCs by virtue of their inherent migratory and tumor-trophic properties. This ability represents a new and potentially powerful approach in the treatment of invasive tumors and has been used as a delivery vehicle for targeting and disseminating therapeutic gene products throughout tumor sites. NSCs, by infiltrating the tumor mass selectively and aggressively, may help to overcome major obstacles that are being faced by current gene therapy strategies.Citation2,Citation10,Citation11
Brain tumor tropism of NSCs can be used to deliver therapeutic molecules, such as genes, proteins, peptides, or small chemical molecules; however, clinical use of NSCs is limited by both ethical and logistical problems, including their isolation and their immunological compatibility in allogenic transplantation.Citation12,Citation13 Therefore, it is essential to find new sources of easily accessible stem cells with tumor-targeting properties which are also useful for autologous transplantation.
The factors involved in brain tumor tropism of NSCs, and the interactions of NSCs within the tumor environment, are not well known.Citation2 From the perspective of viewing stem cell biology as a means to track and help in the eradication of tumors, we have reviewed the literature to highlight the information on the mechanism of organization, regulation, and function of stem cell tropism and tumor growth suppression in glioma brain tumors. At this point nearly all of the data are limited to in vitro and animal studies and there is no evidence of clinical trials.
Stem cell administration, fate and distribution around gliomas
It has been shown that stem cells exhibit extensive tropism toward tumor sites and infiltrate tumor foci when implanted intraventricularly and intracranially within normal tissue on the side of a lesion, into the contralateral hemisphere, and through the peripheral, intravascular circulation.Citation14–Citation16
Intravascular delivery of stem cells is advantageous because it obviates invasive surgical interventions and because repeated injections over an extended period are clinically feasible. In the study by Nakamizo et al, injection of IFN-β from human mesenchymal stem cells into the internal carotid artery significantly increased survival of animals bearing established intracranial gliomas.Citation15 Interestingly, the authors found that when human mesenchymal stem cells (hMSCs) were injected into tail veins, the majority of stem cells were filtered by the lungs. Brown et al have suggested that intravascular administration of NSCs was an effective delivery vehicle for targeting and disseminating therapeutic agents to invasive tumors of neural and nonneural origin, both within and outside the brain.Citation17 Tang et al showed that most NSCs migrate to the brain tumor through an intraventricular path.Citation16 Based on the study by Kim et al, 50 minutes after contralateral hemispheric NSC injection, around 10% of the NSCs migrated to the tumor region. Five days after injection, the number of NSCs increased slowly, reaching a significant increase by 15 days post-injection. Changes in tumor volume showed similar patterns. The rate of NSC migration was approximately 175 μm/min. In the absence of in vivo tumor cell inoculation, the number of NSCs increased approximately 1.7-fold during day one; however, the proliferation of NSCs began to decrease 5 days after injection.Citation18
Nakamizo et al found that the intratumoral injection of 2.5 × 104 hMSCs did not extend animal survival. Based on this, they concluded that at least 25% of cells (2.5 × 105 hMSCs interferon [IFN]-β) must have integrated into the tumor for a significant increase in animal survival, so intra-arterial injection of at least 1 × 106 hMSCs is needed.Citation15 Although the beneficial effects of different stem cells have been shown in animal models to some extent, the most effective type of stem cell, the more convenient and efficient route of delivery and the optimal number of stem cells in treatment of gliomas have not yet been elucidated and require further research.
Mechanism of stem cell migration toward gliomas
Although the brain is completely formed and integrated a few weeks after birth, it maintains some degree of plasticity throughout life, including axonal remodeling, synaptogenesis, neural cell birth, migration, and integration. The dentate gyrus (DG) of the hippocampus and the subventricular zone (SVZ) are the two main neurogenic niches in the adult brain. Neural stem cells live in these structures and produce progenitors that migrate toward their ultimate locations, including the granular cell layer of the DG and olfactory bulb, respectively.Citation19 However, the vast majority of adult glial progenitors reside outside the neurogenic niches (DG and SVZ). Under normal circumstances, these resident adult progenitors and their glial progeny do not migrate, although they can be stimulated to migrate under pathological conditions.Citation19–Citation21
The role of NSCs in both the physiological and pathological processes in the brain has not been clearly explained. Normal NSCs possess the capability to migrate extensively toward the tumor mass and to linger in and around neoplastic regions of the brain.Citation10 The tropism of NSCs toward brain tumors may provide an additional tool for the treatment of malignant brain tumors. The creation of potential NSC-based therapies has been studied, and this type of therapy involves the delivery of gene products to specific areas of the CNS that can selectively target malignant brain tumor cells and maximize the capability of their delivery.Citation12,Citation14
Many brain tumor behaviors unexpectedly resemble the intrinsic properties of neural stem/progenitor cells.Citation22,Citation23 This has generated recent concern about providing stem cells to help eliminate tumors. There is also concern about the fact that stem cell biology may be somehow integral to the origination and/or production of the neoplasm itself. Yet, based on the unrivalled efficiency of NSCs to migrate throughout the brain and target invasive tumors, the transplantation of NSCs offers a new potential therapeutic approach as a cell-based delivery system for gene therapy in brain tumors. On the one hand, both stem cells and cancer cells are thought to be capable of unlimited proliferation. Yet, on the other hand, many tumors and cancer cell lines express stem cell markers, suggesting that either cancer cells look like stem cells or those cancers contain stem-like cells.Citation22,Citation24,Citation25
During development or after xenograft inoculation, normal NSCs also display high levels of motility throughout the brain.Citation26,Citation27 This property is especially discernible when NSCs are inoculated immediately after injury.Citation8,Citation28 or during tumorigenesis.Citation9,Citation29 Such findings provide evidence that NSCs and brain tumor stem cells may respond to migratory signals in similar ways.Citation22
Cell migration is an important multistep process that leads to organism development, tissue repair, and regeneration. In addition, it drives disease progression in cancer and inflammation.Citation30 Every step of the cell migration process relates to extracellular factors that act on the cell itself through molecular pathways and intracellular signaling cascades.Citation2
In normal brains, secreted proteins, which act as chemoattractants or chemorepellants, coupled with proteins that are implicated in cell–cell or cell–matrix interactions, play pivotal roles in the regulation of neural progenitor cell migration. In addition, recent data suggest that gliomas originate from the transformation of neural stem cells or progenitor cells,Citation24,Citation31–Citation33 and that glioma cell infiltration reiterates key aspects of glial progenitor migration. The factors that are implicated in such cell migrations and recruitments are just beginning to be understood. Many observations show that brain lesions and neurological diseases provoke neural stem/progenitor cell migration toward altered structures, such as tumors. Inflammation, which has long been contemplated as thoroughly devastating to brain repair, is now known to produce some positive effects on stem/progenitor cell recruitment through the regulation of growth factor signaling and the secretion of a number of chemoattractant cytokines. This knowledge is critical for the development of new therapeutic strategies.Citation19
Cytokines, such as vascular endothelial cell growth factors (VEGFs), tumor growth factors (TGFs), epidermal growth factors (EGFs), platelet-derived growth factors (PDGF), monocyte chemoattractant protein-1 (MCP-1), and interleukin-8 (IL-8), which are released from the neoplasm or inflammatory tissues, are all possible candidates for tropism of stem cells.Citation34–Citation36 Mesenchymal cells augment VEGF-induced angiogenesis in vitro.Citation37 It is recognized that the factors released from cancer cells promote the movement of endothelial cell progenitors and stromal cell progenitors from the bone marrow toward the tumorCitation38,Citation39 or tissues surrounding the tumor, which enhances the formation of tumor-stroma.Citation40
Gondi et al have observed that human umbilical cord blood stem cells (hUCBs) show tropism toward glioma cells in vitro, in vivo, and ex vivo. They concluded that this migration relies partially on the expression levels of platelet-derived growth factor-D (PDGF-D) from glioma cells. These investigators have also pointed out that a local concentration gradient of PDGF-D is sufficient to cause migration of hUCBs toward brain slice cultures.Citation41
NSC tumor tropism
NSCs show extensive tropism toward the tumor itself or toward the CNS degeneration. In vitro studies have shown that NSCs did not migrate toward sites where a needle was inserted to imitate tissue damage that takes place during the establishment of a tumor bed, but where the actual implantation of glioblastoma cells did not take place.Citation10 This suggests that the tumor itself possesses at least some of the tropic cues necessary to cause NSC migration. Nonetheless, in other previously reported experimental situations in which significant neuronal death took place.Citation42 NSC differentiation was altered by apparent trophic influences. Therefore, the signals to which the NSCs respond are most likely complex, from multiple sources, and may represent a mixture of attractants, adhesion molecules, substrate molecules and chemokines of broader biological significance. These findings suggest that migration can be unexpectedly extensive, even in an adult brain, and along non-stereotypical routes, if pathology (ie, a tumor) is present.Citation10 Little is known about the signals and factors that influence the tumor tropism of NSCs or their interactions within the tumor environment. It has been speculated that soluble factors, which are over-expressed by tumor cells, may be an important signal for the long-range attraction of NSCs from distant sites.
Some empirical evidence indicates that upregulation of stromal cell-derived factor (SDF-1)Citation43 and VEGFCitation35 serve as soluble chemotactic factors that induce NSC tropism toward gliomas; however, the observation that even microsatellites and infiltrating glioma cells that are distant from the main tumor mass are targeted by NSCs suggests that additional local signals exist that guide NSCs. The migration of glioma cells during invasion is associated with a complex and continuous remodeling of the pre-existing normal extracellular matrix (ECM) of the brain.Citation44 In vitro and in vivo studies have shown that unlike the pre-existing normal ECM, the ECM of gliomas and their migration pathways consist mainly of tenascin, fibronectin, laminin, vitronectin, and different types of collagen.Citation44–Citation47
Laminin, fibronectin, tenascin-C, and collagen I are localized within the basement membrane of existing and newly formed blood vessels.Citation44 These basement membrane proteins, which comprise the reconstituted basement membrane Matrigel™ (BD Biosciences, Franklin Lakes, NJ), allowed the migration of HB1.F3-hNSCs in monolayer migration assays.Citation2
The ECM is extensively modified when gliomas progress and invade the brain. Ziu et alCitation2 analyzed the effects of tumor-ECM compounds from six glioblastoma cell lines on NSC motility. They found that NSC migration was highly dependent on tumor-produced ECM. Laminin and tenascin-C were the most permissive and the strongest inducers of human NSC migration, respectively. Different components of ECM produced by glioma cells positively affect the degree of NSC adhesion and migration. They also suggested that NSC migration is modulated by the ECM of malignant gliomas. These findings showed that the ECM plays a crucial role in NSC migration toward tumor cells, which reinforced the idea that cell migration is a complex process. This is further supported by the fact that the migratory rate of HB1. F3-hNSCs on normal Matrigel was significantly higher than on growth factor-depleted Matrigel.Citation2
HMSC tumor tropism
Nakamizo et al concluded that the cell-specific capacity of hMSCs to localize in human gliomas seems to be an intrinsic property of this cell type. The results from this study indicate that migration of hMSCs toward glioma tumors may be mediated, at least in part, by growth factors and chemokines; however, this group showed that despite the presence of a wide range of growth factors within tumors, there is selectivity of hMSCs for specific factors. For example, whereas fibroblast growth factor (FGF) and VEGF had little effect on hMSC migration, PDGF, EGF, and SDF-1a enhanced hMSC tropism. Moreover, a cocktail of antibodies that block PDGF-BB, EGF, and SDF-1a was able to attenuate the migration of hMSCs toward conditioned medium derived from U87 cells.Citation15
Nakamura et al showed that cultured rat glioma cells stimulate the migration of rat MSCs.Citation48 Soluble factors released from 9 L glioma cells mediated the activation of MSC migration. Cytokines, such as hepatocyte growth factor (HGF),Citation36 VEGFCitation37,Citation49 TGFs,Citation36,Citation37,Citation49,Citation50 FGFs,Citation36 PDGF,Citation36,Citation51 MCP-1, and IL-8,Citation52,Citation53 which are released from the neoplasm or inflammatory tissues, are all possible candidates. These factors, which are secreted from cancer cells, encourage the migration of endothelial cell progenitors and stromal cell progenitors from the bone marrow toward the cancer bed or tissue surrounding the tumor and enhance the formation of tumor-stroma. Similar mechanisms would be expected for the migration of implanted MSCs and tumor-stromal formation in gliomas. Rat MSCs introduced into tumors were basically distributed at the border zones between normal rat brain parenchyma and tumors.Citation48
In vitro Matrigel invasion assays showed that conditioned media from gliomas, but not from fibroblasts or astrocytes, supported the migration of hMSCs, and that PDGF, EGF, or SDF-1a, but not basic FGF or VEGF, enhanced hMSC migration.Citation15
In order to evaluate the capability of hMSCs to track human gliomas, Nakamizo et al injected hMSCs directly into the opposite side of the cerebral hemisphere of an established human glioma and showed that the hMSCs were capable of migrating into the xenograft.Citation15 hMSCs may integrate into glioma tumors to contribute to the mesenchymal elements of the tumor and differentiate into glial and neuronal cells.Citation54,Citation55 Despite the fact that hMSCs may provide a microenvironment that is favorable for tumor growth, they may also have the capacity as a cellular vehicle for delivery of therapeutic agents to glioma tumors. Further explanation of the fundamental mechanism of hMSCs tropism toward gliomas may give insights into methods that can be used to increase the efficiency of the engraftment process.Citation15
Modified stem cell therapy
By using genetically modified stem cells to secrete anti-neoplastic compounds, it may be possible to achieve high levels of one or more chemotherapeutic agents at the site of a tumor.
Different approaches are being undertaken by several researchers, all of whom are focusing on a variety of potentially therapeutic genes that could exert better efficacy with fewer side effects when expressed in close proximity to the tumor mass. Antineoplastic compounds have been divided into three categories, including the prodrug-converting enzymes, viral vectors, and immune-response modulators.Citation14
Genetic strategies are also being developed to deliver genetically engineered NSCs to the sites of brain tumors. Candidate genes include those that encode proteins that induce differentiation of neoplastic cells and/or their signal transduction mediators, cell cycle modulators, apoptosis-promoting agents, antiangiogenesis factors, immune-enhancing agents, and oncolytic factors.Citation10,Citation56
Lee et al evaluated the therapeutic efficacy of genetically modified NSCs encoding cytosine deaminase (CD) and IFN-β, a proinflammatory cytokine gene, in treating brain stem gliomas. They added 5-fluorocytosine to kill dividing cells, including human NSCs encoding CD and IFN-β. Histological analyses showed a 59% reduction in tumor volume in the treated group, and apoptosis was 2.33-fold higher in the treated group than in the control group.Citation57 The therapeutic actions of CD and IFN-β are different. CD acts as prodrug-activating enzyme and IFN-β has the antiangiogenic effect and immune response.Citation58 Nearly the same gene therapy with human NSCs had significant therapeutic benefit in experimental gliomas.Citation59 In another study, NSCs were modified to produce CD. Administration of these NSCs caused an 80% reduction in tumor masses when animals were treated with the systemic prodrug 5-fluorocytosine.Citation17
Antitumor effects of intracranial administration of gene modified NSC expressing IL-4,Citation12 IL-12,Citation7 or tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)Citation6 have also been reported.
Gene modification of MSCs by infection with an adenoviral vector encoding human IL-2 clearly augmented the antitumor effect and further prolonged the survival of tumor-bearing rats.Citation48
In experimental glioma models, Nakamizo et al found that hMSCs that were engineered to produce IFN-β would provide a high degree of local intratumoral delivery, with a limited degree of systemic toxicity. They used an adenoviral vector to transfer the IFN-β gene into hMSCs and found that these engineered hMSCs (hMSC IFN-β) released high levels of IFN-β and were capable of directly killing human glioma cell lines that were grown in vitro. These studies provided the proof of principle that hMSCs can be engineered to release a soluble factor into brain tumors. The authors suggested that IFN-β is itself a good therapeutic agent worthy of assessment in patients with gliomas. The same approach can be exploited in the delivery of other agents with antitumor activity. Methods to maximize transfection of therapeutic genes to hMSCs and to separate transfected from nontransfected cells are challenges for the ultimate application of this and other stem cell approaches to treating tumors.Citation15 Although their studies have focused on bone marrow-derived hMSCs, Lee et al have suggested that other cells in the bone marrow may also be useful as delivery vehicles for brain tumors.Citation29
Using live imaging and tumor measurements, Goren et al reported that encapsulated hMSC-PEX (Pexin) injected next to glioblastoma tumors in nude mice significantly reduced tumor volume (87%) and weight (83%). The authors clearly demonstrated that hMSCs are the best cell type for microencapsulation cell-based therapy, which brought this technology closer to clinical application.Citation60
Germano et al showed that modified embryonic stem cells expressing transgenic hTRAIL induced apoptosis in human malignant glioma cells while sparing normal cells.Citation61
All of the above observations imply that modified stem cells carrying therapeutic genes may successfully suppress the growth of glioma tumors.
Unmodified stem cell therapy
NSCs have been shown to mobilize and juxtapose themselves along aggressively advancing tumor cells.Citation10 Glass et al demonstrated that endogenous neural precursors migrated from the SVZ toward a tumor mass and surrounded the bulk of the tumor in an established intracranial glioma mouse model. Furthermore, endogenous neural precursor cells did not manifest a pathotropic movement when implanted with other non-neoplastic lesions, which suggests a specific tropism toward brain tumors. Unmodified NSCs that were cocultured with glioblastoma multiform cells showed both a suppression of tumor growth and an induction of tumor cell apoptosis in mice, which improved their survival.Citation62 Another previous study has provided evidence of the antitumor activity of NSCs and showed their migration toward tumor cells.Citation63
Staflin et al showed that transplanted neural progenitor cells respond to queues from a tumor, home to, and exert an antitumor effect on the pre-established glioma. Transplanted NSCs significantly decreased the tumor volume by approximately 67% compared to untreated, control tumors after 1 to 2 weeks. Furthermore, these early effects could be translated into increased survival times of the animals treated with neural progenitor cell grafts 3 days after intrastriatal tumor injection. In contrast, there was no activation or migration of endogenous SVZ neuroblasts in response to intrastriatal syngeneic tumors. They concluded that neuronal precursor cells possess the ability to influence tumor growth and respond to queues from the tumor or tumor microenvironment, thereby demonstrating cross-talk between the cells.Citation64
To evaluate whether the increased survival that was observed after inoculation of MSC-IL2 or MSC was associated with the inhibition of tumor growth, Nakamura et al monitored tumor growth volume by magnetic resonance imaging (MRI) every 7 days after intracranial injection of tumors. The 9 L glioma was clearly visible as an enhanced area in the coronal section of the brain. Fourteen days after tumor inoculation, glioma growth progression was observed in the brain of untreated rats and reached a lethal volume. In contrast, significantly smaller tumor volumes were present in brains of animals treated with MSC-IL2 or unmodified MSCs (P < 0.01, compared with untreated controls 14 days after tumor inoculation). A significant difference in tumor volume was not observed between the groups treated with unmodified MSCs and MSCs-IL2s by day 14; however, the therapeutic effect of MSCs expressing IL-2 was clearly visible by MRI 21 days after tumor injection. At this time, tumors treated with unmodified MSCs had reached near-lethal volumes, but those tumors treated with IL-2-expressing MSCs resulted in smaller tumors. The observed changes in glioma tumor volume were consistent with the survival durations in the different treatment groups. The prolonged survival in glioma-bearing rats treated in this way might depend on a direct antitumor effect of the MSCs themselves.Citation48
It has been reported that in the cerebral infarction model, implanted MSCs mediate neural protection through the inhibition of neuronal apoptosis, and this protective effect is thought to be due to neurotrophic factors, such as nerve growth factor (NGF), which are released from MSCs.Citation65 The protective effect of MSCs on normal brain parenchyma may also contribute to prolonged survival of glioma-bearing rats treated by MSC implantation.Citation66
MSCs generate several neurotrophic factors, including NGF, which can induce differentiation and growth inhibition of rat glioma cells in vitro.Citation48,Citation67 Kang et al demonstrated another cytotoxic mechanism of rat MSCs, which involved the differentiation of rat MSCs into immune effector cells;Citation68 however, differentiation of hUCB-derived MSCs into immune effector cells has not been demonstrated, although a variety of cytokines could activate these cells.Citation69
MSCs have been found to secrete large amounts of angiogenic factors, such as angiopoietin-1 (Ang1). Ang1 inhibits tumor-vascular leakage and tumor growth in vivo.Citation70 Ang1 released from MSCs influences the antitumor effects of MSCs. Ang1 may also protect brain parenchyma from lethal cerebral edema via suppression of vascular leakage. MSCs mainly localize between the edge of the tumor and normal parenchyma and make a capsule-like structure. This capsule-like structure of MSCs may act as a barrier that prevents the spread of glioma cells into normal parenchyma. Thus, implantation of MSCs may be beneficial for the treatment of gliomas both because of their antitumor effects and their protective effects on normal brain tissue.Citation66
Pisati et al evaluated tumor targeting and anti-tumor activity of human skin-derived stem cells (hSDSCs). This group showed that when hSDSCs were injected directly into glioblastomas in mice, the hSDSCs distributed themselves throughout the tumor mass and reduced tumor vessel density and angiogenic sprouts. The hSDSCs also differentiated into pericyte cells and produced high levels of human actor-β1 with low expression of VEGF, all of which may decrease tumor growth and prolong animal survival.Citation67
The ability of hUCBs to inhibit established intracranial tumors was observed by Gondi et al The results of this study demonstrated that hUCBs are capable of inducing apoptosis in human glioma cells.Citation34,Citation41
Endogenous stem cells
Many observations have shown that brain lesions and neurological diseases trigger neural stem/progenitor cell activation and migration toward the pathological structures. Normal NSCs exhibit a high degree of motility throughout the brain after xenograft injection.Citation26,Citation27 In fact, the results of one study indicate that endogenous precursor cells are attracted by tumor cells, the presence of precursor cells is anti-tumorigenic, and this cellular interaction decreases with age.Citation62
The factors implicated in such cell migrations and recruitments are just beginning to be understood. Some factors have been reported to enhance stem cell migration toward glioblastoma cells, such as transmembrane protein 18, MCP-1, MIP-1, and IL-8.Citation50,Citation71 These factors and ischemic cerebral tissue enhance human bone marrow stromal cell migration in interface culture.Citation50,Citation53
A recombinant human TGF-β1 fusion protein with a collagen-binding domain promotes migration, growth, and differentiation of bone marrow mesenchymal cells.Citation50
It has long been considered that inflammation is largely devastating for brain repair, and it is now known to produce some positive effects on stem/progenitor cell recruitment via the regulation of growth factor signaling and the secretion of a number of chemoattractant cytokines. This knowledge is crucial for the development of new therapeutic strategies. One of these strategies could consist of increasing the mobilization of endogenous progenitor cells that could replace lost cells and improve functional recovery. It is not yet known why newly added neurons do not originate directly in the place in which they need to be located. Progenitor cell migration may provide an additional level of control for cell positioning, and the preservation of stem cell niches also represents a potential source of cells for brain repair. Yet, this may be costly for the organism, and it also requires specific features that restrict the structures to locations where they can persist. This idea implies that cells need to be able to migrate from these discrete niches to their final destinations.Citation19
Concluding remarks
Summary
In this review, we concentrated on stem cell migration toward glioma tumors and provided some suggested mechanisms by which these stem cells may suppress tumor growth. Brain lesions, tumors, and neurological diseases trigger migration of neural stem cell/progenitor cells toward these altered structures. An understanding of the factors involved in to such cell migration events is a necessary step in delineating the critical pathways that control NSC tropism. The factors that are involved in migration of NSCs can be divided into two main groups: chemoattractant cytokines, including HGF, EGF, VEGF, TGF, FGF, PDGF, MPC-1, IL-8, IL-4, and SDF-1aCitation15,Citation35–Citation37,Citation41,Citation43,Citation49,Citation50,Citation72 and extracellular matrix compounds, such as tenascin, fibronectin, laminin, vitronectin, various types of collagen, and hyaluronic acid ().Citation2,Citation44–Citation47,Citation73,Citation74
Figure 1 Stem cell migration is related to two groups of inducing factors of chemo-attractant cytokines and extracellular matrix.
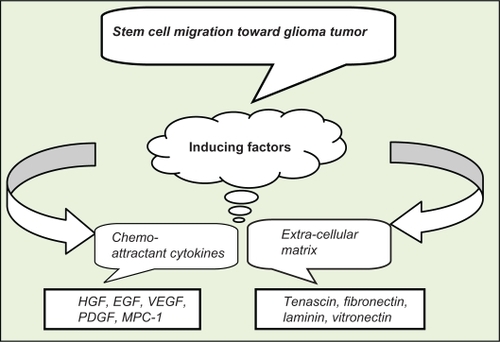
It seems that there is selectivity of hMSCs for specific factors. For example, FGF and VEGF have little effect on hMSC migration, but PDGF, EGF, and SDF-1a enhance hMSC tropism.Citation15
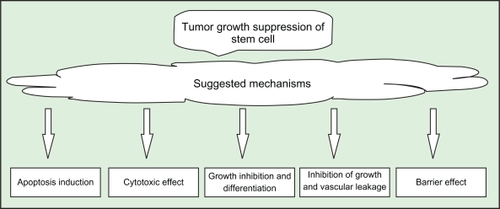
While the mechanisms by which modified stem cells suppress tumor growth are more often studied (see Achanta et alCitation14), the precise mechanisms by which unmodified stem cells suppress tumor growth are not completely understood. We suppose that biochemical immunological and physical effects are responsible for growth inhibition of glioma tumor. A few mechanisms are suggested in some reports, including the following ():
Differentiation and growth inhibition of glioma cells in vitro, which may be due to the production of NGF by the MSCs.Citation48,Citation65,Citation75
A cytotoxic mechanism that involves the differentiation of rat MSCs into immune effector cells.Citation68,Citation69
Apoptosis, in which hUCBs may be capable of inducing apoptosis in human glioma cells.Citation34,Citation41,Citation76
Inhibition of tumor growth and vascular leakage, which may be caused by the production of Ang1.Citation70 Ang1 may also protect the brain parenchyma from lethal cerebral edema by reducing vascular leakage.Citation48,Citation70 Ang1 inhibits tumor-vascular leakage and tumor growth in vivo.Citation70
Formation of a barrier in which MSCs may prevent the spread of glioma cells into normal parenchyma.Citation48
Future insights
An increasing knowledge about the factors that are necessary for stem cell survival, attraction, and their interactions with the tumor environment is necessary for the development of rational therapeutic strategies using stem cell technology.Citation2 Some of the following issues should be considered in further studies of cell-based therapies for the treatment of tumors:
Stem cell type: we are currently performing the experiments necessary to determine whether there is any difference in the potency of tumor growth suppression and tropism among multiple types of stem cells.
Factors that induce stem cell migration: different factors may have varying effects on the tropism of different types of stem cells.
Timing of the effect: there was no significant difference in tumor volume between groups treated with unmodified MSCs and IL-2-expressing MSCs after 14 days of treatment. The therapeutic effect of the IL-2 gene modification was, however, clearly visible by MRI 21 days after tumor inoculation.Citation15
Route of administration: importantly, regional delivery of IFN-β-expressing hMSCs by injection into the internal carotid artery significantly extended the survival of animals harboring established intracranial gliomas. Conversely, intravenous injection of IFN-β did not extend animal survival.Citation15
Dose dependency of antitumor effects: the most appropriate dose of stem cells for treatment of tumors remains unknown and needs to be studied. Nakamizo et al estimate that at least 25% of the cells (2.5 × 105) must have integrated into the tumor for a significant effect to be observed on tumor growth suppression.Citation15
Endogenous production of stem cells: considering that normal stem cells exhibit a high degree of motility throughout the brain, and that tumor cells attract endogenous precursor cells, this innate behavior of endogenous NSCs may be helpful for suppressing tumor growth. If this is true, enhancing the endogenous production of stem cells may be a considerable issue for further studies.
Neutralization of the growth-inhibitory components of endogenous neuronal stem cells: this may help to increase the capacity of attraction.
Microenvironmental changes: chemical,Citation77 physical, and mechanical interactions can affect the ECM, and cell growth and differentiation can be normalized by modulating cell adhesion to the ECM. Embryonic tissues may reverse cancerous growth by restoring these normal microenvironmental cues,Citation78 and it is possible that the migration of either endogenous or exogenous stem cells toward gliomas may change the microenvironment and suppress tumor growth.
Acknowledgements
The authors gratefully acknowledge Mrs Jamileh Mahdavi, Dr Seyed Pouyan Jalali and Dr Mohammad Eskandari for their editorial assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
- SurawiczTSMcCarthyBJKupelianVJukichPJBrunerJMDavisFGDescriptive epidemiology of primary brain and CNS tumors: results from the Central Brain Tumor Registry of the United States, 1990–1994Neuro Oncol199911142511554386
- ZiuMSchmidtNOCargioliTGAboodyKSBlackPMCarrollRSGlioma-produced extracellular matrix influences brain tumor tropism of human neural stem cellsJ Neurooncol200679212513316598423
- GieseABjerkvigRBerensMEWestphalMCost of migration: invasion of malignant gliomas and implications for treatmentJ Clin Oncol 1520032181624163612697889
- QiuJAiLRamachandranCInvasion suppressor cystatin E/M (CST6): high-level cell type-specific expression in normal brain and epigenetic silencing in gliomasLab Invest200888991092518607344
- DunnIFBlackPMThe neurosurgeon as local oncologist: cellular and molecular neurosurgery in malignant glioma therapyNeurosurgery200352614111422 discussion 1422–1414.12762886
- EhteshamMKabosPGutierrezMAInduction of glioblastoma apoptosis using neural stem cell-mediated delivery of tumor necrosis factor-related apoptosis-inducing ligandCancer Res200262247170717412499252
- EhteshamMKabosPKabosovaANeumanTBlackKLYuJSThe use of interleukin 12-secreting neural stem cells for the treatment of intracranial gliomaCancer Res200262205657566312384520
- HayashiTIwaiMIkedaTNeural precursor cells division and migration in neonatal rat brain after ischemic/hypoxic injuryBrain Res200510381414915748871
- LiSGaoYTokuyamaTGenetically engineered neural stem cells migrate and suppress glioma cell growth at distant intracranial sitesCancer Lett2007251222022717196326
- AboodyKSBrownARainovNGNeural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomasProc Natl Acad Sci U S A20009723128461285111070094
- KimSUGenetically engineered human neural stem cells for brain repair in neurological diseasesBrain Dev200729419320117303360
- BenedettiSPirolaBPolloBGene therapy of experimental brain tumors using neural progenitor cellsNat Med20006444745010742153
- BosmanFTStamenkovicIFunctional structure and composition of the extracellular matrixJ Pathol2003200442342812845610
- AchantaPSedora RomanNIQuinones-HinojosaAGliomagenesis and the use of neural stem cells in brain tumor treatmentAnticancer Agents Med Chem201010212113020184546
- NakamizoAMariniFAmanoTHuman bone marrow-derived mesenchymal stem cells in the treatment of gliomasCancer Res20056583307331815833864
- TangYShahKMesserliSMSnyderEBreakefieldXWeisslederRIn vivo tracking of neural progenitor cell migration to glioblastomasHum Gene Ther200314131247125412952596
- BrownABYangWSchmidtNOIntravascular delivery of neural stem cell lines to target intracranial and extracranial tumors of neural and non-neural originHum Gene Ther200314181777178514670128
- KimJHLeeJEKimSUChoKGStereological analysis on migration of human neural stem cells in the brain of rats bearing gliomaNeurosurgery2010662333342 discussion 342.20087133
- CayreMCanollPGoldmanJECell migration in the normal and pathological postnatal mammalian brainProg Neurobiol2009881416319428961
- GohEMaDMingGSongHAdult neural stem cells and repair of the adult central nervous systemJ Hematother Stem Cell Res200312667167914977476
- MaDBonaguidiMMingGSongHAdult neural stem cells in the mammalian central nervous systemCell research200919667268219436263
- ColleoniFTorrenteYThe new challenge of stem cell: brain tumour therapyCancer Lett2008272111118621474
- VescoviALGalliRReynoldsBABrain tumour stem cellsNat Rev Cancer20066642543616723989
- FomchenkoEIHollandECStem cells and brain cancerExp Cell Res2005306232332915925587
- GalderisiUCipollaroMGiordanoAStem cells and brain cancerCell Death Differ200613151116123777
- DirksPBGlioma migration: clues from the biology of neural progenitor cells and embryonic CNS cell migrationJ Neurooncol200153220321211716071
- WatsonDJWaltonRMMagnitskySGBulteJWPoptaniHWolfeJHStructure-specific patterns of neural stem cell engraftment after transplantation in the adult mouse brainHum Gene Ther200617769370416839269
- ChangYCShyuWCLinSZLiHRegenerative therapy for strokeCell Transplant200716217118117474298
- LeeJElkahlounAGMessinaSACellular and genetic characterization of human adult bone marrow-derived neural stem-like cells: a potential antiglioma cellular vectorCancer Res200363248877888914695205
- RidleyAJSchwartzMABurridgeKCell migration: integrating signals from front to backScience200330256511704170914657486
- PanagiotakosGTabarVBrain tumor stem cellsCurr Neurol Neurosci Rep20077321522017488587
- ShirasAChettiarSTShepalVRajendranGPrasadGRShastryPSpontaneous transformation of human adult nontumorigenic stem cells to cancer stem cells is driven by genomic instability in a human model of glioblastomaStem Cells20072561478148917332509
- XieZBrain tumor stem cellsNeurochem Res200934122055206619856100
- GondiCSGogineniVRChettyCInduction of apoptosis in glioma cells requires cell-to-cell contact with human umbilical cord blood stem cellsInt J Oncol20103651165117320372790
- SchmidtNPrzyleckiWYangWBrain tumor tropism of transplanted human neural stem cells is induced by vascular endothelial growth factorNeoplasia (New York, NY)200576623
- YamamotoSWakimotoHAoyagiMHirakawaKHamadaHModulation of motility and proliferation of glioma cells by hepatocyte growth factorJpn J Cancer Res19978865645779263534
- TilleJCPepperMSMesenchymal cells potentiate vascular endothelial growth factor-induced angiogenesis in vitroExp Cell Res2002280217919112413884
- CoussensLMWerbZInflammation and cancerNature2002420691786086712490959
- De PalmaMVenneriMARocaCNaldiniLTargeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cellsNat Med20039678979512740570
- WeaverVMFischerAHPetersonOWBissellMJThe importance of the microenvironment in breast cancer progression: recapitulation of mammary tumorigenesis using a unique human mammary epithelial cell model and a three-dimensional culture assayBiochem Cell Biol19967468338519164652
- GondiCSVeeravalliKKGorantlaBHuman umbilical cord blood stem cells show PDGF-D-dependent glioma cell tropism in vitro and in vivoNeuro Oncol201012545346520406896
- SnyderEYoonCFlaxJMacklisJMultipotent neural precursors can differentiate toward replacement of neurons undergoing targeted apoptotic degeneration in adult mouse neocortexProc Natl Acad Sci U S A19979421116639326667
- EhteshamMYuanXKabosPGlioma tropic neural stem cells consist of astrocytic precursors and their migratory capacity is mediated by CXCR4Neoplasia20046328729315153341
- GieseAWestphalMGlioma invasion in the central nervous systemNeurosurgery1996392235250 discussion 250–232.8832660
- BellailACHunterSBBratDJTanCVan MeirEGMicroregional extracellular matrix heterogeneity in brain modulates glioma cell invasionInt J Biochem Cell Biol20043661046106915094120
- FriedlanderDRZagzagDShiffBMigration of brain tumor cells on extracellular matrix proteins in vitro correlates with tumor type and grade and involves alphaV and beta1 integrinsCancer Res1996568193919478620517
- MahesparanRReadTALund-JohansenMSkaftnesmoKOBjerkvigREngebraatenOExpression of extracellular matrix components in a highly infiltrative in vivo glioma modelActa Neuropathol20031051495712471461
- NakamuraKItoYKawanoYAntitumor effect of genetically engineered mesenchymal stem cells in a rat glioma modelGene Ther200411141155116415141157
- SchichorCBirnbaumTEtminanNVascular endothelial growth factor A contributes to glioma-induced migration of human marrow stromal cells (hMSC)Exp Neurol2006199230131016574102
- AndradesJAHanBBecerraJSorgenteNHallFLNimniMEA recombinant human TGF-beta1 fusion protein with collagen-binding domain promotes migration, growth, and differentiation of bone marrow mesenchymal cellsExp Cell Res1999250248549810413602
- YuJUstachCKimHRPlatelet-derived growth factor signaling and human cancerJ Biochem Mol Biol2003361495912542975
- WangLLiYChenJIschemic cerebral tissue and MCP-1 enhance rat bone marrow stromal cell migration in interface cultureExp Hematol200230783183612135683
- WangLLiYChenXMCP-1, MIP-1, IL-8 and ischemic cerebral tissue enhance human bone marrow stromal cell migration in interface cultureHematology20027211311712186702
- KopenGCProckopDJPhinneyDGMarrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brainsProc Natl Acad Sci U S A19999619107111071610485891
- WoodburyDSchwarzEJProckopDJBlackIBAdult rat and human bone marrow stromal cells differentiate into neuronsJ Neurosci Res200061436437010931522
- YaoKKomataTKondoYKanzawaTKondoSGermanoIMolecular response of human glioblastoma multiforme cells to ionizing radiation: cell cycle arrest, modulation of cyclin-dependent kinase inhibitors, and autophagyJ Neurosurg200398237838412593626
- LeeDHAhnYKimSUTargeting rat brainstem glioma using human neural stem cells and human mesenchymal stem cellsClin Cancer Res200915154925493419638465
- KimSKKimSUParkIHHuman neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regressionClin Cancer Res200612185550555617000692
- ItoSNatsumeAShimatoSHuman neural stem cells transduced with IFN-beta and cytosine deaminase genes intensify bystander effect in experimental gliomaCancer Gene Ther201017529930619893595
- GorenADahanNGorenEBaruchLMachlufMEncapsulated human mesenchymal stem cells: a unique hypoimmunogenic platform for long-term cellular therapyFaseb J2010241223119726759
- GermanoIMUzzamanMBenvenisteRJZaurovaMKellerGApoptosis in human glioblastoma cells produced using embryonic stem cell-derived astrocytes expressing tumor necrosis factor-related apoptosis-inducing ligandJ Neurosurg20061051889516871882
- GlassRSynowitzMKronenbergGGlioblastoma-induced attraction of endogenous neural precursor cells is associated with improved survivalJ Neurosci200525102637264615758174
- StaflinKHonethGKalliomakiSKjellmanCEdvardsenKLindvallMNeural progenitor cell lines inhibit rat tumor growth in vivoCancer Res200464155347535415289341
- StaflinKLindvallMZuchnerTLundbergCInstructive cross-talk between neural progenitor cells and gliomasJ Neurosci Res200785102147215917526014
- LiYChenJChenXGHuman marrow stromal cell therapy for stroke in rat: neurotrophins and functional recoveryNeurology200259451452312196642
- HamadaHKobuneMNakamuraKMesenchymal stem cells (MSC) as therapeutic cytoreagents for gene therapyCancer Sci200596314915615771617
- PisatiFBelicchiMAcerbiFEffect of human skin-derived stem cells on vessel architecture, tumor growth, and tumor invasion in brain tumor animal modelsCancer Res20076773054306317409412
- KangSGJeunSSLimJYCytotoxicity of rat marrow stromal cells against malignant glioma cellsChilds Nerv Syst200521752853815933882
- KangSGJeunSSLimJYCytotoxicity of human umbilical cord blood-derived mesenchymal stem cells against human malignant glioma cellsChilds Nerv Syst200824329330217968556
- StoeltzingOAhmadSALiuWAngiopoietin-1 inhibits vascular permeability, angiogenesis, and growth of hepatic colon cancer tumorsCancer Res200363123370337712810673
- JurvansuuJZhaoYLeungDSTransmembrane protein 18 enhances the tropism of neural stem cells for glioma cellsCancer Res200868124614462218559506
- RempelSADudasSGeSGutierrezJAIdentification and localization of the cytokine SDF1 and its receptor, CXC chemokine receptor 4, to regions of necrosis and angiogenesis in human glioblastomaClin Cancer Res20006110211110656438
- OhnishiTHiragaSIzumotoSRole of fibronectin-stimulated tumor cell migration in glioma invasion in vivo: clinical significance of fibronectin and fibronectin receptor expressed in human glioma tissuesClin Exp Metastasis199816872974110211986
- SobeihMMCorfasGExtracellular factors that regulate neuronal migration in the central nervous systemInt J Dev Neurosci2002203–534935712175873
- KimuraSYoshinoAKatayamaYWatanabeTFukushimaTGrowth control of C6 glioma in vivo by nerve growth factorJ Neurooncol200259319920512241115
- DasariVRVelpulaKKKaurKCord blood stem cell-mediated induction of apoptosis in glioma downregulates X-linked inhibitor of apoptosis protein (XIAP)PLoS One201057e1181320676365
- CharlesNOzawaTSquatritoMPerivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cellsCell Stem Cell20106214115220144787
- IngberDCan cancer be reversed by engineering the tumor microenvironment?2008