Abstract
Cancer progression is a highly complex process that is driven by a constellation of deregulated signaling pathways and key molecular events. In non-small-cell lung cancer (NSCLC), as in several other cancer types, the epidermal growth factor receptor (EGFR) and its downstream signaling components represent a key axis that has been found not only to trigger cancer progression but also to support advanced disease leading to metastasis. Two major therapeutic approaches comprising monoclonal antibodies and small molecule tyrosine kinase inhibitors have so far been used to target this pathway, with a combination of positive, negative, and inconsequential results, as judged by patient survival indices. Since these drugs are expensive and not all patients derive benefits from taking them, it has become both pertinent and paramount to identify biomarkers that can predict not only beneficial response but also resistance. This review focuses on the chimeric monoclonal antibody, cetuximab, its application in the treatment of NSCLC, and the biomarkers that may guide its use in the clinical setting. A special emphasis is placed on the EGFR, including its structural and mechanistic attributes.
Statistical data made available by the World Health Organization show that lung cancers are the leading cause of cancer-related mortality in both males and females worldwide.Citation1,Citation2 Non-small-cell lung cancer (NSCLC) is one of the two major subtypes and accounts for over 85% of cases.Citation3 Lung cancer has been epidemiologically associated largely with cigarette smoking,Citation4 but lifestyle, diet, passive smoking, and occupational exposure have all been found to play contributory roles.Citation5–Citation8 Although early stage cancer is curable, over 70% of patients present with locally advanced or metastatic disease at the time of diagnosis.Citation9 Chemotherapy has been shown to be beneficial in patients with resected early disease and remains the backbone of therapy for patients with advanced disease, but it is notorious for its side effects,Citation10 and the overall 5-year survival rate is still under 15%.Citation11 Recently, the identification of drugs able to hit molecular targets has been a refreshing addition to the arsenal of tools that can be used against lung cancer.Citation12 One such target is the epidermal growth factor receptor (EGFR),which is overexpressed in up to 80% of patients with NSCLC.Citation13 EGFR expression in resected patient tissues has been correlated with advanced disease and poor survival.Citation14–Citation16 Because EGFR is overexpressed in lung cancer, it thus becomes a logical drug target; therefore, two classes of inhibitors, including monoclonal antibodies and small molecular inhibitors against EGFR, have been developed.Citation17,Citation18
Epidermal growth factor receptor
The EGFR is a cell surface glycoprotein receptor belonging to the ErbB family of proteins, a subfamily of four closely related proteins comprising EGFR (ErbB-1), HER2/c-neu (ErbB-2), Her 3 (ErbB-3), and Her 4 (ErbB-4).Citation19 The extracellular domain of EGFR has four distinct subdomains consisting of two sets of tandem repeats that compose the ligand binding sites.Citation20,Citation21 In the absence of any ligand, the extracellular domain appears to be locked into an auto inhibitory configuration.Citation20 Scatchard analysis indicated that the receptor possesses two different binding affinities; however, the mechanisms regulating the different binding affinities still remain obscure.Citation22
EGFR has a single transmembrane domain consisting of 23 amino acids that attach the receptor to the membrane and function in receptor dimerization.Citation23 Furthermore, this domain also promotes dimer formation when attached to the extracellular portion,Citation24 and mutations in the HER2/c-neu transmembrane domain have been demonstrated to favor its dimerization.Citation25 Moreover, exposure to a peptide corresponding to the transmembrane domain of EGFR results in the inhibition of receptor autophosphorylation and downstream signaling events.Citation26 It is highly likely that the transmembrane domain plays a part in the alignment of the intracellular kinase domain upon dimerization.Citation27,Citation28
The intracellular domain consists primarily of the tyrosine kinase domain (approximately 260 amino acids), a juxtamembrane region (approximately 40 amino acids), and a carboxyl terminal regulatory region domain (approximately 232 amino acids).Citation29 Numerous autophosphorylation sites are located on the intracellular domain of the receptor, which acts as an anchor for SH2 (Src homology 2) domain proteins.Citation30 Analysis of its crystal structure indicated that the tyrosine kinase remains in a constitutively active configuration but is not accessible because of the regulatory region at the carboxy terminus.Citation31,Citation32 Ligand binding enhances the proximal alignment of the receptor, leading to trans-phosphorylation tyrosine residues favoring conformational changes that remove inhibitory constrains on the kinase domain.Citation33 With this configuration, EGFR is now able to phosphorylate tyrosine residues on other molecules, including phospholipase C γ,Citation34 cytoskeletal-associated proteinsCitation35 and protein 4.1.Citation36 Moreover, residues including serine, threonine, and even tyrosine can be phosphorylated by nonreceptor kinases.Citation37,Citation38 These sites are phosphorylated by downstream kinases that are part of the EGFR activation cascade,Citation30 which includes protein kinase C,Citation39 Src,Citation40 and PKA.Citation41 Furthermore, phosphotyrosines can act as anchor sites for various proteins that contain SH-2 or phosphotyrosine binding domains, and they thus act as a recruitment hub for members of various cell signaling cascades.Citation30 It is important to stress that EGFR can also copartner with other receptors outside its family and mediate signal transduction. Several studies indicated that the EGFR transactivation mechanism is subject to different regulatory influences.Citation42,Citation43
EGFR signaling
The binding of EGF to its receptor initiates a mitogenic signaling cascade via several pathways, including the Ras/Raf mitogen-activated protein kinase (MAPK), phosphatidylinositol 3 kinase (PI3K)-Akt, JNK, and the Stat3/Stat5 pathways as shown in .Citation19,Citation44,Citation45 These pathways converge to enhance DNA synthesis, cell proliferation, angiogenesis, invasion, and metastasis.Citation19,Citation44,Citation45 Activation of the EGFR pathway orchestrates increased autophosphorylation of tyrosine kinases, which leads to a series of intracellular events culminating in enhanced metastatic propensities and angiogenesis.Citation46
Figure 1 The EGFR signaling cascade.
Abbreviation: EGFR, epidermal growth factor receptor.
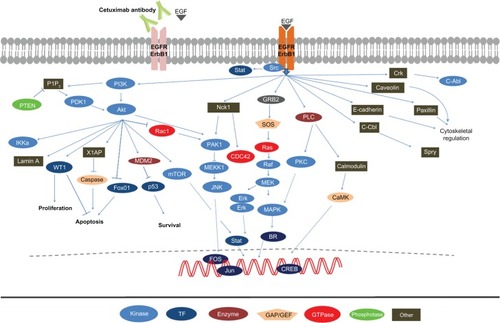
The RAS proto-oncogene family (K-ras, H-ras, N-ras and R-ras) encode four highly homologous 21 kDa membrane bound proteins involved in signal transduction. These proteins exist in either an active state bound to guanosine triphosphate (GTP) or an inactive state bound to guanosine diphosphate (GDP).Citation47 Activating point mutations confer oncogenic potential through a loss of an intrinsic GTPase activity, resulting in an inability to cleave GTP to GDP and culminating in unabated cell proliferation downstream of EGF signaling.Citation48 Activating RAS mutations occur in approximately 15% to 20% of NSCLCs, and in lung cancer, 90% of these mutations are located in the K-ras gene (80% in codon 12, and the remainder in codons 13 and 61), with H-ras and N-ras mutations only occasionally documented.Citation49 K-ras mutations are mutually exclusive in EGFR and Erbb2 mutations and confer resistance to EGFR, tyrosine kinase inhibitors (TKIs), and chemotherapy.Citation50,Citation51 Moreover, whereas K-ras mutations are primarily observed in lung adenocarcinomas of smokers, EGFR mutations are primarily observed in lung adenocarcinomas of neversmokers.Citation52,Citation53
The PI3K/Akt pathway is a key regulator of cell growth, proliferation, and survival. It is commonly activated in lung cancer by changes in several of its components including PI3K, PTEN, Akt, EGFR, and K-ras.Citation54 In lung cancer, activation of PI3K/Akt is considered a relatively early event and results in cell survival through an inhibition of apoptosis. This activation occurs either through the binding of the SH-2 domain of P-85 to phosphotyrosine residues of activated receptor tyrosine kinase or more commonly through the amplification of PIK3CA, which encodes the catalytic subunits of PI3K.Citation54,Citation55 Akt, a serine/threonine kinase acting downstream of PI3K, can also have mutations that lead to pathway inactivation. In addition, PTEN regulates the PI3K/Akt pathway via phosphatase activity on phosphatidylinositol 3,4,5 triphosphate (PIP3), which is commonly suppressed in lung cancer by inactivating mutations or loss of expression.Citation56,Citation57
Signal transducers and activators of transcription (STAT) are cytoplasmic transcription factors that, upon activation, translocate into the nucleus where they mediate gene expression of several downstream targets. STAT signaling is a key intrinsic pathway for cancer inflammation and is activated in tumor cells, often resulting in the induction of inflammation-associated genes.Citation58 To date, seven STAT proteins (1–6) have been identified in mammalian cells.Citation59 STAT proteins are important in establishing immune responses in the tumor microenvironment to promote or inhibit cancer progression. In addition, sustained activation of STAT3, and to some extent STAT5, leads to increased tumor cell proliferation, survival, and invasion.Citation58,Citation60 STAT3 is activated by growth factor receptor tyrosine kinases, such as EGFR and platelet-derived growth factor receptors, as well as nonreceptor tyrosine kinases, such as Src.Citation61,Citation62 STAT3 and STAT5 proteins have been found overexpressed in resected NSCLC tissues.Citation63,Citation64
Therapeutic options targeting EGFR in NSCLC
Monoclonal antibodies targeting the extracellular domain of EGFR together with small molecule TKIs have been exploited pharmacologically to block EGFR activation.
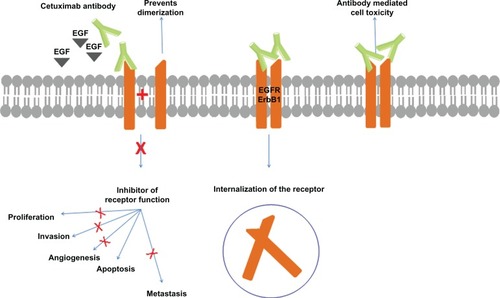
Cetuximab (marketed as Erbitux®; Dako, Copenhagen, Denmark) is a 152 kDa chimeric monoclonal antibody of the immunoglobulin G1 subclass produced in mammalian cell culture by mouse myeloma cells (Sp2/0). It was obtained by attaching the variable regions of the murine monoclonal antibody M225 against EGFR to constant regions of the human IgG1.Citation65,Citation66 It has two identical heavy chains consisting of 449 amino acids each and two light chains of 214 amino acids each.Citation67 Cetuximab has a 5- to 10-fold higher affinity for EGFR than the native ligand, resulting in inhibition of the receptor function.Citation68 It is also able to mediate antibody-dependent, cell-mediated cytotoxicity,Citation69 and receptor downregulation leading to a mitigation of EGFR activity that does not affect other HER family receptors.Citation70 A general overview of mechanisms by which cetuximab exerts its activity is shown in .
Figure 2 The mechanism of cetuximab action.
Abbreviation: EGFR, epidermal growth factor receptor.
Biomarkers in cetuximab therapy
The National Institute of Health (NIH) defines a biomarker as a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.Citation71 In the context of cetuximab therapy, it is applicable mostly to molecules that have been or can be used to assess both sensitivity and resistance to therapy, with the goal of predicting patient subgroups that would most likely benefit or not benefit from therapy. To date, the following biomarkers have been evaluated in the assessment of the efficacy of cetuximab treatment in NSCLC.
EGFR expression, copy number evaluation, and mutation status
One of the first studies assessing combination chemotherapy with cetuximab in a Phase II trial, in which patients evenly distributed across two arms received cisplatin or carboplatin and gemcitabine with cetuximab in one arm and without cetuximab in the other arm, showed an increased response rate, progression free survival and overall survival in the cetuximab group.Citation72 A similar Phase II study in which cisplatin and vinorelbine were administered with or without cetuximab also showed enhanced survival indices in the cetuximab arm.Citation73 Interestingly, both studies were conducted without any preselection for EGFR status. However, a large Phase III trial investigating paclitaxel or docetaxel and carboplatin, with or without cetuximab in 676 patients with NSCLC, found no notable differences in the primary end point of progression free survival, nor in the secondary end point of overall response rate (ORR), as judged by an independent radiological review committee when the two arms were compared.Citation74,Citation75 This group evaluated EGFR protein expression by IHC, gene copy number by fluorescent in situ hybridization (FISH), and EGFR mutation status by sequencing and did not find any significant difference for all of the biomarkers assessed.Citation76
The efficacy of cetuximab was also evaluated as a mono-therapy in a group of patients that were largely EGFR positive (n = 60/66), who had been previously treated with other chemotherapeutic regimens (number of prior regimens: 1 = 28, 2 = 27, and ≥3 = 11), where the response rate was found to be similar to pemetrexed, docetaxel, and erlotinib in similar groups of patients, even though these patients were heavily pretreated.Citation77
The FLEX study assessed EGFR expression in the tumor tissues using immunohistochemistry and defined EGFR positivity as a prerequisite for study inclusion, with tumor immunohistochemical data available for over 99.6% of enrolled patients. An increased benefit from chemotherapy plus cetuximab was observed in patients with IHC scores greater than 150. Overall and median survival rates were also higher in the chemotherapy plus cetuximab group as compared to the chemotherapy alone group in patients with high EGFR expression.Citation78
In a somewhat different approach, a Phase II clinical trial evaluated EGFR gene copy number by FISH as a predictive index for cetuximab efficacy. This trial enrolled 229 patients with advanced NSCLC, who were divided into two groups receiving paclitaxel and carboplatin with or without cetuximab, and found a significantly higher survival benefit in FISH-positive EGFR patients. Median progression-free survival and median survival time were both twice as long in the FISH-positive patients as in the FISH-negative patients, whereas the disease control rate was 81% in FISH-positive and 55% in FISH-negative patients, respectively. Complete response/partial response was also numerically higher in FISH-positive (45%) versus FISH-negative (26%) patients.Citation79
A meta-analysis looking at four trials in which 2018 previously untreated NSCLC patients were ultimately analyzed concluded that cetuximab improved overall survival and overall response rate.Citation80 The meta-analysis did not consider any biomarkers, but nonetheless tried to show the overall benefits of cetuximab. Nevertheless, however, our critical review of all studies on EGFR showed that EGFR currently cannot be considered a reliable biomarker for consistent response in NSCLC.
K-ras
In the abrogation of the EGFR function, it became evident that in addition to EGFR, other key downstream molecules were equally important. Several reports have shown that constitutive activation of key downstream components renders the EGFR blockade by antibodies and/or TKI ineffective. One of these essential downstream factors is the small G protein proto-oncogene K-ras (Kirsten rat sarcoma 2 viral oncogene homologue). K-ras can acquire activating mutations in exon 2, thus isolating the pathway from the effect of EGFR and rendering EGFR inhibitors ineffective.Citation81–Citation84 K-ras mutations are mutually exclusive to EGFR mutations in NSCLC and are more associated with smoking.Citation85,Citation86 Generally, K-ras mutations have been tied to poor outcomes, as confirmed by a meta-analysis of 28 studies encompassing all stages of NSCLC, which showed that tumors with K-ras mutations were associated with poor prognosis.Citation47
In the bid to appraise the use of K-ras a as predictive biomarker in cetuximab therapy, however, a few clinical trials have integrated its analysis into their protocols. The Khambata-Ford et al trial discovered that 17% of the patients from their patient pool harbored K-ras mutations, as detected by sequencing. However, they were unable to find any significant clinical correlation that could be attributable to this biomarker in the context of cetuximab treatment.Citation76 Reevaluation of tissues used for the FLEX study for KRAS mutations in codons 12 and 13 with polymerase chain reaction-based assays revealed 17% positive samples out of 375 patients, in which however, they found no significant predictive or prognostic outcomes differentiating mutant and nonmutant K-ras cases with the administration of cetuximab.Citation87 A Phase II selection design trial of chemotherapy together with concurrent or subsequent cetuximab addition in advanced stage NSCLC patients evaluated the significance of K-ras and did not find any significant association with any efficacy parameters in both K-ras and wild-type patients.Citation88
Phosphatase and tensin homolog (PTEN)
Thus far, only one study has sought to evaluate PTEN as a biomarker for cetuximab therapy in NSCLC. Data stemming from the FLEX study discussed above found 196 out of 303 patients to be PTEN-positive as assessed by immunohistochemical analysis. Patients in both the cetuximab-plus-chemotherapy and chemotherapy-alone groups experienced better overall survival if their tumors expressed PTEN compared with those whose tumors were negative for expression. Although this finding was not significant, the authors still inferred that the absence of PTEN expression might be a marker of poor prognosis.Citation87,Citation89
Biomarkers in ongoing clinical trials
At present, a number of clinical trials are still evaluating the efficacy of cetuximab in combination with other treatment modalities inclusive of radiotherapy, in combination with TKIs, and other chemotherapeutic drugs (). Most of these trials are also assessing biomarker status that could be predictive of prognostic value. These trials are studying EGFR expression, KRAS in individualized or combined trials with fluoro-2-depoxy-D-glucose-positrion emission tomography/computed tomography, MAPK, phosphorylated AKT, p27, and Ki-67 biomarkers.
Table 1 An overview of ongoing clinical trials evaluating biomarker status in cetuximab therapy
Putative biomarkers in preclinical studies
In a quest to find alternative biomarkers for cetuximab in NSCLC, especially in cases of cetuximab resistance, several groups have employed different strategies to identify and analyze markers of resistance. In one such study, our group showed that low E-cadherin and high urokinase-type plasminogen activator receptor (u-PAR), but not EGFR, was associated with resistance to cetuximab in seven NSCLC cell lines. This same expression pattern was also observed in 63% of patients with progressive disease while on cetuximab therapy, implying that low E-cadherin and high u-PAR are markers of cetuximab resistance.Citation90 Furthermore, Yonesaka and colleagues convincingly showed that ERBB2 amplification is a unique mechanism of drug resistance to cetuximab in NSCLC.Citation91 Interestingly, they were able to show that ERBB amplified NSCLC cells remain sensitive to gefitinib, but not to cetuximab. Therefore, a phenomenon likely to be associated with gefitinib but not cetuximab is also able to inhibit ERBB2 in clinically achievable concentrations.Citation91 The expression of amphiregulin (a growth factor regulator related to EGF and transforming growth factor-alpha) expression was also found to predict sensitivity to cetuximab in EGFR wild-type cancers.Citation92
Discussion
Conflicts of interest exist between pharmaceutical companies that want to market their drugs, physicians that are looking for drugs that will best meet the needs of their patients, and the very ill patients that want to get better at all costs. A good predictive biomarker would be an optimal solution to these conflicts of interest. However, as experience has shown, robust, valid, sensitive, and specific biomarkers are few and far between. In the case of cetuximab, overwhelming evidence, particularly in meta-analysis studies, has shown that patients with advanced NSCLC derive benefit, especially when this antibody is combined with cytotoxic chemotherapy. Cetuximab has even been found to be of benefit after the failure of gefitinib, regardless of EGFR mutational status.Citation93
Existing data on EGFR protein expression, evaluated through IHC, suggested that patients with high scores stand to gain when cetuximab is included in the therapy, which supports its further evaluation as a candidate biomarker. Tangible evidence, however, also exists showing that not all patients benefit. The problem with IHC is that protocols vary, which could have considerable consequences on the outcome. Even in the FLEX study, where this biomarker was evaluated, in an effort to obtain a high degree of consistency in the analysis, all the individuals involved in the interpretation of the results had to be collectively tutored.
The EGFR gene copy number detected by FISH may be another potential biomarker for the selection of NSCLC patients for treatment with EGFR-directed therapies. Patients with FISH-positive tumors have demonstrated a higher disease control rate compared to patients with FISH-negative tumors. Furthermore, survival favored FISH-positive patients receiving concurrent therapy. In the BMS099 trial, EGFR FISH positivity was seen in 54 of 104 (52%) patients, but was neither prognostic nor of predictive value with regard to cetuximab efficacy.Citation76 Thus, EGFR FISH positivity as a predictive factor of benefit from cetuximab therapy remains undecided and needs further exploration.
It was interesting to observe that with small molecule TKIs, a preferential response was observed in females, patients with adenocarcinomas, Asians, and neversmokers.Citation94–Citation97 A more in-depth analysis of these subgroups revealed that specific activating mutations in the tyrosine kinase domain of the EGFR gene were responsible for the observed benefit from TKIs.Citation94,Citation98 In agreement with the findings in NSCLC cell line studies that these mutations were associated with sensitivity to gefitinib but not cetuximab,Citation99 these mutations did not seem to affect patients’ response to cetuximab in the Phase III trials. In addition, no significant treatment-specific correlations between EGFR mutation status and progression free survival, overall survival, or response rate were observed in the BMS099 trial.Citation76 Therefore, it is safe to conclude that EGFR mutations are not useful as biomarkers in cetuximab therapy.
In colorectal cancer, KRAS mutation status was found to be a useful marker of resistance because the benefit of cetuximab was found to be limited to patients with KRAS wild-type tumors.Citation100,Citation101 The results from two Phase II trials that compared platinum-based chemotherapy with cetuximab (concurrent/sequential) and with bevacizumab in patients with advanced NSCLC,Citation88 however, showed no differences in progression-free survival or overall survival with cetuximab in relation to K-ras mutation status. The Phase III trials that evaluated K-ras mutations in NSCLCCitation76,Citation87 also found that K-ras mutation status had no impact on progression-free survival, overall survival, or response rate in relation to cetuximab administration. The observed differences between colorectal and NSCLC might be the result of alternative routes of signal transduction in NSCLC that render K-ras insignificant and impressively show that every tumor entity needs to be individually considered when establishing biomarkers for novel therapeutics. An increasing number of putative biomarkers are still in the preclinical pipeline, and it will be interesting to see which ones will be brought over into clinical trials. Thus far, the evidence of their potential use is limited to a few studies, which need to be reinforced by more groups and follow-up studies.
Conclusion
EGFR protein expression still has the potential to advance to a biomarker that is able to predict favorable outcomes from cetuximab administration in NSCLC. It is rather disappointing that with all the advances in molecular cancer research and the several clinical trials that have evaluated cetuximab in the context of NSCLC, no clear significant biomarker has so far been discovered. A major contributing reason that could account for the observed findings is that the methods for the detection of EGFR positivity as well as other potential biomarkers are by no means standardized, which leaves room for a great deal of improvement. Secondly, the analysis conducted on some of the biomarkers as in the case of K-ras mutations was done on a small subset of patients, where statistical validity is difficult to establish. However, the possibility of other unidentified molecular mediators important in the response of NSCLC to cetuximab cannot be ruled out, and only when these mediators are identified can the desired outcomes be achieved. Moreover, in our opinion, the clinical trials do not all have the same endpoints regarding the survival indices being evaluated. To resolve these issues, multicentered clinical trials should be organized such that a significant number of patients with similar clinical backgrounds are included and the approach/method used for biomarker detection is standardized. Ongoing clinical trials and preclinical studies indicate that more biomarkers could soon be in contention.
Acknowledgements
HA was supported by Alfried Krupp von Bohlen and Halbach Foundation (Award for Young Full Professors), Essen, Hella-Bühler-Foundation, Heidelberg, Dr Ingrid zu Solms Foundation, Frankfurt/Main, the Hector Foundation, Weinheim, Germany, the FRONTIER Excellence Initiative of the University of Heidelberg, the BMBF, Bonn, Germany, the Walter Schulz Foundation, Munich, Germany, the Deutsche Krebshilfe, Bonn, Germany, the DKFZ-MOST German-Israeli program, Heidelberg, Germany, and a Cancer Grant by Genome Biosciences, Germany.
Disclosure
The authors report no conflicts of interest in this work.
References
- FerlayJShinHBrayFFormanDMathersCParkinDGLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10Lyon, FranceInternational Agency for Research on Cancer2010 http://globocan.iarc.fr. Accessed May 24, 2011.
- FerlayJShinHRBrayFFormanDMathersCParkinDMEstimates of worldwide burden of cancer in 2008: GLOBOCAN 2008Int J Cancer2010127122893291721351269
- LaskinJJSandlerABJohnsonDHNon-Small Cell Lung CancerPhiladelphia, PASaunders2005
- AlbergAJBrockMVSametJMEpidemiology of lung cancer: looking to the futureJ Clin Oncol200523143175318515886304
- VineisPAiroldiLVegliaFEnvironmental tobacco smoke and risk of respiratory cancer and chronic obstructive pulmonary disease in former smokers and never smokers in the EPIC prospective studyBMJ2005330748627715681570
- TardonALeeWJDelgado-RodriguezMLeisure-time physical activity and lung cancer: a meta-analysisCancer Causes Control200516438939715953981
- LeeIMPhysical activity and cancer prevention – data from epidemiologic studiesMed Sci Sports Exerc200335111823182714600545
- BoffettaPEpidemiology of environmental and occupational cancerOncogene200423386392640315322513
- MolinaJRYangPCassiviSDSchildSEAdjeiAANon-small cell lung cancer: epidemiology, risk factors, treatment, and survivorshipMayo Clin Proc200883558459418452692
- BarlesiFJacotWAstoulPPujolJLSecond-line treatment for advanced non-small cell lung cancer: a systematic reviewLung Cancer200651215917216360238
- MokTSWuYLYuCJRandomized, placebo-controlled, phase II study of sequential erlotinib and chemotherapy as first-line treatment for advanced non-small-cell lung cancerJ Clin Oncol200927305080508719738125
- OkamotoIEpidermal growth factor receptor in relation to tumor development: EGFR-targeted anticancer therapyFEBS J2010277230931519922468
- MendelsohnJBaselgaJStatus of epidermal growth factor receptor antagonists in the biology and treatment of cancerJ Clin Oncol200321142787279912860957
- BrabenderJDanenbergKDMetzgerREpidermal growth factor receptor and HER2-neu mRNA expression in non-small cell lung cancer Is correlated with survivalClin Cancer Res2001771850185511448895
- BiancoRGelardiTDamianoVCiardielloFTortoraGMechanisms of resistance to EGFR inhibitorsTarget Oncol2007213137
- SalomonDSBrandtRCiardielloFNormannoNEpidermal growth factor-related peptides and their receptors in human malignanciesCrit Rev Oncol Hematol19951931832327612182
- CiardielloFTortoraGEGFR antagonists in cancer treatmentN Engl J Med2008358111160117418337605
- NormannoNTejparSMorgilloFDeLAVanCECiardielloFImplications for KRAS status and EGFR-targeted therapies in metastatic CRCNat Rev Clin Oncol20096951952719636327
- YardenYSliwkowskiMXUntangling the ErbB signalling networkNat Rev Mol Cell Biol20012212713711252954
- FergusonKMBergerMBMendrolaJMChoHSLeahyDJLemmonMAEGF activates its receptor by removing interactions that autoinhibit ectodomain dimerizationMol Cell200311250751712620237
- BurgessAWChoHSEigenbrotCAn open-and-shut case? Recent insights into the activation of EGF/ErbB receptorsMol Cell200312354155214527402
- KingACCuatrecasasPResolution of high and low affinity epidermal growth factor receptors. Inhibition of high affinity component by low temperature, cycloheximide, and phorbol estersJ Biol Chem19822576305330606277923
- SchlessingerJLigand-induced, receptor-mediated dimerization and activation of EGF receptorCell2002110666967212297041
- TannerKGKyteJDimerization of the extracellular domain of the receptor for epidermal growth factor containing the membrane-spanning segment in response to treatment with epidermal growth factorJ Biol Chem199927450359853599010585488
- BargmannCIHungMCWeinbergRAMultiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185Cell19864556496572871941
- BennasrouneAFickovaMGardinATransmembrane peptides as inhibitors of ErbB receptor signalingMol Biol Cell20041573464347415146055
- MorikiTMaruyamaHMaruyamaINActivation of preformed EGF receptor dimers by ligand-induced rotation of the transmembrane domainJ Mol Biol200131151011102611531336
- BellCATynanJAHartKCMeyerANRobertsonSCDonoghueDJRotational coupling of the transmembrane and kinase domains of the Neu receptor tyrosine kinaseMol Biol Cell200011103589359911029057
- WaltonGMChenWSRosenfeldMGGillGNAnalysis of deletions of the carboxyl terminus of the epidermal growth factor receptor reveals self-phosphorylation at tyrosine 992 and enhanced in vivo tyrosine phosphorylation of cell substratesJ Biol Chem19902653175017541688559
- SchlessingerJCell signaling by receptor tyrosine kinasesCell2000103221122511057895
- StamosJSliwkowskiMXEigenbrotCStructure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitorJ Biol Chem200227748462654627212196540
- WeberWBerticsPJGillGNImmunoaffinity purification of the epidermal growth factor receptor. Stoichiometry of binding and kinetics of self-phosphorylationJ Biol Chem19842592314631146366094567
- FergusonKMActive and inactive conformations of the epidermal growth factor receptorBiochem Soc Trans200432Pt 574274515494003
- NishibeSCarpenterGTyrosine phosphorylation and the regulation of cell growth: growth factor-stimulated tyrosine phosphorylation of phospholipase CSemin Cancer Biol1990142852922103503
- AkiyamaTKadowakiTNishidaESubstrate specificities of tyrosine-specific protein kinases toward cytoskeletal proteins in vitroJ Biol Chem19862613114797148033771552
- SubrahmanyamGBerticsPJAndersonRAPhosphorylation of protein 4.1 on tyrosine-418 modulates its function in vitroProc Natl Acad Sci U S A19918812522252261647028
- HeisermannGJGillGNEpidermal growth factor receptor threonine and serine residues phosphorylated in vivoJ Biol Chem19882632613152131583138233
- DownwardJParkerPWaterfieldMDAutophosphorylation sites on the epidermal growth factor receptorNature198431159854834856090945
- HunterTLingNCooperJAProtein kinase C phosphorylation of the EGF receptor at a threonine residue close to the cytoplasmic face of the plasma membraneNature198431159854804836090944
- SatoKSatoAAotoMFukamiYc-Src phosphorylates epidermal growth factor receptor on tyrosine 845Biochem Biophys Res Commun19952153107810877488034
- BarbierAJPoppletonHMYigzawYTransmodulation of epidermal growth factor receptor function by cyclic AMP-dependent protein kinaseJ Biol Chem199927420140671407310318821
- DaubHWeissFUWallaschCUllrichARole of transactivation of the EGF receptor in signalling by G-protein-coupled receptorsNature199637965655575608596637
- ZwickEHackelPOPrenzelNUllrichAThe EGF receptor as central transducer of heterologous signalling systemsTrends Pharmacol Sci1999201040841210577253
- ScaltritiMBaselgaJThe epidermal growth factor receptor pathway: a model for targeted therapyClin Cancer Res200612185268527217000658
- OdaKMatsuokaYFunahashiAKitanoHA comprehensive pathway map of epidermal growth factor receptor signalingMol Syst Biol20051200516729045
- KolchWPittAFunctional proteomics to dissect tyrosine kinase signalling pathways in cancerNat Rev Cancer201010961862920720570
- MascauxCIanninoNMartinBThe role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysisBr J Cancer200592113113915597105
- ShieldsJMPruittKMcFallAShaubADerCJUnderstanding Ras: ‘It ain’t over ‘til it’s over’Trends Cell Biol200010414715410740269
- RodenhuisSSlebosRJClinical significance of ras oncogene activation in human lung cancerCancer Res199252Suppl 92665s2669s1562997
- PaoWWangTYRielyGJKRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinibPLoS Med200521e1715696205
- EberhardDAJohnsonBEAmlerLCMutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinibJ Clin Oncol200523255900590916043828
- RielyGJKrisMGRosenbaumDFrequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinomaClin Cancer Res200814185731573418794081
- SunSSchillerJHGazdarAFLung cancer in never smokers – a different diseaseNat Rev Cancer200771077879017882278
- VivancoISawyersCLThe phosphatidylinositol 3-kinase AKT pathway in human cancerNat Rev Cancer20022748950112094235
- YamamotoHShigematsuHNomuraMPIK3CA mutations and copy number gains in human lung cancersCancer Res200868176913692118757405
- SoriaJCLeeHYLeeJILack of PTEN expression in non-small cell lung cancer could be related to promoter methylationClin Cancer Res2002851178118412006535
- MarsitCJZhengSAldapeKPTEN expression in non-small-cell lung cancer: evaluating its relation to tumor characteristics, allelic loss, and epigenetic alterationHum Pathol200536776877616084946
- YuHPardollDJoveRSTATs in cancer inflammation and immunity: a leading role for STAT3Nat Rev Cancer200991179880919851315
- DarnellJEJrSTATs and gene regulationScience19972775332163016359287210
- YuHJoveRThe STATs of cancer – new molecular targets come of ageNat Rev Cancer2004429710514964307
- KimLCSongLHauraEBSrc kinases as therapeutic targets for cancerNat Rev Clin Oncol200961058759519787002
- AggarwalBBKunnumakkaraABHarikumarKBSignal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship?Ann N Y Acad Sci20091171597619723038
- GantiAKEpidermal growth factor receptor signaling in nonsmall cell lung cancerCancer Invest201028551552520073576
- Sanchez-CejaSGReyes-MaldonadoEVazquez-ManriquezMELopez-LunaJJBelmontAGutierrez-CastellanosSDifferential expression of STAT5 and Bcl-xL, and high expression of Neu and STAT3 in non-small-cell lung carcinomaLung Cancer200654216316816959370
- MaemondoMInoueAKobayashiKGefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFRN Engl J Med2010362252380238820573926
- MendelsohnJKawamotoHybrid cell lines that produce monoclonal antibodies to epidermal growth factor receptor United States Patent: 4,943,533.7201990
- HumbletYCetuximab: an IgG(1) monoclonal antibody for the treatment of epidermal growth factor receptor-expressing tumoursExpert Opin Pharmacother2004571621163315212612
- GoldsteinNIPrewettMZuklysKRockwellPMendelsohnJBiological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft modelClin Cancer Res1995111131113189815926
- HsuYFAjonaDCorralesLComplement activation mediates cetuximab inhibition of non-small cell lung cancer tumor growth in vivoMol Cancer2010913920529262
- RansonMSliwkowskiMXPerspectives on anti-HER monoclonal antibodiesOncology200263Suppl 1172412422051
- AtkinsonAJColburnWADeGruttolaVGBiomarkers and surrogate endpoints: Preferred definitions and conceptual frameworkClin Pharmacol Ther2001693899511240971
- ButtsCABodkinDMiddlemanELRandomized phase II study of gemcitabine plus cisplatin or carboplatin, with or without cetuximab, as first-line therapy for patients with advanced or metastatic non-small-cell lung cancerJ Clin Oncol200725365777578418089875
- RosellRRobinetGSzczesnaARandomized phase II study of cetuximab plus cisplatin/vinorelbine compared with cisplatin/vinorelbine alone as first-line therapy in EGFR-expressing advanced non-small-cell lung cancerAnn Oncol200819236236917947225
- LynchTJPatelTDreisbachLCetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: results of the randomized multicenter phase III trial BMS099J Clin Oncol201028691191720100966
- Bristol-Myers Squibb, ImClone LLCStudy of taxane/carboplatin +/– cetuximab as first-line treatment for patients with advanced/metastatic non-small cell lung cancerNCT001122942012 Available from: http://clinicaltrials.gov/show/NCT00112294. Accessed on June 18, 2012.
- Khambata-FordSHarbisonCTHartLLAnalysis of potential predictive markers of cetuximab benefit in BMS099, a phase III study of cetuximab and first-line taxane/carboplatin in advanced non-small-cell lung cancerJ Clin Oncol201028691892720100958
- HannaNLilenbaumRAnsariRPhase II trial of cetuximab in patients with previously treated non-small-cell lung cancerJ Clin Oncol200624335253525817114658
- PirkerRPereiraJRvonPJEGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX studyLancet Oncol2012131334222056021
- HirschFRHerbstRSOlsenCIncreased EGFR gene copy number detected by fluorescent in situ hybridization predicts outcome in non-small-cell lung cancer patients treated with cetuximab and chemotherapyJ Clin Oncol200826203351335718612151
- LinHJiangJLiangXZhouXHuangRChemotherapy with cetuximab or chemotherapy alone for untreated advanced non-small-cell lung cancer: a systematic review and meta-analysisLung Cancer2010701576220149474
- AmadoRGWolfMPeetersMWild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancerJ Clin Oncol200826101626163418316791
- FransenKKlintenasMOsterstromADimbergJMonsteinHJSoderkvistPMutation analysis of the BRAF, ARAF and RAF-1 genes in human colorectal adenocarcinomasCarcinogenesis200425452753314688025
- DeRWPiessevauxHDeSJKRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximabAnn Oncol200819350851517998284
- DiFFBlanchardFCharbonnierFClinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by cetuximab plus chemotherapyBr J Cancer20079681166116917375050
- KosakaTYatabeYEndohHKuwanoHTakahashiTMitsudomiTmutations of the epidermal growth factor receptor gene in lung cancerCancer Res200464248919892315604253
- TamIYSChungLPSuenWSDistinct epidermal growth factor receptor and kras mutation patterns in non-small cell lung cancer patients with different tobacco exposure and clinicopathologic featuresClin Cancer Res20061251647165316533793
- O’ByrneKJGatzemeierUBondarenkoIMolecular biomarkers in non-small-cell lung cancer: a retrospective analysis of data from the phase 3 FLEX studyLancet Oncol201112879580521782507
- HerbstRSKellyKChanskyKPhase II selection design trial of concurrent chemotherapy and cetuximab versus chemotherapy followed by cetuximab in advanced-stage non-small-cell lung cancer: Southwest Oncology Group study S0342J Clin Oncol201028314747475420921467
- MerckKGaAStudy of cisplatin/vinorelbine +/– cetuximab as First-line treatment of advanced non-small cell lung cancer (FLEX)NCT001487982012 Available from: http://clinicaltrials.gov/ct2/show/NCT00148798?term=NCT00148798&rank=1. Accessed on June 18, 2012.
- NikolovaDAAsanganiIANelsonLDFetuximab attenuates metastasis and u-PAR eExpression in non-small cell lung cancer: u-PAR and E-Cadherin are novel biomarkers of cetuximab sensitivityCancer Res20096962461247019276367
- YonesakaKZejnullahuKOkamotoIActivation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximabSci Transl Med201139999ra86
- YonesakaKZejnullahuKLindemanNAutocrine production of amphiregulin predicts sensitivity to both gefitinib and cetuximab in EGFR wild-type cancersClin Cancer Res200814216963697318980991
- WuJYYangCHHsuYCUse of cetuximab after failure of gefitinib in patients with advanced non-small-cell lung cancerClin Lung Cancer201011425726320630828
- PaezJGJannePALeeJCEGFR mutations in lung cancer: correlation with clinical response to gefitinib therapyScience200430456761497150015118125
- PaoWMillerVZakowskiMEGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinibProc Natl Acad Sci U S A200410136133061331115329413
- HuangSFLiuHPLiLHHigh frequency of epidermal growth factor receptor mutations with complex patterns in non-small cell lung cancers related to gefitinib responsiveness in TaiwanClin Cancer Res200410248195820315623594
- HerbstRSGiacconeGSchillerJHGefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial – INTACT 2J Clin Oncol200422578579414990633
- LynchTJBellDWSordellaRActivating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinibN Engl J Med2004350212129213915118073
- MukoharaTEngelmanJAHannaNHDifferential effects of gefitinib and cetuximab on non-small-cell lung cancers bearing epidermal growth factor receptor mutationsJ Natl Cancer Inst200597161185119416106023
- VanCEKohneCHHitreECetuximab and chemotherapy as initial treatment for metastatic colorectal cancerN Engl J Med2009360141408141719339720
- TolJKoopmanMCatsAChemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancerN Engl J Med2009360656357219196673
- LurjeGLenzHJEGFR signaling and drug discoveryOncology200977640041020130423
- GovindanRCetuximab in advanced non-small cell lung cancerClin Cancer Res20041012 Pt 24241s4244s15217966
- PennellNALynchTJJrCombined inhibition of the VEGFR and EGFR signaling pathways in the treatment of NSCLCOncologist200914439941119357226
- KruserTJWheelerDLMechanisms of resistance to HER family targeting antibodiesExp Cell Res201031671083110020064507
- RossiABriaEMaionePPalazzoloGFalangaMGridelliCThe role of cetuximab and other epidermal growth factor receptor monoclonal antibodies in the treatment of advanced non-small cell lung cancerRev Recent Clin Trials20083321722718782080
- CapdevilaJElezEMacarullaTRamosFJRuiz-EcharriMTaberneroJAnti-epidermal growth factor receptor monoclonal antibodies in cancer treatmentCancer Treat Rev200935435436319269105
- CiardielloFDeVFOrdituraMTortoraGThe role of EGFR inhibitors in non-small cell lung cancerCurr Opin Onco2004162130135
- BrandTMIidaMLiCWheelerDLThe nuclear epidermal growth factor receptor signaling network and its role in cancerDiscov Med2011126641943222127113