Abstract
Proteasomal inhibition revolutionized myeloma therapies in this decade of novel agents. The only US Food and Drug Administration approved proteasome inhibitor so far, bortezomib effectively targets the constitutive proteasome subunit β5 of the 26S proteasome. Bortezomib induces high and quality response rates that are durable. However, myeloma cells acquire resistance to bortezomib through various mechanisms. Further, grade 3/4 peripheral neuropathy is seen in up to a quarter of patients treated with bortezomib. While the recent change in the mode of administration via the subcutaneous route is associated with a lower incidence of grade 3/4 peripheral neuropathy, it remains a major concern. The second generation proteasome inhibitors are promising, with increased preclinical efficacy and a better administration schedule. The current review spotlights the second generation proteasome inhibitors with special focus on the safety and efficacy of carfilzomib, an epoxyketone with lesser peripheral neuropathy, which exhibits irreversible proteasome inhibition. In this article, we review the pharmacology and preclinical and clinical efficacy and safety of carfilzomib alone and in combination with other chemotherapeutic agents in the various lymphoid neoplasms and multiple myeloma as well as ongoing clinical trials.
Introduction
The proteasome is a validated target in the management of multiple myeloma (MM) and lymphoma. Combinations of proteasome inhibition with other conventional or targeted agents have proven quite successful for both newly diagnosed and relapsed patients. These combinations have significantly improved overall response rates, complete response, progression free survival, and overall survival. This review summarizes the biologic rational for why this pathway is so important in plasma cell disorders as well as second generation agents in development, including the mechanism of proteasomal inhibition (the ubiquitin-proteasome system); various novel proteasome inhibitors (PIs) under evaluation, with a focus on carfilzomib; the preclinical activity of carfilzomib in myeloma and various lymphoid neoplasms; and the efficacy data from the early Phase trials, and concludes by summarizing the ongoing trials with carfilzomib for refractory lymphoid neoplasms and myeloma.
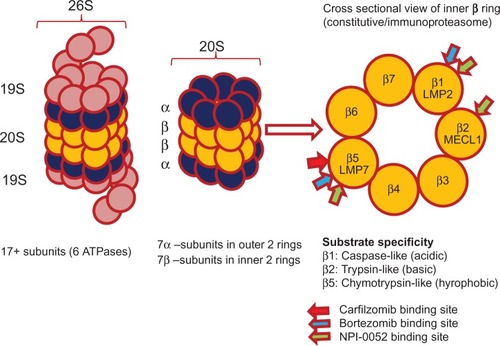
The ubiquitin proteasome system is a multicatalytic proteinase complex that degrades a wide variety of protein substrates within normal and transformed cells to maintain cellular homeostasis.Citation1 The intracellular proteins that regulate cellular processes such as cell-cycle control, apoptosis, deoxyribonucleic acid (DNA) repair, and response to oxidative stress are controlled by it. Intracellular proteins targeted for degradation by the proteasome are first ubiquitinated via the ubiquitin conjugation system, which requires the coordinated reactions of three enzymes: the ubiquitin-activating enzyme, ubiquitin-conjugating enzyme, and ubiquitin ligases. Once ubiquitinated, proteins targeted for degradation bind to the 26S proteasome. The 26S proteasome has a molecular mass of 200 kilo daltons (kDa) and contains a 20S proteolytic hollow core structure and two 19S regulatory caps to regulate the entry of target proteins (). A 19S regulatory subunit hosts multiple ATPase active sites and ubiquitin binding sites that recognize the ubiquitinated proteins, transferring them to the catalytic core for proteolysis.Citation2,Citation3 The 20S core contains two outer rings (α), comprising 7 α subunits (α1–α7), and two inner rings (β), comprising 7 β-subunits (β1–β7). The core has three active enzymatic sites: a chymotrypsin-like (CT-L) on the β5 subunit, trypsin-like (T-L) on the β2 subunit, and a caspase-like (C-L) site on the β1 subunit ().Citation4,Citation5
The ubiquitinated proteins are cleaved within the 20S core by one or more of the three separate threonine protease activities – CT-L for hydrophobic residues, T- L for basic residues, and C-L for acidic residues – though the significance of the different proteolytic sites and the efficacy of inhibitors vary with the protein substrate.Citation6 CT-L activity represents the target for most clinical PIs. Increased levels of CT-L activity and the resultant protein breakdown have been associated with malignancies, suggesting a vulnerable target. It is the most sensitive protease site for inactivation, with resultant accumulation of ubiquitinated proteins and induction of apoptosis.Citation7,Citation8
Proteasomes are present in their constitutive form and an immunoproteasome form, and are predominantly seen in cells of hemopoietic origin. The immunoproteasome is assembled with alternative subunits whose substrate specificity is altered relative to the normal proteasome. The alternative β forms, denoted as β1i (LMP2), β2i (MECL1), and β5i (LMP7), are expressed in response to the exposure to proinflammatory signals, such as cytokines, in particular, interferon gamma.Citation9 The most currently available PIs target both the constitutive and immunoproteasome, and emerging evidence suggests that dual targeting enhances efficacy ().Citation10
Proteasome inhibitors
Bortezomib (PS-341) is the only PI approved by the US Food and Drug Administration for both newly diagnosed and relapsed myeloma patients as well as relapsed mantle cell lymphoma patients.Citation11 The introduction of bortezomib containing induction regimens specifically combining with the immunomodulatory drugs thalidomide or lenalidomide has significantly enhanced the overall response rates and the depth of responses.Citation12,Citation13 A recent meta-analysis by Nooka et al suggests an improvement in overall survival associated with bortezomib containing inductions not seen with other agents.Citation14 Through the addition of bortezomib to the induction regimens in transplant-eligible patients with MM, not only was there improvement in the overall response rate (ORR) and deepening in the magnitude of the responses seen, but there was also the prolonging of progression free survival and overall survival compared with the nonbortezomib containing induction regimens.Citation14 In relapsed/refractory MM (R/R MM), bortezomib combination therapies induced responses and prolonged survival in poor risk patients, as defined by the presence of high risk cytogenetic abnormalities.Citation13 Without a doubt, bortezomib has revolutionized myeloma therapies in this decade of novel agents. However, adverse side effects, including peripheral neuropathy (PN) and bortezomib-resistance, are inevitable and the currently established dosing of bortezomib administration, either intravenous or subcutaneously, on days 1, 4, 8, and 11 of a 21-day cycle can be arduous.
The incidence of grade (G) 3/4 PN is seen in up to 28% of the patientsCitation15 receiving bortezomib and the incidence is greater in the initial course of therapy, but plateaus after five cycles.Citation16,Citation17 In the process of inhibiting the proteasome, bortezomib results in the breakdown of some essential proteases, which is presumed to be associated with the observed neurotoxicity. Even though the recent change in the mode of administration via the subcutaneous (SC) route is associated with less incidence of G3-PN,Citation18 in 38% of patients, it continues to remain a major problem given the association of any grade PN. The reduced incidence is thought to be related to lowering the maximum concentration exposure of bortezomib when given subcutaneously, without affecting the efficacy.
Bortezomib is a boronate reversible PI () that primarily targets the constitutive proteasome subunit β5 of the 26S proteasome (). Myeloma cells acquire resistance to bortezomib by modulating the cell signal pathways (eg, decreased proteasome inhibition with high levels of heat shock protein 27), altering the proteasome complex (eg, subunit β5 gene mutation and over expression of subunit β5 protein, leading to impaired binding of bortezomib),Citation19 or escalating alternate methods of protein degradation, thereby effciently bypassing the proteasome inhibition (clearance of the protein via the aggresome pathway).Citation20 The most promising options to overcome the above stated issues are second generation PIs with better schedules of administration, preferably oral, that can circumvent bortezomib-resistance mechanisms. We have summarized a few novel PIs currently under clinical evaluation that have shown activity, even in bortezomib resistant cell lines.
Table 1 Various novel proteasome inhibitors
MLN9708
MLN9708 (Millennium Pharmaceuticals) is a second-generation, reversible PI, boronate analog of bortezomib () which has good oral bioavailability and hydrolyzes to the active form, MLN2238. Preclinically, in vitro studies have shown improved efficacy of MLN2238 compared to bortezomib in the lymphoma models. In vivo, MLN9708 demonstrated significant antimyeloma activity.21 Two Phase I studies with MLN9708 in R/R MM as a single agent22,23 and one Phase I study in combination with lenalidomide and dexamethasone in newly diagnosed patients24 were presented at ASH 2011. In the relapsed/refractory Phase I trials, biweekly22 and weekly23 dose escalation cohorts were evaluated to establish the maximum tolerated dose (MTD). Both schedules were well tolerated and demonstrated disease stabilization for 12.9 months and 9.5 months in 61% and 45% patients, respectively (). Fatigue, thrombocytopenia, nausea, and neutropenia were the most common >G3 serious adverse events (SAEs). Neutropenia was not observed in newly diagnosed patients at an MTD of 2.97 mg/m2, sug-gesting that heavily pretreated patients may be more prone to neutropenia. MLN9708 in combination with lenalidomide and dexamethasone demonstrated ≥partial response ($PR) rates in 100% patients and ≥very good partial response (≥VGPR) per International Myeloma Working Group uniform response criteria25 in 60% patients. These results are encouraging for the possibility of a highly efficacious, oral triplet-regimen in the induction therapy for those newly diagnosed with MM.
Table 2 Other proteasome inhibitors in multiple myeloma: safety, tolerability, and efficacy
CEP-18770
CEP-18770 (Cephalon) is another second generation reversible PI that is orally active (). In myeloma xenograft models, CEP-18770 demonstrated sustained pharmacodynamic activity and potent anti-tumor activity with tumor regressions.26,27 Overall median survival was improved in a systemic model of human MM.27 Addition-ally, CEP-18770 exhibited a favorable toxicity profile with human bone marrow progenitors and bone marrow-derived stromal cells. A Phase I study of CEP 18770 in patients with solid tumors and NHL was completed.28 In patients with R/R MM, a Phase I/II study to determine the MTD of CEP-18770 and to evaluate the antitumor activity is currently being evaluated.Citation29 In the same patient population, another Phase I/II study combining CEP-18770 with lenalidomide and dexamethasoneCitation30 is ongoing based on the efficacy and tolerability from the xenograft models.Citation31
NPI-0052 (salinosporamide A, marizomib)
NPI-0052 (Nereus Pharmaceuticals) is a nonpeptide irreversible PI that induces activity by covalently modifying the active site threonine residues of the 20S proteasome (). It is an orally available agent that inhibits CT-L and T-L activities at much lower concentrations, though higher concentrations are required to inhibit C-L activity ().Citation32 Preclinical studies show significant activity and a large therapeutic index in hematologic and solid tumor models.Citation33 Efficacy was noted in MM tumor models resistant to bortezomib.Citation34 A Phase I trial of NPI-0052 in patients with R/R MM was evaluated in 32 patients at doses ranging from 0.025 mg/mCitation2–0.7 mg/mCitation2. Inhibition of CT-L activity at days 1 and 15 was 73% and 99%, respectively, and 28% of patients achieved a durable stable disease (SD) duration . 6 months.Citation35 Preclinically, NPI-0052 in combination with lenalidomide triggered in vitro and in vivo synergistic cytotoxicity in MM. Apoptosis was primarily dependent on caspase-8 signaling. In animal tumor model studies, this combination was well tolerated, inhibited tumor growth, and prolonged survival.Citation36 Other Phase I trials of NPI-0052 in patients with refractory solid tumors, lymphomas,Citation37 and R/R MM alone or in combination are ongoing.Citation38 Trials regarding the efficacy of NPI-0052 in combination with vorinostat in patients with non-small-cell lung cancer, pancreatic cancer, melanoma, or lymphomaCitation39 are ongoing.
ONX 0912 (oprozomib)
ONX 0912 (Onyx Pharmaceuticals, San Francisco, California, USA) is an irreversible tripeptide epoxyketone PI that is orally bioavailable (). Similar to bortezomib, it predominantly inhibits CT-L activity of β5 subunit of the proteasome. ONX 0912 was shown to induce apoptosis in MM cells resistant to bortezomib in vitro. In xenograft models, it significantly reduced tumor growth and prolonged survival. Anti-MM activity was shown to be enhanced when ONX 0912 was used in combination with bortezomib, lenalidomide, and dexamethasone or a pan-histone deacetylase (HDAC) inhibitor.Citation40 A Phase I study of ONX 0912 in patients with advanced refractory or recurrent solid tumors showed an acceptable safety profile and levels of proteasome inhibition > 80% when given daily for 5 days every 2 weeks in patients with advanced solid tumors. Nausea, vomiting, and diarrhea were the most common adverse events and MTD was not reached. Dose-escalation was started at 30 mg and is currently escalating at 180 mg in 30 mg increments. Stable disease of >3 month duration has been observed in three patients with prostate cancer, liposarcoma, and non-small-cell lung cancer.Citation41 Another Phase I/II study in patients with Waldenstrom’s macroglobulinemia (WM), MM, and mantle cell (MCL)Citation42 is planned, but not open for patient recruitment yet.
Carfilzomib
Carfilzomib (Onyx Pharmaceuticals) is a second generation irreversible epoxyketone analogue of YU-101Citation43 with increased aqueous solubility due to the addition of a NH2-terminal morpholino moiety (). It inhibits CT-L activity, resulting in sustained proteasomal inhibition. It also exhibits less selectivity for T- L and C-L proteases, which may reduce toxicities that are seen with bortezomib, especially neurotoxicity.Citation16,Citation44,Citation45 We will further review the preclinical and clinical data of carfilzomib in patients with refractory lymphoid neoplasms and R/R MM.
Preclinical activity of carfilzomib in AML/ALL/CLL
The functional (antiproliferative and proapoptotic) effects of bortezomib and carfilzomib were compared by Stapnes et al on primary human acute myeloid leukemia (AML) cells in vitro. Both drugs inhibited autocrine- and cytokine-dependent proliferation of primary AML blasts in a dose dependent manner (bortezomib: 21 nmol/l–24.8 nmol/l and carfilzomib: 9.8 nmol/l–10.4 nmol/l). AML cells incubated in the presence of bortezomib or carfilzomib showed an increase in the percentage of apoptotic cells and a decrease in cell viability, suggesting the proapoptotic nature of both drugs. The tested functional effects of bortezomib and carfilzomib were identified in AML blasts, even in the presence of nonleukemic stromal cells, after coculturing leukemia cells with bone marrow stromal cells in the in vitro model. Similar trends were observed with the acute lymphoblastic leukemia (ALL) cells in vitro.Citation60 In combination with PIs, cytotoxic agents used for AML therapy, idarubicin, and cytarabine caused a dose-dependent decrease in cell viability after 18 hours of in vitro incubation relative to the drugs tested alone. Further, HDAC inhibitors (HDACis), such as valproic acid, which are finding a place in AML therapy, when combined with bortezomib (10 nmol/l) caused 79% inhibition, whereas valproic acid alone caused no significant inhibition and bortezomib caused 62% growth inhibition when tested alone.Citation60 Antiproliferative and proapoptotic effects of carfilzomib are greater than bortezomib.
The differential sensitivity of pediatric ALL cells to bortezomib, carfilzomib, ONX 0912, and ONX 0914 was examined for the drug concentrations that are required for 50% cell death (LC50) and for correlation with the protein expression levels of the constitutive proteasome and immunoproteasome subunits.Citation61 LC50 was determined in 29 pediatric ALL and twelve AML patient samples after drug exposure for 4 days using the MTT cytotoxicity assay. ALL cells were significantly more sensitive for bortezomib than AML cells (median LC50:6.0 nM vs 14.2 nM, respectively, P = 0.002). The corresponding sensitivity for carfilzomib (median LC50) was 4.1 nM and 20.1 nM (P = 0.00). Expression of constitutive proteasome subunits was higher in AML cells than in ALL cells. LC50 concentrations of carfilzomib significantly correlated with beta 5 and beta 1 expression. ALL cells were more sensitive to PIs than AML. This may be due to lower constitutive proteasome unit expression in these cells. ALL/AML cells display sensitivity to PIs without cross-resistance between them. Carfilzomib is promising for bypassing bortezomib resistant cells.
In the chronic lymphocytic lymphoma (CLL) cells, the mechanism of carfilzomib action is unknown, though pre-clinical data demonstrated potent cytotoxicity of carfilzomib in primary CLL cells. Gupta et al evaluated the effect of carfilzomib to inhibit the chymotrypsin-like proteasome subunit in CLL cells. Carfilzomib causes CLL cell death ex vivo by a caspase-dependent apoptotic pathway, indicated by Poly (ADP-ribose) polymerase (PARP) cleavage and rescued by the broad caspase inhibitor Boc-D-fmk. Further, the pro-apoptotic protein Noxa is increased following carfilzomib treatment.Citation62 Carfilzomib causes apoptosis in primary CLL cells irrespective of p53 status, which is of tremendous clinical significance given resistance to standard therapies and dismal overall survival. Proteasome inhibition as a relevant therapeutic target in CLL requires further exploration.
Preclinical activity of carfilzomib in lymphoma
Like bortezomib, carfilzomib induces growth inhibition of mantle cell lymphoma cells both in vitro and in vivo. In vitro, the anti-tumor effects of carfilzomib and bortezomib were the same, but in vivo, carfilzomib was well tolerated, significantly inhibited tumor growth, and showed prolonged survival relative to bortezomib-treated mice, indicating the therapeutic potential of carfilzomib in MCL therapy.Citation63 In the B-cell lymphoma cell lines that are resistant to rituximab-chemotherapy, carfilzomib showed significant activity.Citation64,Citation65 Compared to bortezomib, carfilzomib exhibited greater dose-dependent and time-dependent cytotoxicity against rituximab-chemotherapy cell lines and the primary tumor cells of patients with R/R B-cell lymphoma by up-regulating proapoptotic proteins and inducing G2/M cell cycle arrest. It also demonstrated the ability to overcome chemoresistance in chemotherapy resistant cell lines and potentiated the anti-tumor activity of paclitaxel and gemcitabine, suggesting that the clinical use of carfilzomib can overcome resistance to conventional chemotherapeutic agents. A possible mechanism of sensitizing resistant cells to chemotherapy is the down-regulation of p52. Carfilzomib targets the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)-p52 pathway by down-regulating the tumor necrosis factor-receptor family B-cell activating factor (BAFF), resulting in lymphoma cell growth inhibition and apoptosis induction.Citation66 Carfilzomib showed single agent activity in WM. Inhibition of CT-L activity was seen in both LMP7 and β5 subunits in WM and immunoglobulin M (IgM) secreting lymphoma cell lines, resulting in increased toxicity in primary WM cells by both activation of caspase-dependent and caspase-independent mechanisms.Citation67
Carfilzomib and HDAC inhibitors
Histone deacetylase inhibitors reverse histone deacetylation, a process involved in the epigenetic regulation of gene expression, thereby promoting cell death and/or differentiation.Citation46 Apoptosis from HDACis occurs through the induction of oxidative injury and disruption of DNA repair, among other processes. Co-administration of HDACis with PIs induces a marked increase in mitochondrial injury and apoptosis. Increased activity is associated with transcription factor NF-κB inactivation, c-Jun NH2-terminal kinase activation, p63 induction, caspase-dependent cleavage of p21CIP1, p27KIP1, and Bcl-2, as well as antiapoptotic protein myeloid cell leukemia sequence 1, X-linked inhibitor of apoptosis, and cyclin D1 downregulation. The combination also induces reactive oxygen species generation that further induces apoptosis.Citation47 In myeloma and lymphoma cells, HDACi inhibits cell growth and induces apoptosis as a single agent and is also synergistic with bortezomib.Citation48–Citation50 Phase I studies combining vorinostat with bortezomib in R/R MM showed promising antimyeloma activity of the regimen,Citation51 which is being further evaluated. Similarly, in MCL, bortezomib-induced apoptosis in human MCL cells was shown to be potentiated with the pan-HDACi, panobinostat.Citation52
Coadministration of carfilzomib, vorinostat, and SNDX-275 was evaluated in MCL cells. At minimally lethal concentrations, this combination induced a sharp increase in mitochondrial injury and apoptosis in both cell lines and primary MCL cells.Citation53 Similar activity was seen in human diffuse large B-cell lymphoma (DLBCL) cells.Citation54 Carfilzomib was also tested in combination with HDAC6-specific inhibitor (WT-161) in MCL. This caused induction of the pro-apoptotic transcription factors and synergistically induced apoptosis of cell lines and primary MCL cells.Citation55 These results support the basis for combining carfilzomib with HDACi in clinical trials. A Phase I study of carfilzomib in combination with vorinostat in R/R B-cell lymphomasCitation56 and a Phase I/II study of carfilzomib, lenalidomide, vorinostat, and dexamethasone in R/R MMCitation57 are ongoing. A Phase I studyCitation58 and a Phase I/II studyCitation59 of carfilzomib in combination with panobinostat in R/R MM are underway.
Preclinical activity of carfilzomib in myeloma
Carfilzomib is structurally dissimilar to bortezomibCitation68 and is less active against nonproteasomal proteases when compared to bortezomib.Citation69 This target, which is specificity relative to bortezomib, may also explain the ability of carfilzomib to overcome resistance to bortezomib both in vitro and in vivo.Citation70 Carfilzomib inhibits proliferation and induces apoptosis in bortezomib-resistant MM cell lines and in primary MM cells from patients with clinically established resistance to bortezomib and other conventional agents.Citation70 The proteasomal binding site affinity and magnitude of proteasomal inhibition of carfilzomib differ from bortezomib in bortezomib sensitive cell lines as well as bortezomib-resistant cell lines.Citation71
Site affinity
Carfilzomib exhibits inhibitory-specificity for 20S catalytic activities. Eighty percent CT-L activity inhibition occurred in the β5 subunit (10 nM), with minimal effects on the C-L or T- L activities at 10-fold higher concentrations.Citation70 Bortezomib targets β1 and β1i subunits in addition to the β5 subunits,Citation72 but does not inhibit the β2 subunit at clinically relevant concentrations. Interestingly, upregulation of the β2 subunit is observed in bortezomib-resistance and combining specific β2 inhibitors to overcome bortezomib-resistance is a potential strategy.Citation73 Kuhn et al demonstrated that exposure to low-dose carfilzomib led to preferential binding specificity for the β5 constitutive 20S proteasome and the β5i immunoproteasome subunits. At higher doses (>1 μM), binding occurred with the β1 and β2 subunits as well as with β1i and β2i.Citation70 At doses of carfilzomib that selectively inhibit both CT-L active sites in the β5 and LMP7 subunits, antitumor responses were seen in MM, non hodgkin lymphoma (NHL), and leukemia cells. Individual chemical inhibitors of the CT-L subunits β5 or LMP7 had no antitumor effects, but combining both inhibitors produced an antitumor effect equivalent to carfilzomib.Citation74
Cytotoxicity
Exposing hematologic tumor cell lines to carfilzomib for a brief period of one hour leads to rapid proteasomal inhibition followed by accumulation of polyubiquitinated proteins and induction of apoptotic cell death.Citation70 Relative to bortezomib, carfilzomib demonstrated increased apoptosis in the cell line models of MM by 1.5 to 2.0 fold through the intrinsic (caspase-9) and extrinsic pathways (caspase-8 and caspase-3). Proapoptotic signaling via Jun-N-terminal kinase (JNK) phosphorylation was also enhanced with carfilzomib compared to bortezomib. In patient-derived purified CD138+ plasma cells, continuous exposure to escalating doses of carfilzomib induced a dose-dependent proteasome inhibition, as measured by the CT-L activity, decreased cellular viability.Citation70
The timing of proteasomal inhibitory activity with carfilzomib was faster and was observed with a shorter duration of exposure.Citation71 One hour following treatment with carfilzomib there was more cytotoxicity than bortezomib for tumor cell lines, specifically hematologic tumor lines.Citation71 Rapid induction of apoptosis occurred during this brief exposure, as evidenced by caspase activation detected as early as 5 hours and maximum annexin V staining occurred by 24 hours. The consequent effects of carfilzomib on apoptosis, growth arrest, or stress response pathways by measuring markers (eg, β-catenin, P21) were more pronounced than with bort-ezomib, which is consistent with the greater effects of carfilzomib on cell viability, apoptosis, and cell cycle progression. Carfilzomib has also been demonstrated to be cytotoxic in bortezomib-resistant tumor cell lines.Citation47,Citation71 The authors demonstrated that prolonging the length of exposure from one hour to 6 hours resulted in markedly increased total proteasome inhibition in both bortezomib and carfilzomib, especially in C-L and T- L sites.Citation71 Inhibition of multiple proteasome active sites restricted the protein turnover to a greater degree than inhibiting CT-L by itself, suggesting a direction for future studies that combines different proteasomal agents such as bortezomib and carfilzomib, which may result in increased proteasomal inhibition.Citation8 Lee et al measured the activity of c20S subunits (β5, β2 and β1) and i20S subunits (LMP7, MECL1, and LMP2). Carfilzomib doses of up to 45 mg/mCitation2 showed ≥80% inhibition of CT-L active sites (β5, LMP7) as well as dose-dependent inhibition of MECL1 and LMP2. Carfilzomib inhibited 63% of all active sites of the immunoproteasome at 45 mg/mCitation2 and 78% at 56 mg/m2.75 Phase I data from Papadopoulos et al administered carfilzomib at 20/56 mg/mCitation2 in patients with relapsed solid tumors, and R/R MM showed encouraging activity with a good safety profile and may provide insight into evaluating whether proteasome inhibition alone would be a predictive marker of clinical response.Citation76
Pharmacokinetics
In rat pharmacokinetic (PK) studies with intravenous (IV) administration, carfilzomib was rapidly cleared from the plasma compartment with a half-life of approximately 15 minutes. The estimated steady-state volume of distribution superseded the blood volume, suggesting significant penetration into peripheral tissues.Citation71 In another PK study,Citation77 plasma half-life ranged from 5 to 20 minutes and plasma clearance values were 195 to 296 mL/min/kg. Carfilzomib binds to the target rapidly and is irreversible. It clears rapidly, but target binding was shown not to be the major mechanism of clearance. Pretreated controls who received a placebo rather than ONX 0912Citation78 to completely inhibit the proteasomal activity (CT-L) showed no significant differences in the PK parameters.Citation77 The Phase I trial with carfilzomib led by O’Connor et al reported pharmacokinetic parameter estimates for three dose levels (11, 15, and 20 mg/mCitation2).Citation79 Variability in plasma concentrations of rapid clearance with a half-life < 30 minutes, as seen in the animal experiments, was observed. The steady state volume of distribution showed good peripheral tissue distribution consistent with studies in rats and monkeys. Elimination occurred through multiple clearance pathways.
Pharmacodynamics
In rat pharmacodynamic with IV administration of carfilzomib, one hour following administration, a dose-dependent suppression of CT-L activity was observed in the blood, heart, adrenals, lung, spleen, bone marrow, and liver. The same activity was not suppressed in brain, suggesting that despite rapid entry into cells, the compound does not readily cross the blood–brain barrier.Citation71 A similar clearance and tissue distribution profile has been reported for bortezomib.Citation80 The in vivo proteasome active site selectivities in mice confirmed that at doses that induced >.80% inhibition of CT-L in blood, bortezomib was able to mediate pronounced inhibition of the CT-L consistent with the active site selectivity profiles observed in cell lines.Citation71 The CT-L activity in vivo is a function of the total dose of carfilzomib administered, not the maximum plasma concentration. Proteasomal inhibition persists even after the drug is cleared systemically, and rapid clearance may prevent its ability to inhibit nonproteasomal targets and may reduce toxicities.Citation77 In the human Phase I trial conducted by O’Connor et al,Citation79 a dose-dependent inhibition of the CT-L proteasome activity in whole blood and peripheral blood mononuclear cells (PBMCs) was seen one hour after carfilzomib dosing. At dose levels > 15 mg/mCitation2,>.75% proteasome inhibition was seen in whole blood and PBMCs after one dose of carfilzomib, and >90% inhibition after five daily doses. Activity normalized to baseline in PBMCs before the next cycle (every 14 days), but not in whole blood, which is thought to be secondary to the inability of erythrocytes to produce new proteins.
Dosing schedules
In rats, proteasome inhibition and recovery was also examined following two dosing schedules: IV carfilzomib administered for two (QDx2) or five (QDx5) consecutive daily doses. No significant differences were observed in the level of inhibition and the rate of recovery of CT-L activity in bone marrow following either dosing schedules compared with a single dose. However, cumulative inhibition of CT-L in blood following QDx2 was more pronounced than QDx5 dosing, likely due to the inability of erythrocytes to recover activity between doses.Citation71
Xenograft models
In the human tumor xenograft models, the antitumor activity of carfilzomib was compared with bortezomib in immuno-compromised mice implanted with established xenografts derived from three tumor cell lines (colorectal adenocarcinoma, B cell lymphoma, and Burkitt’s lymphoma).Citation71 MTD for bortezomib in mouse strains was 1 mg/kg D1 and for carfilzomib was 5 mg/kg. In all three models, at MTD, carfilzomib was more efficacious than bortezomib. Dose-reduction or schedule-alteration of carfilzomib to mimic the bortezomib dosing schedule resulted in decreased efficacy. Both the in vivo and xenograft studies reveal that intensive dosing schedules yield greater proteasomal inhibition in solid tumors and the hematologic tumor models without excessive toxicity.
Animal toxicity studies
In the animal toxicology studies, carfilzomib was administered to rats and monkeys for 5 consecutive days every 2 weeks. A severely toxic dose (dose that causes death or irreversible severe toxicity) in 10% of rodents (STD 10) was 12 mg/mCitation2, with greater than 90% proteasome inhibition in red blood cells one hour after dosing. A dose-dependent decrease in proteasome activity was demonstrated in animals, and equivalent levels of proteasome inhibition were achieved with administration of carfilzomib as either an IV push or an IV infusion. The dose limiting toxicities (DLTs) in animals included toxicity to the gastrointestinal tract, bone marrow, pulmonary, and cardiovascular systems. Carfilzomib did not cross the blood–brain barrier, with similar results reported in rat PD studies by Demo et al.Citation71 Neurotoxicity or behavioral abnormalities were not seen immediately or at 6 to 9 months. In the chronic toxicity studies, carfilzomib at clinical doses was tolerable. DLTs included effects on the gastrointestinal, renal, pulmonary, and cardiovascular systems. As with bortezomib, transient neutrophilia and cyclical thrombocytopenia was seen, which was likely due to a reversible effect on mega-karyocytes.Citation81 Animal toxicity studies supported the tolerability of carfilzomib at doses achieving excellent proteasome inhibition. Based on the animal studies, two Phase I clinical trials in R/R hematological malignancies, PR-171-001Citation79 and PR-171-002,Citation82 with two dosing schedules from the xenograft modelsCitation71 (QDx2 and QDx5) were completed.
Carfilzomib monotherapy: phase I trials
PX-171-001 evaluated the safety and efficacy of carfilzomib in R/R hematologic malignancies, with a dose schedule of carfilzomib administered as an IV push consecutively on days 1–5 every 14 days in eight dose groups (1.2, 2.4, 4, 6, 8.4, 11, 15, and 20 mg/mCitation2); 29 patients (10-MM, 15-NHL, 3-HL, and 1-WM) were enrolled on the study. The median number of 14-day cycles per patient was 4.8, and the mean cumulative carfilzomib exposure was 233 mg/mCitation2. No DLTs were observed in the initial seven dose groups (1.2–15 mg/mCitation2), but one of three patients in the eighth dose group at 20 mg/ mCitation2 level experienced G3 febrile neutropenia. One of two additional patients enrolled in the same group also experienced prolonged G4 thrombocytopenia with central nervous system (CNS) progression. The 15 mg/mCitation2 dose level was determined to be the MTD. Hematological toxicities were less common and included G2 anemia (G2-3 and G3: two patients) and thrombocytopenia (G1-2 and G4: two patients). Nonhematologic toxicities, mainly G1-2 gastrointestinal symptoms, were seen (mild to moderate nausea, 48%; diarrhea, 35%; and constipation, 21%). Respiratory symptoms (cough, 28%; dyspnea, 28%; and exertional dyspnea, 21%) were common. The responses seen include one unconfirmed CR (uCR) in an MCL patient, one PR in an MM patient, two minor responses (MR) – one in an MM patient and one in an WM patient – and SD in a third of patients in the study.
An alternative dosing schedule of carfilzomib administration was tested by dosing carfilzomib on 2 consecutive days, as opposed to the split dosing schedule.Citation71 In PX-171-002,Citation82 during the dose escalation (1.2 to 27 mg/mCitation2) portion of the trial, 37 subjects received carfilzomib on days 1, 2, 8, 9, 15, and 16 of a 28-day cycle. At 15 mg/mCitation2, maximal proteasome inhibition (80%) was achieved one hour after dosing. At and beyond this dose level, among the 16 patients enrolled, five durable responses (134 to 392 days) occurred (four PRs and one MR in an MM patient). Six patients had SD (two MM patients and four lymphoma patients). The responses were rapid in onset, beginning in some subjects after one to two doses. DLTs at 27 mg/mCitation2 were hypoxia, thrombocytopenia, (G2–G4), and elevated creatinine (G2). In the PX-171-001study, the most common reason for discontinuation of the study was the inability to maintain the scheduled dosing among patients who completed at least six cycles of treatment. PX-171-002 demonstrated that consecutive biweekly dosing (days 1, 2, 8, 9, 15, and 16 of a 28-day cycle) of carfilzomib at doses of 20 mg/mCitation2 leads to sustained proteasome inhibition and is well tolerated. Due to the encouraging safety profile of proteasomal inhibition and the clinical efficacy of carfilzomib as a single agent in the Phase I studies, Phase II studies of carfilzomib (PX-171-003 and PX-171-004) were initiated in patients with R/R MM.
Carfilzomib monotherapy: phase II trials
Two Phase II clinical studies evaluated carfilzomib in MM patients: PX-171-003-A0Citation83 (N = 46) in relapsed and refractory MM and PX-171-004Citation84 (N = 129) in relapsed MM. In both studies, patients received carfilzomib 20 mg/mCitation2 IV on days 1, 2, 8, 9, 15, and 16 every 28 days for up to twelve cycles. In the PX-171-003-A0 trial, patients received a median of three cycles and 13 patients completed ≥six cycles. The most common adverse events were fatigue, anemia, thrombocytopenia, nausea, upper respiratory infections, increased creatinine, and diarrhea. PN occurred in <10% of patients with one G3 in a patient with pre-existing G2 PN. The treatment-emergent PN rate was low with G3/4 2.2%, despite the fact that 78% of patients had G1/2 PN at enrollment. The response rate in PX-171-003-A0 was 18% PR, 7% MR, and 41% SD in R/R MM patients; 80% of patients achieved response during the first cycle. Of note, five of the 23 patients with bortezomib-refractory disease achieved an MR or PR. The median time to progression was 5.1 months, with a duration of response (DOR) of 7.4 months. One of the MM patients achieved a PR when the dose was escalated to 27 mg/mCitation2 after the patient began to progress on 20 mg/mCitation2. Subsequently, a “stepped up” dosing schedule incorporated a higher dose of 20/27 mg/mCitation2 in order to maximize the clinical benefit of carfilzomib. Patients were enrolled in a stepped up dosing on PX-171-003-A1Citation85 and received 20 mg/mCitation2 for the first cycle and 27 mg/mCitation2 thereafter.
In PX-171-003-A1, a Phase IIb trial, a total of 266 patients were enrolled and 257 were response-evaluable. The ORR was 24% with a median DOR of 7.4 months. Of the 229 patients that had available cytogenetics, 71 had ≥one cytogenetic abnormality. Among these patients, the ORR was very similar to standard risk patients and achieved an ORR of 28%, with a median DOR of seven months. Median overall survival was 15.5 months. The most common treatment-emergent AEs ≥ G3 SAEs were mostly hematologic events; 27 patients completed twelve cycles and continued on the extension protocol of PX-171-010. Notably, durable responses were achieved in 36% of patients with bortezomib and immunomodulatory drug refractory MM and those with unfavorable cytogenetics. This long term follow up did not demonstrate any cumulative toxicities.
In PX-171-004,Citation84 R/R MM patients received 20 mg/mCitation2 carfilzomib in cohort 1 (n = 94) and stepped up dosing with 20 mg/mCitation2 for the first cycle and 27 mg/mCitation2 thereafter in cohort 2 (n = 70). The study included patients who had never received bortezomib treatment (n = 129) and patients who had received prior bortezomib (n = 35). The subset of patients that were bortezomib treated had an ORR of 18%. Median DOR was 9 months and median time to progression (TTP) was 5.3 months.Citation84,Citation86 Among the nonbortezomib patients, 80% received a transplant in cohort 1 and 67% received a transplant in cohort 2. At baseline, 52% had a PN of any grade, 15% patients had unfavorable cytogenetics, and another 15% had creatinine clearance (CrCl) < 50 mL/ min. Altogether, the median number of cycles received was >6.5 in both groups. Response rates for cohorts 1 and 2 were ORR 42% and 52%; ≥VGPR rates were 17% and 29%; and CR was 3% and 2%, respectively. In patients with unfavorable cytogenetics, the ORR was 36.8%. The median duration of response was 13.1 (7.2-NE) months in cohort 1 and not reached in cohort 2 at a median follow up of 13.8 months. Median progression free survival was 8.3 months. The most common G3/4 toxicities were lymphopenia (14%/19%), anemia (12%/17%), thrombocytopenia (15%/11%), neutropenia (12%/14%), pneumonia (14%/11%), fatigue (12%/1%), and dyspnea (5%/6%). G1/2 PN was seen in 14% and 19% of patients and G 3/4 in 2% and 0%. AEs led to discontinuation of the drug in 22% and 10% of patients, with 29% and 42% of patients completing twelve cycles. The median TTP was 7.6 and 5.3 months in these two groups, respectively. Carfilzomib can induce very high levels of response in patients who have not previously been treated with bortezomib, and even in bortezomib-treated patients, substantial anti-tumor activity was observed. Notably, disease control was achieved in 65% of patients with progressive MM upon entering the study. Patients that had undergone > twelve cycles had good tolerability, and cumulative toxicity has not been observed.
PX-171-005Citation87 is a Phase II trial that evaluated the safety and efficacy of carfilzomib in R/R MM patients with varying degrees of renal insufficiency. A quarter of the total enrolled patients (n = 39) had normal (CrCl 80 mL/min), mild (50–79), moderate (30–49), and severe (<30) renal functions. Patients received carfilzomib at a dose of 15 mg/mCitation2 IV on days 1, 2, 8, 9, 15, and 16 every 28 days for cycle 1, escalating to 20 mg/mCitation2 in cycle 2 and to 27 mg/mCitation2 in cycle 3. G3/4 AEs included anemia, thrombocytopenia, fatigue, increased creatinine, and mental status changes. Carfilzomib was dissociated with t 3/4 at 30–60 minutes and undetectable in plasma within three hours. Proteasome inhibition ranged from 75%–89% at one hour post-dose at doses of 15–20 mg/mCitation2. Thirteen patients achieved ≥MR and another 13 patients achieved SD (37% each). Dose adjustments were not required, suggesting manageable toxicity in renal failure patients.
PX-171-007Citation76 is a Phase Ib/2 study in R/R MM and relapsed solid tumors, designed to evaluate a 30-minute infusion of carfilzomib using a modified stepped-up dosing regimen, based on preclinical safety data showing that a slower infusion was better tolerated than a 2 to 10 minute infusion. MTD of 56 mg/mCitation2 was given on days 1, 2, 8, 9, 15, and 16 of a 28-day cycle. Of a total of 33 patients (four at 36 mg/mCitation2; three at 45 mg/mCitation2; 24 at 56 mg/mCitation2; and two at 70 mg/mCitation2), five received $ten cycles, suggesting tolerability to a prolonged infusion schedule at higher doses. DLTs occurred in two patients treated at 70 mg/mCitation2. The ORR of the MTD cohort (56 mg/mCitation2) was 60% (stringent complete response (sCR) 5%; VGPR 20%, PR 35%; MR 5%; and SD 20%) with ≥G3 AEs in the 20/56 mg/mCitation2 cohort with thrombocytopenia (38%), anemia (21%), and hypertension (13%). Proteasomal inhibition of chymotrypsin-like activity in PBMCs was ≥95% at ≥56 mg/mCitation2 compared to >80% at 20 mg/mCitation2. These encouraging data support prolonging infusion times, which enables higher dose tolerability and a higher magnitude of proteasome inhibition. summarizes the efficacy, safety, and tolerability of carfilzomib in Phase I/II trials.
Table 3 Efficacy, safety, and tolerability of carfilzomib in phase I/II trials
Carfilzomib in combination with lenalidomide and dexamethasone
PX-171-006Citation88 is an ongoing Phase II study in patients with relapsed MM in which carfilzomib is administered in combination with lenalidomide and dexamethasone (CRd). Carfilzomib was administered at 20 mg/mCitation2 on days 1 and 2 of cycle 1; 27 mg/mCitation2 was thereafter administered on days 1, 2, 8, 9, 15, and 16; lenalidomide was administered at 25 mg per os (PO) on days 1–21; and 40 mg dexamethasone PO was administered on days 1, 8, 15, and 22 of a 28-day cycle. Among the 52 patients enrolled, no DLTs were reported and 11.5% of the patients had SAEs (6/52). Hematologic AEs including G3/4 neutropenia (n = 12), anemia (n = 8), and thrombocytopenia (n = 8) were manageable. The ORR was 78% (CR/sCR 18%; VGPR 22%; PR 38%; MR 2%; and SD 8%) and prolonged administration of this regimen with manageable toxicities was possible (14–23 months). Based on these results, a large Phase III trial, ASPIRE, comparing CRd and Rd (PX-171-009) was initiated in July 2010 and is currently accruing patients with a final accrual goal of 700 patients.Citation89
Another Phase I/II study with CRd studied frontline therapy in MM.Citation90 Transplant eligible candidates received four cycles of CRd (carfzomib 20/27/36 mg/mCitation2 on days 1, 2, 8, 9, 15, and 16; lenalidomide 25 mg on days 1–21; and dexamethasone 40/20 mg on days 1, 8, 15, and 22 of a 28-day cycle); patients then underwent stem cell harvest and received four more cycles of CRd (dexamethasone reduced to 20 mg on days 1, 8, 15, and 22 of a 28-day cycle), followed by a maintenance phase from cycle 9 (reduced to 20 mg on days 1, 8, 15, and 22 of a 28-day cycle) onward until disease progression or toxicity. Transplant ineligible patients received eight cycles of CRd and proceeded to the maintenance phase from cycle 9 until disease progression or toxicity. In Phase II, CRd was administered at the maximum planned dose (carfilzomib 36 mg/mCitation2 on days 1, 2, 8, 9, 15, and 16; lenalidomide 25 mg on days 1–21, and dexamethasone 40/20 mg on days 1, 8, 15, and 22 of a 28-day cycle). Evaluating toxicities from cycles 1–8, the most common G1/2 hematological toxicity was thrombocytopenia and the most common nonhematological toxicity was hyperglycemia. Most common G3/4 hematological toxicities included anemia and neutropenia. Dose reductions were required in 31% of patients (carfilzomib 14%; lenalidomide 22%; and dexamethasone 4%). No G3/4 PN was seen among the cohort, but G1/2 PN was seen in 22% of patients. The ORR was 94% with ≥VGPR rates of 65% and sCR/CR/near complete response (nCR) rates of 53%. Patients with unfavorable cytogenetics (deletion 13 by metaphase, hypodiploidy, t(4;14), t(14;16), or deletion 17p) had ≥VGPR rates of 75% and sCR/CR/nCR rates of 56%. Given that the median change in M-protein from cycle 1 day 1 (C1D1) to C3D1 was 82%, most patients achieved the initial cytoreduction by C3. Continuing the same dose of lenalidomide at 25 mg until C4 and further lowering the doses of lenalidomide after stem cell harvesting may decrease the incidence of hematological toxicities associated with this combination. The study design also signifes the importance of maintenance regimens, as 24 of 27 patients continued CRd maintenance beyond cycle 9, and most patients did not require dose modifications. After twelve cycles or more, 100% of patients achieved ≥VGPR rates favoring this regimen, making it an excellent option for frontline therapies in myeloma. Together, these results suggest that the combination of CRd is active and well tolerated with no significant overlapping toxicities. Importantly, lenalidomide-associated neutropenia and thrombocytopenia do not appear to be exacerbated by concurrent treatment with carfilzomib, even up to 27 mg/mCitation2, suggesting that carfilzomib will combine well with other anti-cancer agents.
Carfilzomib in combination with thalidomide and dexamethasone
The European Myeloma Network evaluated the combination of carfilzomib with thalidomide and dexamethasone (Carthadex) as induction and consolidation therapy in transplant eligible patients with MM based on the VTD (bortezomib/thalidomide/dexamethasone) data from Cavo et al, resulting in the highest CR rate after induction and after high dose therapy and transplant in Phase III trials.Citation91 The study evaluated 45 patients for response and safety with the combination of carfilzomib 20 mg/mCitation2 on days 1 and 2 and 27 mg/mCitation2 on days 8, 9, 15, and 16, and thalidomide 200 mg daily and dexamethasone 40 mg on days 1, 8, 15, and 22 of a 28-day cycle. After four such cycles, patients were harvested and received high dose chemotherapy with melphalan 200 mg/mCitation2. Post-transplant, in the consolidation phase, patients received four more cycles of CTd (carfilzomib 27 mg/mCitation2 on days 1, 2, 8, 9, 15, and 16; thalidomide 50 mg daily; and dexamethasone 40 mg on days 1, 8, 15, and 22 of a 28-day cycle). Two SAEs were reported in cycle 1. One patient progressed at cycle 4 and another progressed at the time of stem cell harvest. This combination resulted in an ORR of 84%, and 16% patients achieved CR/sCR. It is very well tolerated, with G1/2 PN in 24% patients and no G3 PN. Dose reductions for thalidomide were done for one of seven patients with G1 PN and three of nine patients with G2 PN. 4% tumor lysis syndrome (TLS) was observed. The ORR for high risk patients (del17p) was 60% and inferior in standard risk patients; however, it is premature to come to conclusions, given the small sample size. Overall, the combination is well tolerated, but the incidence of PN may be further reduced with second and third generation immunomodulatory drugs.
Safety data of carfilzomib
The selectivity of carfilzomib for the proteasome is regarded as the major reason for the low rates of PN relative to bortezomib. Safety data have been compiled for 526 patients enrolled in the trials PX-171-003-A0, PX-171-003-A1, PX-171-004, and PX-171-005.Citation92 Overall, 94 (19%) patients received ≥twelve cycles of carfilzomib with a mean (SD) 5.7 (4.2) cycles. Almost all patients experienced a treatment-emergent AE; the most common AEs of any grade were fatigue, anemia, and nausea. The most frequently reported nonhematological AEs occurring in ≥30% of patients included fatigue (55%), nausea (45%), dyspnea (35%), diarrhea (33%), and pyrexia (30%). The most common $ G3 nonhematological AE occurring in ≥10% of patients was pneumonia (11%). More than 50% patients were treated at 20/27 mg/mCitation2, and fewer than 10% required dose reduction. 18% of patients completed ≥twelve cycles and are participating in an ongoing Phase II study (PX-171-010)Citation93 to monitor long-term safety. No evidence of cumulative toxicity was seen in these heavily pretreated patients with R/R MM.
Hematological safety
Since the majority of patients with R/R MM exhibit therapy-related myelosuppression, hematologic safety data for MM patients is of essential importance. The most frequently reported hematological AEs occurring in ≥30% of patients included anemia (47%) and thrombocytopenia (36%). The most common ≥ G3 hematological AEs occurring in $10% of patients were thrombocytopenia (23%), anemia (22%), lymphopenia (18%), and neutropenia (10%). Platelet counts predictably decreased and reached a nadir by day 8 of a 28-day treatment cycle and returned to normal by the first day of the next cycle. There was no evidence of cumulative thrombocytopenia. There were no G5 hematological events and no G4 or 5 bleeding reactions associated with thrombocytopenia. Febrile neutropenia was reported in 1% of patients.Citation92
PN
Overall PN was reported in 14% of patients across all studies. Although 378 (72%) patients had baseline PN (≤G2), only 13% reported treatment-emergent symptoms during the study. Seven (1.3%) patients experienced maximum G3 PN AEs, and no ≥G4 events were reported; one patient (0.2%) discontinued treatment due to a PN AE and five patients (1%) required dose modifcation or discontinuation due to PN. Martin et alCitation94 evaluated the efficacy and tolerability of carfilzomib in R/R MM patients with a baseline PN of G1-2 from the Phase IIb trail (PX-171-003-A1); 206 (77%) had PN G1-2 at study entry. The >PR rate was 24% and the median DOR was 7.4 months for these heavily pretreated R/R MM patients (median lines of therapy were 13 and median disease duration was 5.9 years) in both the overall and PN-baseline cohorts. These results suggest that PN has no impact on the depth or durability of responses, or on the tolerability of carfilzomib and that it can safely be administered in heavily pretreated R/R MM patients.
Special considerations
Unfavorable cytogenetics
Jakubowaik et alCitation95 evaluated the outcomes of patients with high risk cytogenetics from the PX-171-003-A1 trial for Phase II patients with R/R MM with carfilzomib as a single agent (20/27 mg/mCitation2 administered on days 1, 2, 8, 9, 15, and 16 every 28 days). Unfavorable cytogenetic were defined as the presence of any of del13 or hypodiploidy by metaphase cytogenetic (CTG) analysis and/or del 17p13, t(4;14), and t(14;16) by fluorescence in situ hybridization. Of the 234 patients, CTG data were available for 203 patients (87%) and fluorescence in situ hybridization data were available for 210 patients (90%); both were available for 178 patients (76%). 75 patients had ≥one unfavorable cytogenetic feature. The ORR for patients with unfavorable CTG was 30%, compared to 23% for patients with no CTG abnormalities. The DOR and TTP were seven months and four months, respectively, for patients with unfavorable CTG, and eight months and five months, respectively, for patients with no CTG abnormality. DOR for patients with t(14;16) was 5.6 months and 4.6 months for patients with del 17p, suggesting that DOR may be shorter in heavily pretreated patients in whom del17p is consistently prevalent. Response rates were similar to patients with no CTG abnormality, with the exception of a lower rate for t(14;16); however, data are limited to three patients.
In the other Phase II study on patients with R/R MM (PX-171-004),Citation84 CTG and fluorescence in situ hybridization data were available for 122 nonbortezomib patients. The ORR for the entire cohort was 48% (the ORR for patients with favorable vs unfavorable CTG was 51% and 37%). The unfavorable CTG group comprising 19 (15%) patients had only seven responses.
In the CRdCitation90 frontline study, no significant differences in response rates were observed in the unfavorable CTG group vs the favorable CTG group (ORR 100% vs 91%;>VGPR 75% vs 61%; and CR 56% vs 52%). In the other frontline trial CTd,Citation91 no significant differences in response rates were observed in the unfavorable CTG group vs the favorable CTG group (90% vs 88%; and >VGPR 55% vs 59%). These results demonstrate that carfilzomib may overcome the poor prognostic outcomes portended by unfavorable cytogenetics; however, the DOR and TTP are of extreme importance in this patient group because they tend to relapse early. The results of the ongoing carfilzomib maintenance therapy in this high risk group are of significant interest.
Elderly patients
In the PX-171-004 studyCitation84 of R/R MM patients, ORR for patients <65 and >65 was 59% and 38%, respectively. Other confounding variables of unfavorable cytogenetics, prior lines of therapy in older patients, and renal functions have not been adjusted for these results. In the frontline setting, as in CRd,Citation90 where 43% of patients were >65 years old, ORR was 94%, suggesting that elderly patients derive the same benefit as younger patients.
Renal failure
From the safety data of 526 patients from the PX-171-003-A0, PX-171-003-A1, PX-171-004 and PX-171-005Citation92 trials, 71% had baseline renal dysfunction (CrCl <50 mL/min). Transient worsening of renal function (defined as a ≥2 fold increase in serum creatinine from baseline that normalizes during the study period) was reported in 6% of patients. Worsening of renal functions was observed in 7% of patients, and among these patients, 2% discontinued the study due to a renal AE; 87% of patients had no episodes of worsening renal function. Based on the pooled analyses and the results from PX-0171-005Citation96, carfilzomib dose and schedule need not be adjusted for baseline renal dysfunction, including in patients with severe renal dysfunctions.
Dose response
A comparison of efficacy based on dose response was analyzed from the results of PX-171-003-A0 (20 mg/mCitation2) and PX-171-003-A1 (20/27 mg/mCitation2); and PX-171-004-cohort 1 (20/27 mg/mCitation2) and PX-171-004-cohort 2 (20/27 mg/mCitation2), based on the increased ORR observed in the comparator arms 003-A1 vs 003-A0 as well as in 004-cohort 2 vs 004-cohort 1.Citation97 A pooled multivariate model was designed to establish the dose–response relationship using the above Phase II studies. Using multivariate logistic regression analysis, the authors demonstrated that the odds of achieving >PR for a given patient treated with 27 mg/mCitation2 were 4.08-fold higher than for a patient receiving 20 mg/mCitation2. Also of interest in this analysis is the increment per-cycle odds of >PR, which were 1.12-fold for each 1 mg/mCitation2 increase in carfilzomib dose. It is of theoretical interest to analyze the dose-toxicity analysis in the group of interest. Ongoing studies (PX-171-007) are currently evaluating higher dosing regimens in relapsed solid tumors and R/R MM.
Speed of response
Prior studies with carfilzomib suggested that initial responses occur rapidly, and the depth of response improves with continued treatment. An exploratory analysisCitation98 to evaluate the time to initial response was completed using patient data from two Phase II clinical trials, PX-171-003-A1 (257 patients) and PX-171-004 (bortezomib-naive patients, 135; bortezomib treated, 35 patients). The speed of responses was rapid across all groups. The median time to achieve a PR was 1.9, 1.7, and 1.4 months in PX-171-003-A1, PX-171-004, (bortezomibnaive), and PX-171-004 (bortezomib treated) patients, respectively. Similar results were observed in the upfront CRd trial,Citation90 with a 42% reduction in paraprotein 2 weeks after initiating therapy, a 68% reduction after cycle 1, and an 82% reduction after cycle 3. A similar trend was observed in the other upfront trial CTdCitation91 with response rates after cycle 1 (67% PR; 9% VGPR; and 2% CR), improving with subsequent cycles (after cycle 2 [50% PR; 30% VGPR; and 10% CR]; after cycle 3 [36% PR; 39% VGPR; and 11% CR], and after cycle 4 [25% PR; 35% VGPR; and 25% CR]).
Duration of treatment
Neurotoxicity is a major limitation for the long term administration of bortezomib.Citation16 In the preclinical chronic animal toxicology studies, carfilzomib did not cause neurotoxicity with long-term administration of 6 to 9 months. Patients treated on PX-171-002, PX-171-003, PX-171-004, and PX-171-005 were included in the analysis to evaluate long-term tolerability.Citation99 Ten percent of patients in studies PX-171-003 and PX-171-005 and approximately 24% of patients in PX-171-004 completed twelve cycles of carfilzomib therapy, and 42 patients enrolled in the expanded protocol (PX-171-010). The median duration of treatment in this cohort was 14 months; one patient continued treatment for longer than 27 months. Cumulative toxicities were not observed; four drug-related SAEs included infection, dyspnea, bronchitis, and asthenia. PN was not seen. The maintenance carfilzomib offered sustained disease control and resulted in excellent long-term tolerability, which is a promising maintenance regime.
Ongoing trials with carfilzomib as a single agent and in combination with other agents
Studies on carfilzomib as a single agent in relapsed/refractory AML, ALL, CLL, SLL, PLL, and T-cell lymphomas and as maintenance therapy in myeloma as well as a dose-finding study of higher doses of carfilzomib are currently ongoing. A Phase III study with carfilzomib as single agent that compares the best supportive care is ongoing. Dose finding studies in myeloma Phase I studies in combination with immunomodulatory drugs (lenalidomide and pomalidomide), HDACi (panobinostat and vorinostat), and cytotoxic therapies (PLD and cytoxan) are currently continuing. In WM, carfilzomib is being evaluated in combination with rituximab and dexamethasone. A large Phase III trial comparing CRd and Rd is also ongoing. Currently ongoing studies are summarized in .
Table 4 Ongoing carfilzomib trials
Acknowledgment
Dr Lonial is supported by the Richard and Annelly Deets Foundation for Multiple Myeloma.
Disclosure
Dr Lonial is a consultant for Millennium, Celgene, Onyx, Novartis, BMS, and Sanofi. All other authors have nothing to disclose.
References
- JungTCatalgolBGruneTThe proteasomal systemMol Aspects Med200930419129619371762
- RablJSmithDMYuYChangS-CGoldbergALChengYMechanism of gate opening in the 20S proteasome by the proteasomal ATPasesMol Cell200830336036818471981
- LiuC-WLiXThompsonDATP binding and ATP hydrolysis play distinct roles in the function of 26S proteasomeMol Cell2006241395017018291
- BraunBCGlickmanMKraftRThe base of the proteasome regulatory particle exhibits chaperone-like activityNat Cell Biol19991422122610559920
- OrlowskiMWilkSCatalytic activities of the 20S proteasome: a multi-catalytic proteinase complexArch Biochem Biophys2000383111611097171
- KeithDWUbiquitination and deubiquitination: targeting of proteins for degradation by the proteasomeSemin Cell Dev Biol200011314114810906270
- DorseyBDIqbalMChatterjeeSDiscovery of a potent, selective, and orally Active proteasome inhibitor for the treatment of cancerJ Med Chem20085141068107218247547
- KisselevA FCallardAGoldbergALImportance of the different proteolytic sites of the proteasome and the efficacy of inhibitors varies with the protein substrateJ Biol Chem2006281138582859016455650
- GriffnTANandiDCruzMImmunoproteasome assembly: cooperative incorporation of interferon γ (IFN-γ)–inducible subunitsJ Exp Med19981871971049419215
- DickLRFlemingPEBuilding on bortezomib: second-generation proteasome inhibitors as anti-cancer therapyDrug Discov Today2010155–624324920116451
- KaneRCFarrellATSridharaRPazdurRUnited States Food and Drug Administration approval summary: bortezomib for the treatment of progressive multiple myeloma after one prior therapyClin Cancer Res200612102955296016707588
- RichardsonPGWellerELonialSLenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myelomaBlood2010116567968620385792
- CavoMTacchettiPPatriarcaFBortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised Phase 3 studyLancet201037697582075208521146205
- NookaAKKaufmanJLBeheraMThe improved efficacy of bortezomib containing induction regimens (BCIR) versus nonbortezomib containing induction regimens (NBCIR) in transplant-eligible patients with multiple myeloma (MM): meta-analysis of Phase III randomized controlled trials (RCTs)ASH Annual Meeting Abstracts2011118213994
- BringhenSLaroccaARossiDEfficacy and safety of once-weekly bortezomib in multiple myeloma patientsBlood2010116234745475320807892
- ArgyriouAAIconomouGKalofonosH PBortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literatureBlood200811251593159918574024
- WindebankAJGrisoldWChemotherapy-induced neuropathyJ Peripher Nerv Syst2008131274618346229
- MoreauPPylypenkoHGrosickiSSubcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, Phase 3, non-inferiority studyLancet Oncol201112543144021507715
- OerlemansRFrankeNEAssarafYGMolecular basis of bortezomib resistance: proteasome subunit β5 (PSMB5) gene mutation and overexpression of PSMB5 proteinBlood200811262489249918565852
- KumarSRajkumarS VMany facets of bortezomib resistance/ susceptibilityBlood200811262177217818779399
- KuppermanELeeECCaoYEvaluation of the proteasome inhibitor MLN9708 in preclinical models of human cancerCancer Res20107051970198020160034
- RichardsonPGBazRWangLInvestigational agent MLN9708, an oral proteasome inhibitor in patients (Pts) with relapsed and/or refractory multiple myeloma (MM): results from the expansion cohorts of a Phase 1 dose-escalation studyASH Annual Meeting Abstracts201111821301
- KumarSBensingerWIReederCBWeekly dosing of the investigational oral proteasome inhibitor MLN9708 in patients with relapsed and/or refractory multiple myeloma: results from a Phase 1 dose-escalation studyASH Annual Meeting Abstracts201111821816
- BerdejaJGRichardsonPGLonialSPhase 1/2 study of oral MLN9708, a novel, investigational proteasome inhibitor in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma (MM)ASH Annual Meeting Abstracts201111821479
- DurieBGMHarousseauJLMiguelJSInternational uniform response criteria for multiple myelomaLeukemia20062091467147316855634
- SanchezELiMSteinbergJAOral dosing of the novel proteasome inhibitor CEP-18770 shows marked anti-myeloma effects in SCID-Hu models of multiple myelomaASH Annual Meeting Abstracts2009114221840
- PivaRRuggeriBWilliamsMCEP-18770: a novel, orally active proteasome inhibitor with a tumor-selective pharmacologic profile competitive with bortezomibBlood200811152765277518057228
- NIHPhase I study of the proteosome inhibitor CEP 18770 in patients with solid tumours or non-Hodgkin’s lymphomasClinicalTrials gov [website on the Internet]Bethesda, MDUS National Library of Medicine2007 Available from: http://clinicaltrials.gov/show/NCT00572637Accessed February 26, 2012
- NIHStudy to determine the maximum tolerated dose and evaluate the efficacy and safety of CEP-18770 in patients with relapsed multiple myeloma refractory to the most recent therapyClinicalTrials gov [website on the Internet]Bethesda, MDUS National Library of Medicine2010 Available from: http://clinicaltrials.gov/show/NCT01023880Accessed February 26, 2012
- NIHCEP-18770 in combination with lenalidomide and dexamethasone in relapsed or refractory multiple myelomaClinicalTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine2011 Available from: http://clinicaltrials.gov/show/NCT01348919Accessed February 26, 2012
- SanchezENicholsCMLamACEfficacy and tolerability of CEP-18770 in combination with dexamethasone and lenalidomide using a SCID-Hu model of multiple myeloma (MM). ASH Annual Meeting AbstractsNovember 182011118212946
- ChauhanDHideshimaTAndersonKCA novel proteasome inhibitor NPI-0052 as an anticancer therapyBr J Cancer20069596196517047643
- SinghAVLloydGKPalladinoMAChauhanDAndersonKCPharmacodynamic and efficacy studies of a novel proteasome inhibitor NPI-0052 in human plasmacytoma Xenograft Mouse ModelASH Annual Meeting Abstracts2008112113665
- ChauhanDCatleyLLiGA novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from BortezomibCancer Cell20058540741916286248
- RichardsonPHofmeisterCJakubowiakAPhase 1 clinical trial of the novel structure proteasome inhibitor NPI-0052 in patients with relapsed and relapsed/refractory multiple myeloma (MM)ASH Annual Meeting Abstracts200911422431
- ChauhanDSinghAVCiccarelliBRichardsonPGPalladinoMAAndersonKCCombination of novel proteasome inhibitor NPI-0052 and lenalidomide trigger in vitro and in vivo synergistic cytotoxicity in multiple myelomaBlood2010115483484519965674
- NIHPhase 1 clinical trial of NPI-0052 in patients with advanced solid tumor malignancies or refractory lymphomaClinicalTrials gov [website on the Internet]Bethesda, MDUS National Library of Medicine2006 Available from: http://clinicaltrials.gov/show/NCT00396864Accessed February 26, 2012
- NIHPhase 1 clinical trial of NPI-0052 in patients with relapsed or relapsed/refractory multiple myelomaClinicalTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine2007 Available from: http://clinicaltrials.gov/show/NCT00461045Accessed February 26, 2012
- NIHProteasome inhibitor NPI-0052 and vorinostat in patients with non-small cell lung cancer, pancreatic cancer, melanoma or lymphomaClinicalTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine2008 Available from: http://clinicaltrials.gov/show/NCT00667082Accessed February 26, 2012
- ChauhanDSinghAVAujayMA novel orally active proteasome inhibitor ONX 0912 triggers in vitro and in vivo cytotoxicity in multiple myelomaBlood2010116234906491520805366
- PapadopoulosKMendelsonDTolcherAA Phase I, open-label, dose-escalation study of the novel oral proteasome inhibitor (PI) ONX 0912 in patients with advanced refractory or recurrent solid tumorsJ Clin Oncol2011Suppl 29Abstr 3075
- NIHOpen-label study of the safety and activity of ONX 0912 in patients with hematological malignanciesClinicalTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine2011 Avilable from: http://clinicaltrials.gov/show/NCT01416428Accessed February 27, 2012
- ElofssonMSplittgerberUMyungJMohanRCrewsCMTowards subunit-specific proteasome inhibitors: synthesis and evaluation of peptide α′, β′-epoxyketonesChemistry and Biology199961181182210574782
- DikicICrosettoNCalatroniSBernasconiPTargeting ubiquitin in cancersEur J Cancer200642183095310217084074
- Arastu-KapurSBallAJAnderlJLBennettMKKirkCJNeurodegeneration induced by bortezomib exposure in vitro occurs via proteasome independent mechanismsASH Annual Meeting Abstracts2009114222859
- BoldenJEPeartMJJohnstoneRWAnticancer activities of histone deacetylase inhibitorsNat Rev Drug Discov20065976978416955068
- FuchsOProvaznikovaDMarinovIKuzelovaKSpickaIAntiproliferative and proapoptotic effects of proteasome inhibitors and their combination with histone deacetylase inhibitors on leukemia cellsCardiovasc Hematol Disord Drug Targets200991627719275578
- HideshimaTRichardsonPGAndersonKCMechanism of action of proteasome inhibitors and deacetylase inhibitors and the biological basis of synergy in multiple myelomaMol Cancer Ther201110112034204222072815
- MtsiadesCSMitsiadesNSMcMullanCJTranscriptional signature of histone deacetylase inhibition in multiple myeloma: biological and clinical implicationsProc Natl Acad Sci U S A2004101254054514695887
- ZhangQLWangLZhangYWThe proteasome inhibitor bortezomib interacts synergistically with the histone deacetylase inhibitor suberoylanilide hydroxamic acid to induce T-leukemia/ lymphoma cells apoptosisLeukemia20092381507151419282831
- BadrosABurgerAMPhilipSPhase I study of vorinostat in combination with bortezomib for relapsed and refractory multiple myelomaClin Cancer Res200915165250525719671864
- RaoRFiskusWYangYCo-treatment with panobinostat enhances bortezomib-induced unfolded protein response, endoplasmic reticulum stress and apoptosis of human mantle cell lymphoma cellsASH Annual Meeting Abstracts200811211887
- DasmahapatraGLemberskyDSonMPCarfilzomib interacts synergistically with histone deacetylase inhibitors in mantle cell lymphoma cells in vitro and in vivoMol Cancer Ther20111091686169721750224
- DasmahapatraGLemberskyDKramerLThe pan-HDAC inhibitor vorinostat potentiates the activity of the proteasome inhibitor carfilzomib in human DLBCL cells in vitro and in vivoBlood2010115224478448720233973
- RaoRFiskusWBalusuRTreatment with histone deacetylase 6-specific inhibitor WT-161 disrupts hsp90 function, abrogates aggresome formation and sensitizes human mantle cell lymphoma cells to lethal ER stress induced by proteasome inhibitor carfilzomibASH Annual Meeting Abstracts2010116212856
- NIHStudy of carfilzomib and vorinostat for relapsed or refractory lymphomaClinicalTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine2011 Available from: http://clinicaltrials.gov/show/NCT01276717Accessed February 27, 2012
- NIHA study of carfilzomib, lenalidomide, vorinostat, and dexamethasone in relapsed and/or refractory multiple myeloma (QUAD)ClinicalTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine2011 Available from: http://clinicaltrials.gov/show/NCT01297764Accessed February 27, 2012
- NIHCarfilzomib plus panobinostat in relapsed/refractory multiple myeloma (MM)ClinicalTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine2011 Available from: http://clinicaltrials.gov/show/NCT01301807Accessed February 27, 2012
- NIHStudy of the combination of panobinostat and carfilzomib in patients qith relapsed/refractory multiple myelomaClinicalTrials gov [website on the Internet]Bethesda, MDUS National Library of Medicine2011 Available from: http://clinicaltrials.gov/show/NCT01496118Accessed February 27, 2012
- StapnesCDøskelandAPHatfeldKThe proteasome inhibitors bortezomib and PR-171 have antiproliferative and proapoptotic effects on primary human acute myeloid leukaemia cellsBr J Haematol2007136681482817341267
- NiewerthDFrankeNJansenGSensitivity of pediatric acute leukemia cells to bortezomib and epoxyketone-based proteasome inhibitors: correlations with proteasome subunit expressionASH Annual Meeting Abstracts2011118211513
- GuptaSVHertleinEKWoyachJAThe novel proteasome inhibitor carfilzomib functions independently of p53 to induce potent cytotoxicity in primary chronic lymphocytic leukemia cells and a defective NF-{kappa}B responseASH Annual Meeting Abstracts2011118213510
- ZhangLQianJOuZCarfilzomib, an irreversible proteasome inhibitor induces long-term growth inhibition of mantle cell lymphoma in vivoASH Annual Meeting Abstracts2011118213740
- GuJHernandez-IlizaliturriFJKaufmanGPMavisCCzuczmanMSCarfilzomib (CFZ): a novel proteasome inhibitor that induces cell cycle arrest and cell death in rituximab-chemotherapy resistant lymphoma and potentiates the anti-tumor activity of chemotherapeutic agentsASH Annual Meeting Abstracts2010116214908
- GuJCzuczmanNMKaufmanGPMavisCHernandez-IlizaliturriFJCzuczmanMSThe novel proteasome inhibitor carfilzomib (CFZ) potentiates the anti-tumor activity of chemotherapeutic agents in rituximab-chemotherapy resistant lymphoma through inducing G2/M cell cycle arrest and cell deathASH Annual Meeting Abstracts2011118214970
- PhamLVTamayoATLiCLeeJFayadLFordRJChemo-resistance in diffuse large cell lymphoma: novel drug combinations targeting NFAT/NF-Kb growth/survival/chemo-resistance signaling pathways in validated novel experimental systemsASH Annual Meeting Abstracts2011118211428
- SaccoAAujayMMorganBCarfilzomib-dependent selective inhibition of the chymotrypsin-like activity of the proteasome leads to antitumor activity in Waldenstrom’s macroglobulinemiaClin Cancer Res20111771753176421355079
- ZhouH-JAujayMABennettMKDesign and synthesis of an orally bioavailable and selective peptide epoxyketone proteasome inhibitor (PR-047)J Med Chem20095293028303819348473
- Arastu-KapurSAnderlJLKrausMNonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: a link to clinical adverse eventsClin Cancer Res20111792734274321364033
- KuhnDJChenQVoorheesPMPotent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against pre-clinical models of multiple myelomaBlood200711093281329017591945
- DemoSDKirkCJAujayMAAntitumora of PR-171, a novel irreversible inhibitor of the proteasomeCancer Res200767136383639117616698
- BerkersCRVerdoesMLichtmanEActivity probe for in vivo profling of the specificity of proteasome inhibitor bortezomibNat Meth200525357362
- KrausMFloreaBBaderJSelective inhibition of the proteasome’s {beta}2 catalytic subunit alone does not induce cytotoxicity, but resen-sitizes bortezomib-refractory myeloma cells for bortezomib treatmentASH Annual Meeting Abstracts2011118212915
- ParlatiFLeeSJAujayMCarfilzomib can induce tumor cell death through selective inhibition of the chymotrypsin-like activity of the proteasomeBlood2009114163439344719671918
- LeeSJWooTMArastu-KapurSWongAFRenauTEKirkCJPotent inhibition of multiple proteasome subunits by carfilzomib in multiple myeloma and solid tumor patientsASH Annual Meeting Abstracts2011118215068
- PapadopoulosKPLeePSinghalSA phase 1b/2 study of prolonged infusion carfilzomib in patients with relapsed and/or refractory (R/R) multiple myeloma: updated efficacy and tolerability from the completed 20/56 mg/m2 expansion cohort of PX-171-007ASH Annual Meeting Abstracts2011118212930
- YangJWangZFangYPharmacokinetics, pharmacodynamics, metabolism, distribution, and excretion of carfilzomib in ratsDrug Metab Dispos201139101873188221752943
- PapadopoulosKMendelsonDTolcherAA Phase I, open-label, dose-escalation study of the novel oral proteasome inhibitor (PI) ONX 0912 in patients with advanced refractory or recurrent solid tumorsJ Clin Oncol2011Suppl 29Abstr 3075
- O’ConnorOAStewartAKValloneMA Phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignanciesClin Cancer Res200915227085709119903785
- OrlowskiRZStinchcombeTEMitchellBSPhase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignanciesJ Clin Oncol200220224420442712431963
- LonialSWallerEKRichardsonPGRisk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myelomaBlood2005106123777378416099887
- AlsinaMTrudelSValloneMMolineauxCKunkelLGoyAPhase 1 single agent antitumor activity of twice weekly consecutive day dosing of the proteasome inhibitor carfilzomib (PR-171) in hematologic malignanciesASH Annual Meeting Abstracts200711011411
- JagannathSVijRStewartKFinal results of PX-171-003-A0, part 1 of an open-label, single-arm, Phase II study of carfilzomib (CFZ) in patients (pts) with relapsed and refractory multiple myeloma (MM)J Clin Oncol200927Suppl 15sAbstr 8504
- VijRKaufmanJLJakubowiakAJFinal results from the bortezomib-naive group of PX-171-004, a Phase 2 study of single-agent carfilzomib in patients with relapsed and/or refractory MMASH Annual Meeting Abstracts201111821813
- SiegelDWangLOrlowskiRZPX-171-004, An ongoing open-label, Phase II study of single-agent carfilzomib (CFZ) in patients with relapsed or refractory myeloma (MM); updated results from the bortezomib-treated cohortASH Annual Meeting Abstracts200911422303
- VijRSiegelDKaufmanJResults of an ongoing open-label, Phase II study of carfilzomib in patients with relapsed and/or refractory multiple myeloma (R/R MM)J Clin Oncol201028Suppl 15sAbstr 8000
- BadrosAVijRMartinTPhase II study of carfilzomib in patients with relapsed/refractory multiple myeloma and renal insufficiencyJ Clin Oncol201028Suppl 15sAbstr 8128
- WangMBensingerWMartinTInterim results from PX-171-006, a Phase (Ph) II multicenter dose-expansion study of carfilzomib (CFZ), lenalidomide (LEN), and low-dose dexamethasone (loDex) in relapsed and/or refractory multiple myeloma (R/R MM)J Clin Oncol2011Suppl 29Abstr 8025
- MoreauPPalumboAStewartAA randomized, multicenter, Phase (Ph) III study comparing carfilzomib (CFZ), lenalidomide (LEN), and dexamethasone (Dex) to LEN and Dex in patients (Pts) with relapsed multiple myeloma (MM)J Clin Oncol2011Suppl 29abstr TPS225
- JakubowiakAJDytfeldDJagannathSFinal results of a frontline phase 1/2 dtudy of carfilzomib, lenalidomide, and low-dose dexamethasone (CRd) in multiple myeloma (MM)ASH Annual Meeting Abstracts201111821631
- SonneveldPHackerEZweegmanSCarfilzomib combined with thalidomide and dexamethasone (CARTHADEX) as induction treatment prior to high-dose melphalan (HDM) in newly diagnosed patients with multiple myeloma (MM). a trial of the European Myeloma Network EMNASH Annual Meeting Abstracts201111821633
- SinghalSSiegelDSMartinTIntegrated safety from Phase 2 studies of monotherapy carfilzomib in patients with relapsed and refractory multiple myeloma (MM): an updated analysisASH Annual Meeting Abstracts2011118211876
- NIHA study of carfilzomib maintenance therapy in subjects previously enrolled in carfilzomib treatment protocolsClinicalTrials gov [website on the Internet]Bethesda, MDUS National Library of Medicine2009 Available from: http://clinicaltrials.gov/show/NCT00884312Accessed February 27, 2012
- MartinTSinghalSBVijRBaseline peripheral neuropathy does not impact the efficacy and tolerability of the novel proteasome inhibitor carfilzomib (CFZ): results of a subset analysis of a Phase 2 trial in patients with relapsed and refractory multiple myeloma (R/R MM)ASH Annual Meeting Abstracts2010116213031
- JakubowaikAJSiegelDSSinghalSUnfavorable cytogenetic characteristics do not adversely impact response rates in patients with relapsed and/or refractory multiple myeloma treated with single-agent carfilzomib on the 003 (A1) studyASH Annual Meeting Abstracts2011118211875
- BadrosAZVijRMartinTPhase I study of carfilzomib in patients (Pts) with relapsed and refractory multiple myeloma (MM) and varying degrees of renal insufficiencyASH Annual Meeting Abstracts2009114223877
- SquiffetPMichielsSSiegelDSVijRRoSBuyseMEMultivariate modelling reveals evidence of a dose-response relationship in Phase 2 studies of single-agent carfilzomibASH Annual Meeting Abstracts2011118211877
- WangLSiegelDSJakubowiakAJThe speed of response to singleagent carfilzomib in patients with relapsed and/or refractory multiple myeloma: an exploratory analysis of results from 2 multicenter Phase 2 clinical yrialsASH Annual Meeting Abstracts2011118213969
- JagannathSVijRKaufmanJLLong-term treatment and tolerability of the novel proteasome inhibitor carfilzomib (CFZ) in patients with relapsed and/or refractory multiple myeloma (R/R MM)ASH Annual Meeting Abstracts2010116211953
- SiegelDMartinTWangMPX-171-003-A1, an open-label, single-arm, Phase (Ph) II study of carfilzomib (CFZ) in patients (pts) with relapsed and refractory multiple myeloma (R/R MM): long-term follow-up and subgroup analysisJ Clin Oncol2011Suppl 29Abstr 8027
- NIHCarfilzomib in patients with relapsed acute myeloid or acute lymphoblastic leukemia (AML ALL)ClinicalTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine2010 Available from: http:/clinicaltrials.gov/show/NCT01137747Accessed February 27, 2012
- NIHStudy of carfilzomib in chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL) or prolymphocytic leukemia (PLL)ClinicalTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine2010 Available from: http:/clinicaltrials.gov/show/NCT01212380Accessed February 27, 2012
- NIHCarfilzomib in treating patients with relapsed or refractory T-cell lymphomaClinicalTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine2011 Available from: http:/clinicaltrials.gov/show/NCT01336920Accessed February 26, 2012
- NIHPhase 1b multicenter study of carfilzomib with lenalidomide and dexamethasone in relapsed multiple myelomaClinicalTrials gov [website on the Internet]Bethesda, MDUS National Library of Medicine2008 Available from http:/clinicaltrials.gov/show/NCT00603447Accessed February 27, 2012
- NIHA safety study of carfilzomib and pomalidomide with dexamethasone in patients with relapsed or refractory multiple myelomaClinicalTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine2011 Available from: http://clinicaltrials.gov/show/NCT01464034Accessed February 27, 2012
- NIHStudy of carfilzomib for multiple myeloma patients who are relapsed/refractory to bortezomib-containing treatmentsClinicalTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine2011 Available from: http://clinicaltri-als.gov/show/NCT01365559Accessed February 27, 2012
- NIHTrial of carfilzomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma (CARMYSAP)ClinicalTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine2010 Available from: http://clinicaltrials.gov/show/NCT01279694Accessed February 27, 2012
- NIHCarfilzomib and pegylated liposomal doxorubicin hydrochloride in treating patients with relapsed or refractory multiple myelomaClinicalTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine2011 Available from: http://clinicaltrials.gov/show/NCT01246063Accessed February 27, 2012
- NIHArry-520 + carfilzomib for multiple myeloma (MM)Clini-calTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine2012 Available from: http://clinicaltrials.gov/show/NCT01372540Accessed February 27, 2012
- NIHCyclophosphamide, carfilzomib, thalidomide, and dexamethasone in treating patients with newly diagnosed active multiple myelomaClinicalTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine2010 Available from: http://clinicaltrials.gov/show/NCT01057225Accessed February 27, 2012
- NIHCarfilzomib and lenalidomide with dexamethasone combination in newly diagnosed, previously untreated multiple myelomaClinicalTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine2009 Available from: http://clinicaltrials.gov/show/NCT01029054Accessed February 27, 2012
- NIHCarfilzomib, rituximab and dexamethasone in Waldenstrom’s macroglobulinemia (CaRD)ClinicalTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine2011 Available from: http://clinicaltrials.gov/show/NCT01470196Accessed February 27, 2012
- NIHInfusional carfilzomib in patients with relapsed or refractory multiple myelomaClinicalTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine2011 Available from: http://clinicaltrials.gov/show/NCT01351623Accessed February 27, 2012
- NIHCarfilzomib, cyclophosphamide and dexamethasone in newly diagnosed multiple myeloma patients (CCD)ClinicalTrials gov [website on the Internet]Bethesda, MDUS National Library of Medicine2011 Available from: http://clinicaltrials.gov/show/NCT01346787Accessed February 27, 2012
- NIHCarfilzomib, lenalidomide, and dexamethasone in new multiple myeloma patientsClinicalTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine2011 Available from: http://clinicaltrials.gov/show/NCT01402284Accessed February 27, 2012
- NIHPhase 3 study comparing carfilzomib, lenalidomide, and dexamethasone (CRd) versus lenalidomide and dexamethasone (Rd) in subjects with relapsed multiple myelomaClinicalTrials gov [website on the Internet]Bethesda, MDUS National Library of Medicine2010 Available from: http://clinicaltrials.gov/show/NCT01080391Accessed February 27, 2012
- NIHA study of carfilzomib vs best supportive care in subjects with relapsed and refractory multiple myeloma (FOCUS)ClinicalTrials gov [website on the Internet]Bethesda, MDUS National Library of Medicine2010 Avilable from: http://clinicaltrials.gov/show/NCT01302392Accessed February 27, 2012