Abstract
Thyroid cancer incidence continues to increase, remaining the most common endocrine malignancy. The need for effective systemic therapies combined with high incidence of driver mutations and overexpression of molecular pathways make refractory thyroid cancer an ideal candidate for treatment with novel agents. Multikinase inhibitors have caused a paradigm shift in the treatment of patients with advanced iodine-refractory thyroid cancer. These agents have shown to be the most effective systemic therapy for this disease not only causing prolonged responses but also improving survival. The activity of these agents inhibiting several pathways simultaneously, such as rearranged during transfection protooncogene, mitogen-activated protein kinase, and angiogenesis, can probably explain the effectiveness in controlling the progression of this malignancy. Several of these agents are currently on clinical studies in patients with differentiated and medullary thyroid cancer and most of them are showing promising clinical activity. With the approval of vandetanib for the treatment of medullary thyroid cancer, a new era in the management of this disease has begun. The molecular rationale for the use of these drugs for thyroid cancer is discussed as well as their promising clinical results.
Introduction
Thyroid cancer continues to be the most common endocrine malignancy with an incidence that is increasing.Citation1 In the United States alone an estimate of 48,020 new cases will be diagnosed in 2011, causing 1740 deaths.Citation1 Differentiated thyroid cancer (DTC) and medullary thyroid cancer (MTC) which originate from follicular and neural crest C cells, respectively, are the most common types. Undifferentiated thyroid cancer is a highly aggressive and uncommon type of thyroid cancer. Most thyroid cancers are effectively treated with surgical resection, thyroid-stimulating hormone suppressive therapy, and ablation of the thyroid remnant with radioactive iodine (RAI). The prognosis is excellent with a 10-year disease-related survival of 85%.Citation2 However, patients with RAI-refractory and metastatic thyroid cancer have few therapeutic options, with response rates <30% with the use of systemic chemotherapy, and usually short-lasting associated toxicities, and no proven survival benefit.Citation3,Citation4
Targeted therapies, anticancer agents developed to inhibit specific molecular pathways that drive various human tumors, have caused a paradigm shift in the treatment of solid and hematologic malignancies. The study of thyroid carcinomas has revealed somatic mutations in several pathways such as B-type Raf kinase (B-Raf), K-Ras, and rearranged during transfection (RET)/papillary thyroid carcinoma (PTC) that are associated with development and progression of approximately 70% of these malignancies.Citation5 Therefore, lack of effective systemic therapies combined with a high incidence of driver mutations and overexpression of molecular pathways make refractory thyroid cancer an ideal candidate for treatment with novel targeted agents. Multikinase inhibitors (MKIs) have been shown to be the most promising targeted therapies against this disease by blocking pathway signaling like angiogenesis and, concomitantly, inhibiting mutated pathways (). The results of the ZETA (Zactima Efficacy in Thyroid Cancer Assessment) trial led the United States Food and Drug Administration to approve vandetanib for the treatment of symptomatic or progressive MTC, constituting this the first targeted agent approved for thyroid cancer.Citation6 The molecular rationale for targeting thyroid cancer with these agents is discussed as well as the promising results of the treatment with MKIs in refractory thyroid carcinoma.
Table 1 Kinases inhibited by multikinase inhibitors with clinical activity in refractory thyroid cancer
Molecular pathways and therapeutic targets in thyroid cancer
Raf/mitogen-activated protein kinase (MAPK) pathway
The activation of the MAPK pathway plays a major role in the carcinogenesis of PTC, the most common thyroid malignancy. Raf, MAPK kinase, and MAPK are all serine/ threonine-selective protein kinases. The MAPK pathway is activated by growth factor-stimulated cell surface receptors such as epidermal growth factor receptor.Citation7 When the cell surface receptor is phosphorylated, it activates Sos which consequently promotes Ras (a guanosine triphosphatase) to change its guanosine diphosphate for a guanosine triphosphate. Activated Ras activates the protein kinase activity of Raf kinase which phosphorylates and activates MAPK kinase.Citation8 Finally, MAPK kinase phosphorylates and activates MAPK that, in turn, can now activate a transcription factor, such as myc. Among the three forms of Raf kinases, B-Raf is the most potent activator of the MAPK pathway.Citation8 About 45% of sporadic PTC can have mutations in the B-Raf gene, making these the most common genetic alteration found in patients with thyroid cancer.Citation9 More than 90% of B-Raf mutations exist in the V600E mutation (T1799A) in exon 15; abnormality is also present in 77.8% of patients with recurrent disease.Citation9 B-Raf V600E mutation has been associated with several adverse pathologic prognostic features in PTC-like extrathyroidal invasion, multicentricity, presence of nodal metastases, and absence of tumor capsule. Moreover, it is associated with an increased rate of tumor recurrence and treatment failure.Citation10,Citation11
Vascular endothelial growth factor (VEGF) pathway
One of the major developments on anticancer therapy of the last two decades is the essential role of angiogenesis in tumor growth and metastasis; therefore, controlling tumorassociated angiogenesis is now a key tactic in limiting cancer progression. VEGF-A, the major mediator of tumor angiogenesis, is part of the VEGF family of structurally related molecules. VEGF-A promotes the proliferation and survival of endothelial cells and increases vascular permeability.Citation12 VEGF-A signals through VEGF receptor 2 (VEGFR-2), the major VEGF signaling receptor that mediates sprouting angiogenesis. The binding of VEGF to VEGFR-2 leads to dimerization of the receptor, followed by intracellular activation of a cascade of different signaling pathways such as Raf/MAPK and phosphatidylinositol 3′ kinase (PI3K)-Akt pathways.Citation13 Both DTC and MTC have been found to express high levels of both angiopoietin-2 and VEGF and upregulation of its main receptor, VEGFR-2, with respect to normal thyroid.Citation14,Citation15 Moreover, increased expression of VEGF in thyroid cancer has been associated with an increase in tumor size, local and distant metastasis, and poor prognosis.Citation14,Citation16
RET pathway
RET protooncogene is located in the chromosome 10q11.2. RET encodes a receptor tyrosine kinase that is expressed in neuroendocrine cells as well as thyroid C cells, adrenal medullary cells, and neural cells (including parasympathetic and sympathetic ganglion cells). The RET receptor consists of an extracellular portion, a transmembrane portion, and an intracellular portion, which contains two tyrosine kinase subdomains (TK1 and TK2) that are involved in the activation of several intracellular signal transduction pathways. In physiological conditions, activation of RET requires the formation of a multimeric complex with a coreceptor of the glycosylphosphatidylinositol-anchored glial cell line-derived neurotrophic factor family α coreceptors and one of their ligands, the glial cell line-derived neurotrophic factor family of ligands.Citation17 The ligand binding leads to formation of the complex and RET dimerization, kinase activation, and signaling to the nucleus. Activation of RET has been shown to signal through multiple pathways, including Ras/ extracellular signal-related kinase pathway, PI3K, and Src, among others ().
Figure 1 Molecular pathways targeted by multikinase inhibitors in refractory thyroid cancer.
Abbreviations: EGFR, epidermal growth factor; ERK, extracellular signal-regulated kinase; GFL, glial cell line-derived neurotrophic factor family of ligands; GFRα, glial cell line-derived neurotrophic factor family α coreceptor; MAPK, mitogen-activated protein kinase; MEK, mitogen-activated protein kinase kinase; PI3K, phosphatidylinositol 3′ kinase; RET, rearranged during transfection; VEGF-A, vascular endothelial growth factor A; VEGFR2, vascular endothelial growth factor receptor-2.
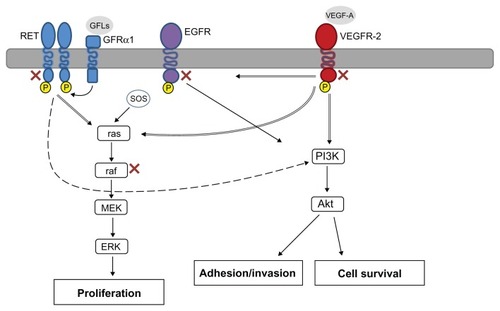
Activating mutations and translocation of RET are a common abnormality of both PTC and MTC. Translocations of RET are seen in ≤30% of PTC, and is commonly seen in cases of PTC associated with radiation exposure.Citation18 Moreover, activating mutations are common in sporadic and hereditary MTC, leading to ligand-independent dimerization and phosphorylation.Citation18
MKIs in thyroid cancer
Vandetanib
Vandetanib is an orally bioavailable agent that is a multimarket kinase inhibitor that also has antiangiogenesis properties (targets VEGFR-2 and VEGFR-3, epidermal growth factor receptor, and RET kinase).Citation19 It was found to be a promising agent for MTC because, besides its effects on angiogenesis, it has inhibitory effects in RET activation, a well-known protooncogene that is predominant in this disease. Two phase II trials have been reported so far using vandetanib in patients with MTC. The first was conducted in patients with locally advanced or metastatic hereditary MTC with RET germline mutation using vandetanib at 300 mg orally per day. A total of 30 patients were accrued and partial response was reported in six patients (20%) with stable disease in other nine subjects (30%). Grade 1–2 adverse events (presented in >50% of the patients) were: rash, diarrhea, fatigue, and nausea. Grade 3 adverse events included asymptomatic QTc prolongation (17%), rash, and diarrhea (both 10%).Citation20 The other phase II trial conducted by Robinson et al used vandetanib in a lower dose (100 mg) in 19 patients, 79% of them with confirmed RET germline mutation.Citation21 Post-progression dose increment to vandetanib 300 mg was allowed in eligible patients. In a preliminary report with a median duration of treatment of 274 days, 14 patients remained on the 100 mg dose and the dose after progression was raised to 300 mg in two patients. From this group of 16 evaluable patients, partial responses were reported in two patients (10%), stable disease for more than 6 months in six patients (31%), and two other patients had disease progression.
The first phase III trial reported using a MKI was the ZETA (Zactima Efficacy in Thyroid Cancer Assessment) trial, a randomized, double-blind phase III trial in patients with locally advanced or metastatic thyroid cancer using 300 mg daily of vandetanib versus placebo.Citation22 The median age of the 331 patients included was 52 years; 56% had a positive RET mutation status. After a median follow-up of 24 months, a statistically significant progression-free survival (PFS), overall response rate, disease control rate, and biochemical response were observed for vandetanib versus placebo. This was the first phase III trial that demonstrated an improved PFS using an MKI in patients with thyroid cancer; thus, vandetanib was approved in April 2011 by the United States Food and Drug Administration for this indication.Citation19 Recently, there have been attempts to combine biologic agents with chemotherapy or among themselves in several trials of head and neck and thyroid cancers. Vandetanib was recently combined with bortezomib, a proteasome inhibitor (approved for the treatment of multiple myeloma).Citation23 The rationale of the trial was based on preclinical evidence which has shown that addition of bortezomib enhanced vandetanib activity against MTC cell lines. This was a phase I trial designed to assess the safety, tolerance, and activity of vandetanib plus bortezomib in 21 patients with MTC. A dose escalation schema with bortezomib (1–1.3 mg/m2) intravenously on days one, four, eight, and eleven and vandetanib (100–300 mg) orally daily (28-day cycles) was in place. Grade 3 toxicities were: hypertension (24%), fatigue (19%), thrombocytopenia (10%), diarrhea (10%), and arthralgia (10%), with keratoacanthoma, hyperkalemia, pulmonary hemorrhage, edema, and prolonged QTc each in one patient (5%). There was one dose limiting toxicity – grade 3 thrombocytopenia at a bortezomib/ vandetanib dose of 1.3/200 in cycle two, but no grade 4–5 toxicities. The 17 patients who had MTC were metastatic. Median calcitonin and carcinoembryonic antigen levels were 5289 and 113, respectively. Five (29%) patients had partial response and eight (47%) attained stable disease. Six patients who attained stable disease maintained their disease control for more than 6 months. The maximum tolerated dose for the combination was bortezomib 1.3 mg/m2 intravenously on days one, four, eight, and eleven and vandetanib at 300 mg orally daily.Citation23
Pazopanib
Pazopanib is a small-molecule inhibitor of VEGFR-1, VEGFR-2, and VEGFR-3, platelet-derived growth factor receptor-β (PDGFR-β), and c-Kit, and is currently approved for the treatment of renal cell carcinoma.Citation24 A phase II trial using pazopanib at 800 mg daily enrolled 39 patients with metastatic, rapidly progressive, RAI-refractory DTC.Citation25 Of the 37 evaluable patients, confirmed partial responses were recorded in 18 patients (49%) and the likelihood of response lasting longer than 1 year was calculated to be 66%. Interestingly, the maximum pazopanib plasma concentration correlated with the maximum change in tumor size and was significantly higher in the 18 patients who achieved confirmed partial response by Response Evaluation Criteria in Solid Tumors criteria when compared to those who did not. Dose reduction was required in 16 (43%) of 37 patients because of adverse effects.Citation25 A ≥30% decrease in thyroglobulin concentrations was observed in 88% of the patients with data available. The most frequent adverse events that required a dose reduction were fatigue, skin and hair hypopigmentation, diarrhea, and nausea. The death of two patients during treatment was attributed to underlying disorders.
Lenvatinib (E7080)
Lenvatinib is an orally administered MKI with a wide therapeutic range targeting several important kinases in thyroid cancers such as VEGFR-1, VEGFR-2, VEGFR-3, fibroblast growth factor receptor-1 (FGFR-1), FGFR-2, FGFR-3, FGFR-4, PDGFR-β, RET, and c-Kit. It also has the benefit of not prolonging the QTc interval like other MKIs.Citation26 A phase II study with lenvatinib 24 mg once daily until disease progression was conducted in 58 patients with advanced RAI-refractory DTC whose disease had progressed during the prior 12 months.Citation27 Confirmed partial responses were observed in 29 patients (50%) based on investigator assessment, with 65% of the responses seen at 8 weeks. Interestingly, the response rate for patients who received prior VEGFR-directed therapy was 41%. Of the patients enrolled, 35% required dose reduction for management of toxicity, and 23% were withdrawn from therapy due to toxicity. The most common adverse events were hypertension, diarrhea, decreased appetite, weight loss, and proteinuria; all them had an incidence of <10% for grade 3. Five patients (8.6%) experienced grade 4 events.Citation27
Sorafenib
Three phase II clinical trials using sorafenib in patients with metastatic RAI-refractory thyroid carcinoma have been published to date. The first trial was conducted by Kloos et al in which 56 patients were enrolled; partial response was seen in six of the 41 patients with PTC included; stable disease lasted > 6 months in 23 patients.Citation28 The median duration of partial response was 7.5 months and median PFS was 15 months. Grade 3 adverse events included hand-foot skin reaction, musculoskeletal pain, and fatigue. Interestingly, a high incidence of the B-Raf mutation was found in 17 of the 22 PTCs (77%) analyzed, with 14 of these mutations being V600E, whereas three other patients had a K601E mutation. No patients with MTC were included and no partial responses were reported in non-PTC patients. The second phase II trial was conducted by Gupta-Abramson et al in 30 patients who were treated with sorafenib 400 mg orally twice daily.Citation29 Seven patients had partial response lasting 18–84 weeks, and 16 patients had stable disease lasting 14–89 weeks; median PFS was 79 weeks. Of note, 95% patients for whom serial thyroglobulin levels were available showed a decrease in thyroglobulin levels, with a mean decrease of 70%. In terms of toxicity, a single patient died of liver failure that was likely to be treatment-related. Although the presence of the B-Raf mutation has been related with poor outcome and the results of these trials are encouraging, the correlation between the presence of B-Raf V600E mutation and clinical response to sorafenib has yet to be elucidated. Preliminary results of another open-label phase II study of sorafenib in 55 patients with metastatic, RAI-refractory thyroid carcinoma conducted by Brose et al reported an increased PFS for patients with B-Raf V600E, compared with that of patients with wild-type B-Raf (84 versus 54 weeks; P = 0.028).Citation30 More recently, another phase II trial by Lam et al looking at patients with advanced MTC was published. Herein, patients were stratified into two different groups: hereditary (arm A) or sporadic (arm B); all patients received sorafenib 400 mg orally twice daily.Citation31 Sixteen patients were enrolled in arm B, and arm A was closed due to a slow accrual of patients with sporadic MTC. One patient achieved partial response (6.3%) and 14 attained stable disease (87.5%), with four of them having stable disease lasting >15 months. The median PFS for arm B was 17.9 months.Citation31
Motesanib
Rosen et al initially reported partial responses in three of seven patients with thyroid cancer enrolled in a phase I study of motesanib in patients with advanced solid tumors.Citation32 Two phase II trials using 125 mg of motesanib diphosphate orally once daily have been conducted in patients with advanced or metastatic RAI-refractory thyroid cancer. The first trial by Sherman et al treated 93 patients with DTC; 57 (61%) of them were PTC.Citation33 The overall response rate was 14% and stable disease was achieved in 67% of the patients and maintained for ≥24 weeks in 35% of the patients; 8% had progression of disease. The median duration of response was 32 weeks and median PFS was 40 weeks. Diarrhea (59%), hypertension (56%), fatigue (46%), and weight loss (40%) were the most common treatment-related adverse events. The second phase II trial by Schlumberger et al also used motesanib at 125 mg daily, but in 91 patients with MTC. Efficacy was somewhat lower than that in the previous study.Citation34 Response rate was characterized by partial remission in two patients (2%), stable disease in 81%, and a median PFS of 48 weeks. Decrease in serum calcitonin and carcinoembryonic antigen during treatment was seen in 83% and 75% of the patients, respectively. The most common treatment-related adverse events were similar to other trials with motesanib including diarrhea, fatigue, hypertension, and anorexia. Hypothyroidism was reported to be considerably worse in 29% of the patients. Circulating biomarkers of angiogenesis and apoptosis were obtained in patients participating in these last two trials. Samples were collected at baseline and up to 4 weeks after the end of the study.Citation35 Levels of soluble VEGFR-1, placental growth factor (PlGF), VEGF, and basic FGF were measured. Results of these biomarkers revealed changes in serum PlGF as early as 1 week into treatment which, in fact, correlated with best tumor response. Those patients who had 4.7-fold increase or greater in PlGF levels after 1 week of treatment were more likely to achieve partial remission than those who reached levels less than the 4.7 cutoff. The response rate among patients with a greater than 4.7-fold increase in PlGF was 30% compared with 3% for those below. There was also a significant separation between responders and nonresponders at a 1.6-fold decrease in VEGFR-2 after 3 weeks of treatment. Baseline serum VEGF levels < 671 pg/mL correlated with significantly longer PFS.Citation35
Sunitinib
Three phase II trials with sunitinib are reported here. The first trial used sunitinib 50 mg daily on a 4-week-on/2-week-off schedule, and included 43 subjects with MTC and DTC.Citation36 The overall response rate in the 31 patients with DTC was 13%, with stable disease in 68% of them. On the other hand, no responses were observed in patients with MTC, although stable disease was observed in the majority of patients (83%).Citation36 Grade 3–4 adverse events included neutropenia, thrombocytopenia, hypertension, fatigue, palmar-plantar erythrodysesthesia, and gastrointestinal symptoms. In the second trial, sunitinib was given at 37.5 mg daily to 2-deoxy- 2-[18F]fluoro-D-glucose positron emission tomography-avid advanced thyroid cancers. Three patients had MTC and 15 patients had DTC; the 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography response rate was observed in seven patients (all of them with DTC histology). The most common grade 3 adverse event was neutropenia (28%); no grade 4 toxicities were reported.Citation37 Another phase II study by Ravaud et al also administered sunitinib at 50 mg/day for 4 weeks every 6 weeks.Citation38 Patients had anaplastic, MTC, or RAI-refractory DTC which had being progressing during the 6 months prior to study start. Seventeen patients were enrolled. Median age was 64.3 years old, metastatic sites included lung, lymph nodes, and bones, the treatment was well tolerated with the main side effects including hypertension, asthenia, mucositis, hand-foot syndrome, and thrombocytopenia. Fifteen subjects were evaluable for response; of those, one patient had evidence of partial remission and 12 patients attained stable disease, with one patient showing >90% decrease of thyroglobulin and another patient with a dramatic decrease of symptoms.
Axitinib
Axitinib (AG-013736) is an oral, potent, and selective small molecule inhibitor of VEGFR-1, VEGFR-2, and VEGFR-3, which was recently approved for the treatment of metastatic kidney cancer. It has inhibitory properties at picomolar concentrations against VEGFR-1, VEGFR-2, and VEGFR-3, and at nanomolar concentrations against PDGFR-β and c-Kit. This agent has been shown to affect VEGF-dependent fenestrations and cause regression of tumor vessels.Citation39–Citation42 The dose of axitinib was determined after a phase I trial in which 36 patients with solid tumors were administered this novel agent.Citation43 The recommended dose is 5 mg orally twice daily. Axitinib demonstrated activity against all histologic subtypes of thyroid cancer and the main side effect was hypertension, which was easily managed with antihypertensives.
A phase II trial evaluated the efficacy of axitinib in patients with a diagnosis of RAI-refractory thyroid carcinoma. A total of 60 patients were enrolled in this study.Citation44 Patients had all histologic subtypes of thyroid cancer and they were treated with 5 mg twice daily. All histologic subtypes achieved a clinical response, with eight partial responses in patients with papillary histology, six in follicular, two in medullary, and one in anaplastic thyroid carcinoma. A total of 23 (38%) patients achieved stable disease and there was a median PFS of 18.1 months. A total of 19 (32%) patients reported at least one grade 3 adverse effect from axitinib. The most common adverse effect was hypertension and there were three associated grade 4 adverse effects, which included cerebrovascular accident, hypertension, and reversible leukoencephalopathy. Finally, a total of eight (13%) patients had to discontinue treatment due to adverse effects.Citation44
Cabozantinib
Cabozantinib (XL184) is a dual inhibitor, exerting its effects over MET and VEGF pathways.Citation45,Citation46 Cabozantinib is a small molecule tyrosine kinase inhibitor that targets multiple receptors such as VEGFR-1, VEGFR-2, c-Met, RET, c-Kit, FMS-like tyrosine kinase receptor 3, Tie-2, and RET/PTC. The recommended dose for cabozantinib was determined at 175 mg daily orally after a phase I trial.Citation47 In this study, eight of 16 patients with MTC achieved partial response and all other patients achieved stable disease. Adverse effects included diarrhea, nausea, fatigue, mucosal inflammation, anorexia, elevated liver enzymes, hypertension, and vomiting. Further studies are on their way to study this promising novel agent.
Imatinib
Imatinib is currently approved by the United States Food and Drug Administration and the European Medicines Agency for the treatment of chronic myelogenous leukemia, dermatofibrosarcoma protuberans, and gastrointestinal stromal tumor.Citation48–Citation52 Imatinib targets the BCR-ABL gene translocation, PDGFR, stem cell factor, and c-Kit, and causes inhibition of proliferation and apoptosis in cells that express them.Citation53 Due to RET overexpression in thyroid carcinoma and the fact that both RET and c-Kit are part of the same subfamily of tyrosine kinase receptors, multiple attempts have been made to exploit the use of imatinib in thyroid carcinomas. A phase II trial treated 15 patients with imatinib at 600 mg daily and in case of clinical response the dose could be escalated to 800 mg daily.Citation54 All patients enrolled in this trial had a confirmed diagnosis of MTC. There were no objective responses observed and the median duration of treatment was 4 months. A similar trial enrolled nine patients with diagnosis of MTC. The patients were treated with imatinib 600 mg daily with a median duration of 13 months. A total of seven patients achieved stable disease at 3 months, but only one of these remained with stable disease at 12 months. There were no clinical responses, and the median PFS was 6 months.Citation55
Conclusion
Systemic chemotherapy has been used for years for advanced or metastatic thyroid carcinomas with low efficacy. Angiogenesis plays a crucial role in tumor progression because tumor angiogenesis is driven by host-derived circulating factors. Nowadays, there are multiple novel therapies that target angiogenesis in clinical trials. The most successful agents target the VEGFRs, with potential targets including the mutant kinases associated with papillary and medullary oncogenesis. VEGFR inhibition has relied on the use of small molecular inhibitors. These drugs inhibit tyrosine kinase activity of VEGFRs as well as other tyrosine kinases. At least one phase III trial has showed improvements in PFS with the use of vandetanib, an MKI of VEGFR, epidermal growth factor receptor, and RET.
As discussed in this manuscript, not all MKIs have the same effect on RAI-refractory thyroid cancers. Some are more effective on certain histologic subtypes (). Thus, it is important to identify biomarkers which can help sort out the best therapeutic choice. Efforts in this regard are underway in clinical trials. As an example, levels of soluble VEGFR-1, PlGF, VEGF, and basic FGF have been studied as biomarkers with the use of motesanib. Initial analyses have shown that their levels may correlate with response and/or PFS. Randomized trials for other agents are currently underway, and the treatment for patients with metastatic or advanced thyroid carcinoma now emphasizes clinical trial opportunities for novel agents with considerable promise.
Table 2 Phase II and III trials reported of multikinase inhibitors in patients with advanced and refractory thyroid cancer
Disclosure
Luis Raez has received research support from Pfizer. All other authors report no conflicts of interest in this work.
References
- SiegelRWardEBrawleyOJemalACancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deathsCA Cancer J Clin201161421223621685461
- Eustatia-RuttenCFCorssmitEPBiermaszNRPereiraAMRomijnJASmitJWSurvival and death causes in differentiated thyroid carcinomaJ Clin Endocrinol Metab200691131331916263822
- GottliebJAHillCSJrChemotherapy of thyroid cancer with adriamycin. Experience with 30 patientsN Engl J Med197429041931974808917
- ShimaokaKSchoenfeldDDeWysWDCreechRHDeContiRA randomized trial of doxorubicin versus doxorubicin plus cisplatin in patients with advanced thyroid carcinomaCancer1985569215521603902203
- NikiforovYEOhoriNPHodakSPImpact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samplesJ Clin Endocrinol Metab201196113390339721880806
- CommanderHWhitesideGPerryCVandetanib: first global approvalDrugs201171101355136521770481
- RobertsPJDerCJTargeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancerOncogene200726223291331017496923
- XingMBRAF mutation in thyroid cancerEndocr Relat Cancer200512224526215947100
- HendersonYCShellenbergerTDWilliamsMDHigh rate of BRAF and RET/PTC dual mutations associated with recurrent papillary thyroid carcinomaClin Cancer Res200915248549119147753
- LupiCGianniniRUgoliniCAssociation of BRAF V600E mutation with poor clinicopathological outcomes in 500 consecutive cases of papillary thyroid carcinomaJ Clin Endocrinol Metab200792114085409017785355
- XingMWestraWHTufanoRPBRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancerJ Clin Endocrinol Metab200590126373637916174717
- WeisSMChereshDATumor angiogenesis: molecular pathways and therapeutic targetsNat Med201117111359137022064426
- KerbelRSTumor angiogenesisN Engl J Med2008358192039204918463380
- BunoneGVigneriPMarianiLExpression of angiogenesis stimulators and inhibitors in human thyroid tumors and correlation with clinical pathological featuresAm J Pathol199915561967197610595926
- KleinMPicardEVignaudJMVascular endothelial growth factor gene and protein: strong expression in thyroiditis and thyroid carcinomaJ Endocrinol19991611414910194527
- LennardCMPatelAWilsonJIntensity of vascular endothelial growth factor expression is associated with increased risk of recurrence and decreased disease-free survival in papillary thyroid cancerSurgery2001129555255811331447
- MologniLDevelopment of RET kinase inhibitors for targeted cancer therapyCurr Med Chem201118216217521110809
- HouvrasYCompleting the arc: targeted inhibition of RET in medullary thyroid cancerJ Clin Oncol201230220020222162569
- LangmuirPBYverAClin Pharmacol Ther20121911718022158569
- WellsSAJrGosnellJEGagelRFVandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancerJ Clin Oncol201028576777220065189
- RobinsonBGPaz-AresLKrebsAVasselliJHaddadRVandetanib (100 mg) in patients with locally advanced or metastatic hereditary medullary thyroid cancerJ Clin Endocrinol Metab20109562664267120371662
- WellsSAJrRobinsonBGGagelRFVandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trialJ Clin Oncol201230213414122025146
- GramzaAWWellsSABalasubramaniamSFojoATPhase I/II trial of vandetanib and bortezomib in adults with locally advanced or metastatic medullary thyroid cancer: phase I results [abstract]J Clin Oncol201129Suppl5565
- SternbergCNDavisIDMardiakJPazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trialJ Clin Oncol20102861061106820100962
- BibleKCSumanVJMolinaJREfficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium studyLancet Oncol2010111096297220851682
- ShermanSIJarzabBCabanillasMEA phase II trial of the multitargeted kinase inhibitor E7080 in advanced radioiodine (RAI)- refractory differentiated thyroid cancer (DTC) [abstract]J Clin Oncol201129Suppl5503
- ShumakerRZhouMRenMFanJMartinezGDarpoBLenvatinib (E7080) does not prolong the QTc interval: results from a thorough QT study in healthy volunteers [abstract]Mol Cancer Ther20111011 Suppl 1C116
- KloosRTRingelMDKnoppMVPhase II trial of sorafenib in metastatic thyroid cancerJ Clin Oncol200927101675168419255327
- Gupta-AbramsonVTroxelABNelloreAPhase II trial of sorafenib in advanced thyroid cancerJ Clin Oncol200826294714471918541894
- BroseMSTroxelABRedlingerMEffect of BRAFV600E on response to sorafenib in advanced thyroid cancer patients [abstract]J Clin Oncol200927Suppl 156002
- LamETRingelMDKloosRTPhase II clinical trial of sorafenib in metastatic medullary thyroid cancerJ Clin Oncol201028142323233020368568
- RosenLSKurzrockRMulayMSafety, pharmacokinetics, and efficacy of AMG 706, an oral multikinase inhibitor, in patients with advanced solid tumorsJ Clin Oncol200725172369237617557949
- ShermanSIWirthLJDrozJPMotesanib diphosphate in progressive differentiated thyroid cancerN Engl J Med20083591314218596272
- SchlumbergerMJEliseiRBastholtLPhase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancerJ Clin Oncol200927233794380119564535
- BassMBShermanSISchlumbergerMJBiomarkers as predictors of response to treatment with motesanib in patients with progressive advanced thyroid cancerJ Clin Endocrinol Metab201095115018502720739388
- CohenEENeedlesBMCullenKJPhase 2 study of sunitinib in refractory thyroid cancer [abstract]J Clin Oncol200826Suppl6025
- GoulartBCarrLMartinsRGPhase II study of sunitinib in iodine refractory, well-differentiated thyroid cancer (WDTC) and metastatic medullary thyroid carcinoma (MTC) [abstract]J Clin Oncol200826Suppl6062
- RavaudAde la FouchardiereCCourbonFSunitinib in patients with refractory advanced thyroid cancer: the THYSU phase II trial [abstract]J Clin Oncol200826Suppl6058
- InaiTMancusoMHashizumeHInhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghostsAm J Pathol20041651355215215160
- MancusoMRDaviasRNorbergSMRapid vascular regrowth in tumors after reversal of VEGF inhibitionJ Clin Invest2006116102610262117016557
- KambaTTamBYHashizumeHVEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculatureAm J Physiol Heart Circ Physiol20062902H560H57616172168
- BaffertFLeTSenninoBCellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signalingAm J Physiol Heart Circ Physiol20062902H547H55916172161
- RugoHSHerbstRSLiuGPhase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: pharmacokinetics and clinical resultsJ Clin Oncol200523245474548316027439
- CohenEERosenLSVokesEEAxitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II studyJ Clin Oncol200826294708471318541897
- JolyAHSimultaneous blockade of VEGF and HGF receptors results in potent anti-angiogenic and anti-tumor effectsEur J Cancer2006Suppl 43517098420
- SenninoBNaylorRMTabruynSPYouWKAftabDTMcDonaldDMReduction of tumor invasiveness and metastasis and prolongation of survival of RIP-Tag2 mice after inhibition of VEGFR plus c-Met by XL184 [abstract]Mol Cancer Ther2011812 Suppl 1A13
- KurzrockRShermanSHongDSA phase I study of XL184, a RET, VEGFR2, and MET kinase inhibitor, in patients (pts) with advanced malignancies, including a subgroup of pts with medullary thyroid cancer (MTC)Paper presented at: AACR-NCI-EORTC International Conference on Molecular Targets and Cancer TherapeuticsOctober 21–24, 2008Geneva, Switzerland.
- DrukerBJTalpazMRestaDJEfficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemiaN Engl J Med2001344141031103711287972
- DemetriGDvon MehrenMBlankeCDEfficacy and safety of imatinib mesylate in advanced gastrointestinal tumorsN Engl J Med2002347747248012181401
- O’BrienSGGuilhotFLarsonRAImatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myelogenous leukemiaN Engl J Med200334811994100412637609
- VerweijJvan OosteromABlayJYImatinib mesylate (STI-571 Glivec, Gleevec) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for this molecular target. Results from the EORTC Soft Tissue and Bone Sarcoma Group phase II studyEur J Cancer200339142006201112957454
- SawyersCLImatinib GIST keeps finding new indications: successful treatment of dermatofibrosarcoma protuberans by targeted inhibition of the platelet-derived growth factor receptorJ Clin Oncol200220173568356912202652
- DrukerBJTamuraSBuchdungerEEffects of a selective inhibitor of the Abl tyrosine kinase on growth of Bcr-Abl positive cellsNat Med1996255615668616716
- de GrootJWBZonnenbergBAQuarles van Ufford-MannessePA phase II trial of imatinib therapy for metastatic medullary thyroid carcinomaJ Clin Endocrinol Metab20079293466346917579194
- Frank-RaueKFabelMDelormeSHaberkornURaueFEfficacy of imatinib mesylate in advanced medullary thyroid carcinomaEur J Endocrinol2007157221522017656601