Abstract
Background
Diffuse alveolar hemorrhage (DAH) is a clinical syndrome with typical symptoms dyspnea and hemoptysis. DAH is a complication of specific diseases, in some cases with acute catastrophic hemoptysis, while other patients present low grade alveolar bleeding with a need of chronic transfusion as in pulmonary hemosiderosis.
Methods
Current literature in the PubMed database and other sources was reviewed in order to evaluate the current treatment recommendations, efficacy of this treatment, and finally the risk of complications after off-label use of rFVIIa in respect to DAH.
Objectives
(i) To elucidate the clinical aspects of alveolar hemorrhage, (ii) to develop a simple diagnostic algorithm in order to separate DAH from other important pulmonary diseases with similar clinical picture and comparably high mortality. Such an algorithm has important therapeutic consequences because these diseases: acute lung injury (ALI), acute respiratory distress syndrome (ARDS) and bronchiolitis obliterans organizing pneumonia (BOOP) have different therapies, (iii) to evaluate and discuss whether local pulmonary administration may improve outcome and reduce mortality in DAH, and (iv) to suggest a treatment schedule.
Results
Hitherto the diagnosis and treatment of DAH has been based on anecdotal reports. The treatment has relied on different unspecific treatment modalities based on a mixture of treatment of the underlying disease and treatment without evidence targeted to stop the alveolar bleeding. However, recently a number of publications have advocated the use of intrapulmonary rFVIIa. Even in severe bleeding DAH has been shown to respond promptly without thromboembolic complication when FVIIa was administered locally via the air side, because the FVIIa does not penetrate the alveolo-capillary membrane to the blood-side. The incidence of DAH (in the US and Europe is 100,000–150,000, and 50,000 patients annually are at risk of developing DAH following hematopoietic stem cell transplant (HSCT) and autoimmune diseases. Finally 50,000–100,000 patients may be falsely categorized as having acute respiratory distress syndrome/acute lung injury (ARDS/ALI) because DAH and ARDS cannot be separated clinically. A new treatment paradigm of DAH is proposed as no other intervention has been able to ensure pulmonary hemostasis in DAH. The diagnosis of DAH is simple, a series of broncho-alveolar washes which become increasingly bloody. This test should be performed in all patients with pulmonary opacities in order to separate ARDS/ALI from DAH. FVIIa administrated via pulmonary route is “drug of choice”, because it stops bleeding in the life-threatening syndrome DAH. Hemostasis is obtained after only one to two small doses of FVIIa (50 μg/kg body weight per dose) and after hemostasis the oxygen transport quickly improves.
Conclusion
Intrapulmonary administration of rFVIIa is recommended as the treatment of choice for DAH and blast lung injury (BLI) because the treatment has been shown to be successful and uncomplicated in spite of the fact that only a small series of DAH has been documented.
Introduction
There is a general lack of understanding of the gap between, on the one hand, the “air side”, where the effective drug-related receptors are located and, on the other hand, the vascular compartment. When administered intravenously biological drugs like FVIIa do not reach the receptors in the alveoli, because they do not pass the alveolo-capillary membrane. “Biologics” refer to biologically manufactured drugs, similar to endogenously key signaling proteins, like FVIIa.
Traditionally, biologics are administered intravenously with the hope that a sufficient concentration of the drug reaches the specific receptors. This requires a high systemic concentration of the drug and is associated with a higher risk of adverse systemic effects than local application at the target site.
The syndrome of diffuse bleeding DAH
The diffuse alveolar hemorrhage (DAH) syndrome has a number of clinical characteristics similar to a number of well-pulmonary documented conditions like acute respiratory distress syndrome (ARDS), acute lung insufficiency (ALI), and bronchiolitis obliterans organizing pneumonia (BOOP) ().
Table 1 DAH – the key points
Diffuse alveolar hemorrhage related to pulmonary receptors in perspective
For many years it has been known that even the air side of the lung has receptors. Rose et al made a functional study of the granulocyte macrophage colony stimulating factor (GM-CSF) receptor, documenting that it was necessary to inhale the drug in order to reach the receptor on the air side (). Intravenous administration of GM-CSF had no effect on the alveolar macrophages whereas the inhaled drug increased the number of macrophages with no interference with systemic monocytes.Citation1
Figure 1 Local pulmonary administration of FVIIa into the airways ensures that the drug reaches its alveolar receptor TF. However, most importantly, taking into account the separation between the two compartments the “air side” and the “systemic side”, there will not be systemic adverse effects in as much as the alveolo-capillary membrane does not allow the transmembranous passage of FVIIa, when inhaled. FVIIa does not pass through the alveolo-capillary membrane either from the (A) alveolus or (B) the blood side.
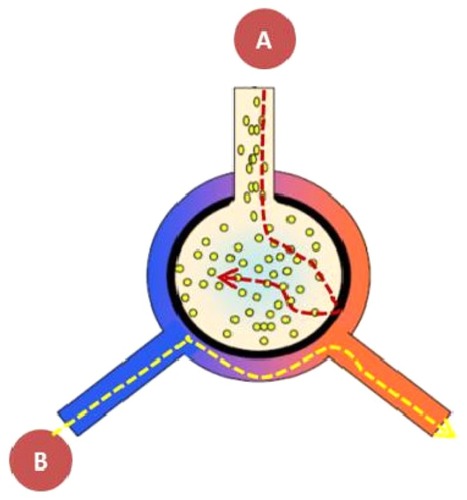
In the normal airspace the hemostatic balance is skewed toward anticoagulation due to increased expression of plasminogen activator and low expression of tissue factor (TF). As soon as the lungs are exposed to an inflammatory process, the anticoagulatory state will swiftly be turned into a procoagulatory state – a primitive host defense reaction in order to stop the invasiveness of bacteria and to hinder bacterial multiplication and dissemination.Citation2
On the air side a whole host of receptors are placed. The complex cross talk between the dynamic receptor expression and the overall effect is hard to predict. It is known that pulmonary receptors ensure pulmonary host defense, ie, the TF and GM-CSF-receptors, both placed on the airside isolated from the blood side.Citation3,Citation4 The different biologics must fulfill certain qualities in order to be able to penetrate the biological membranes. First of all the molecular size must be small, most likely smaller than 15–20 kDa, like insulin with a molecular size of 8 kDa. Secondly, the drug must be lipophilic in order to cross the alveolo-capillary membrane to reach the peripheral airways. However, no newly developed therapeutic recombinant drug fulfills the low size criteria in as much as all the wild type proteins are all hydrophilic FVIIa and GM-CSF.
Pathophysiology of DAH
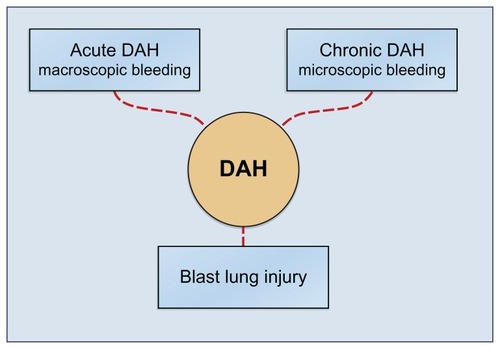
DAH is characterized by damage to the alveolar-capillary basement membrane allowing red blood cells to enter the alveolar spaces. Most frequently DAH is a symptom of pulmonary capillaritis as seen in autoimmune diseases or after hematopoietic stem cell transplant (HSCT). DAH may also occur as a result of diffuse alveolar damage in acute respiratory distress syndrome/acute lung injury (ARDS/ALI). Further the damage may be the result of physicochemical factors like blast lung injury (BLI), toxic drug effects (eg, cytotoxic drugs, crack cocaine inhalation), and radiation therapy ().
Figure 2 The DAH syndrome is the common denominator of multiple diseases and conditions. DAH is the general alveolar response to multiple underlying diseases and conditions. The syndrome DAH manifests itself in three subsets: the acute catastrophic DAH, the chronic DAH and finally as the instant blast lung injury. The acute DAH has been treated with local intrapulmonary FVIIa administration. The two other subsets could potentially also be treated from the air side, an intervention which remains to be shown.
Epidemiology of DAH
In hematological stem cell transplantation (HSCT) recipients, DAH is an infrequent cause of death both in early and late phases after transplantation.Citation5,Citation6 DAH after HSCT is a devastating complication that carries an overall mortality of 16%–70%.Citation7,Citation8 As a result of life-threatening multiple organ dysfunctions, 15%– 40% of HSCT recipients receive intensive care unit support, the majority of whom require mechanical ventilation.Citation9–Citation11 The mortality rate of HSCT recipients receiving invasive ventilation used to exceed 90%.Citation10,Citation12 Although more recent studies have shown improvement in outcome, the mortality rate of HSCT recipients receiving mechanical ventilation is still high.Citation10,Citation13–Citation15 It is estimated that 50,000 HSCT procedures took place in 2007 in the USA, and 80,000 worldwide, and that 25%–40% of HSCT recipients are admitted to the medical intensive care unit (ICU) for the management of pulmonary complications to HSCT.Citation7,Citation11,Citation16,Citation17
A considerable number of patients will also develop DAH due to other causes than HSCT, eg, infections, bronchoscopy, autoimmune diseases, HIV (Kaposi’s sarcoma), and transplantations, ie, DAH has been reported in 75% of patients with Kaposi’s sarcoma,Citation18 in 66% of patients with SLE,Citation19 5%–10% of patients with Goodpasture syndrome.Citation20 Fatal DAH has been reported in approximately 10%–41% of lung autopsies of HSCT patients that succumbed to HSCT related complications.Citation6,Citation21 Further, DAH is reported to develop in 40% of patients admitted to the ICU for respiratory failure (ARDS/ALI). The annual incidence of ARDS/ ALI in the USA and EU admitted to ICU is over 500,000 patients.
The syndrome of DAH
The multiple causes of DAH are shown in Citation22 together with the treatment of the underlying cause of DAH and symptomatic treatment of the ongoing alveolar bleeding. Formerly, the management strategy was limited to the optimization of each of the specific diseases and underlying disorders as a prophylactic measure toward prevention of DAH syndrome. Most of such prophylactic interventions have been treatment with steroids, anti-infective measures, eg, anti-cytomegalovirus pneumonia therapy, plasmapheresis, platelet transfusion, and coagulation factors. These interventions are alone focused on prophylaxis of the stereotype syndrome alveolar bleeding.
Table 2 Overview of diseasesyndromes and other causes leading to DAH and the classical options of treatment
The diagnosis of DAH
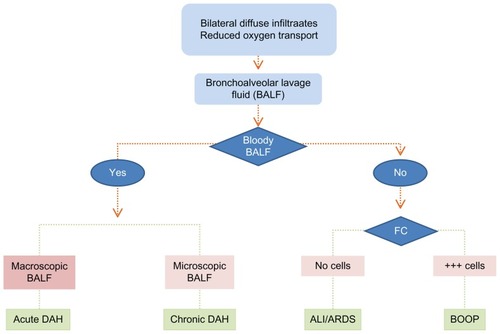
The algorithmic scheme is depicted in , based on the fact that the signs and symptoms are a common denominator of DAH, ARDS and BOOP: acute pulmonary insufficiency with reduced O2 transport capacity and confluent opacities on chest film. It is imperative to distinguish between the specific diagnoses of these conditions, and in as much as they have a very high mortality, it is of utmost importance to diagnose correctly because the specific therapies are different. The key to the diagnosis, as it appears in , is the finding of (i) a macroscopically progressively hemorrhagic aliquot in a series of bronchoalveolar lavage fluid (BALF) findings that denote a severe DAH syndrome, or (ii) measurements of an increased hemoglobin concentration in the BALF corresponding to a slow bleeding (pulmonary hemosiderosis), or (iii) absence of bloody return in the BALF excluding the DAH diagnosis. The remaining two conditions are separated by a simple flow-cytometry (FC) on the BALF, where the BOOP is characterized by abundant inflammatory cells, and the ARDS diagnosis is based on a BALF without inflammatory cells and without bloody return.
Figure 3 The diagnostic algorithm for DAH, BOOP and ARDS. The clinical signs and symptoms of DAH, ALI/ARDS and BOOP are identical, ie, chest film with confluent opacities, acute pulmonary insufficiency with reduced O2 transport capacity. It is imperative to separate between the specific diagnoses of each of these conditions, in as much as they all have a high mortality; it is of utmost importance to diagnose correctly because the specific therapies are different. The simple algorithmic separation is based on progressively hemorrhagic aliquot of a series of BALF’s either macroscopically which denote a severe DAH syndrome or measurements of an increased hemoglobin concentration in the BALF which corresponds to a slow bleeding DAH like in IPH. Provided there is no bloody return at the BALF the DAH diagnosis is excluded. The remaining two conditions are separated by a simple flow-cytometry on the BALF, where the BOOP is characterized by abundant inflammatory cells, and the ARDS diagnosis is based on a BALF without inflammatory cells or bloody return.
DAH in childhood and adolescence
DAH occurs in any age group secondary to the same underlying diseases and conditions. However, the major difference is that the DAH syndrome is characterized by microscopic alveolar bleeding with chronic transfusion need. Hemoptysis is seldom. The chronic erythrocyte intraalveolar bleeding often surpasses the metabolic clearance of iron originating from the erythrocyte metabolism of the alveolar macrophage. This leads to an alveolar iron overload. These patients don’t have a considerable acute mortality, but a severely reduced long term life-expectancy due to pulmonary fibrosis secondary to accumulated iron in the alveolar space.
Pulmonary hemosiderosis
The idiopathic pulmonary hemosiderosis syndrome (IPH) is a DAH condition, however, without known underlying cause, ie, the incidence and prevalence of IPH is unknown. There are approximately 500 cases reported in the literature, primarily in children (80%). The etiology of IPH is not clear, in spite of many theories. Clinically, the signs and symptoms of IPH are identical to other microalveolar bleedings like the acquired pulmonary hemosiderosis secondary to known underlying diseases, ie, exacerbations concomitantly with cough, dyspnea to fulminant respiratory failure. The chronic state is accompanied with variable degrees of iron deficiency anemia, present in most patients. Spirometry shows a restrictive pattern due to pulmonary fibrosis in the chronic condition. Lung biopsies from both IPH and secondary pulmonary hemosiderosis patients often show severe thickening of the alveolar wall and hemosiderin-loaded alveolar macrophages that also appear in sputum.
The blast lung injury – a dynamic process with a “window of opportunity”
A blast lung injury (BLI) is based on the disruption of the alveolo-capillary membrane after an explosion, with a significant pressure wave entailing diffuse alveolar bleeding ().Citation23
Table 3 Overview of BLI – a dynamic conditionCitation23
Based on the pathophysiology, BLI can be characterized into distinct phases of a dynamic process ().Citation24–Citation26 This leads to (i) diffuse bleeding and (ii) hemolysis which quantitatively surpasses the clearance rate of free iron (Fe++) from the alveoli unless the diffuse alveolar bleeding is stopped early.
Table 4 After a major blast the three phases have their own distinct problemsCitation24–Citation26
It seems that there is a “window of opportunity” of 3 hours in which the BLI is reversible (). If the hemorrhage is not brought to a halt, the severity of the pulmonary dysfunction increases over the next 4 days into irreversible BLI and fatal pulmonary failure. The BLI occurs when soldiers in the battlefield or the civilian population are exposed to bomb attacks. Inhalation of FVIIa is an obvious measure to treat the BLI within the window of opportunity, which could be an indication for use of FVIIa in respect to the military and homeland use, because BLI affects soldiers in the battlefield as well as victims of terror attacks.
Table 5 Diffuse alveolar hemorrhage – the treatment paradigm
BLI – signs, symptoms and outcome
BLI secondary to high explosives causes life-threatening dysfunction of the lungs of soldiers exposed to a blast. The incidence of BLI is not known, but may be as high as 1000–5000 persons annually.
There are to date no reports of the prevalence of DAH in blast lungs because it is a military secret. BLI may occur in the absence of any external signs of trauma, as seen in a series of 517 blast casualties, where approximately 20% were immediately fatal.Citation23
Treatment of DAH
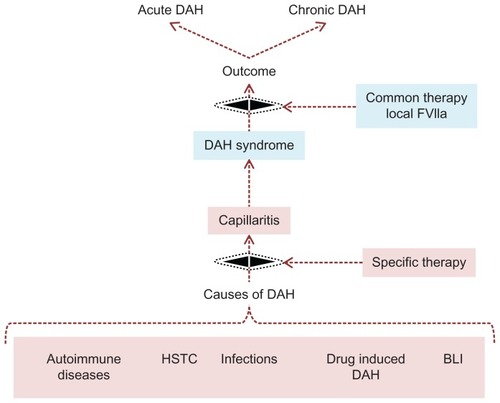
Hitherto the separation between symptomatic intervention towards life threatening DAH and the optimization of the underlying disease has not been properly addressed – a fact which has caused confusion concerning the discussion of the best way to administer FVIIa as an intravenous infusion or as an airway deposition, ie, as a BALF administration or as an inhalation ().
Figure 4 It is essential to separate cause and the effect of DAH. It is important to separate the treatment of multiple underlying causes of DAH from the common complicating denominator DAH syndrome, because the latter is simply treated with local pulmonary FVIIa.
A common denominator for these studies is that the trials generally have only a very small number of patients included, thereby evading proper statistical evaluation.Citation27,Citation28 In 2006 a study was published with a sufficient number of patients to reach a statistical significant effect of local FVIIa. Before this time treatment was a mixed intervention of questionable effect with steroids, anti-infective measures, plasmapheresis, platelet transfusion, and intravenous infusion of FVlla and other procoagulation factors in spite of the fact that DAH bleeding is not due to factor deficiency.Citation29,Citation30
As late as in 2011 it is still suggested that treatment of DAH should be based “on the underlying cause of hemorrhage, with corticosteroids as a mainstay of therapy in most cases”,Citation22 despite of the first series of a systematic treatment of DAH with local intrapulmonary FVIIa in 2006.Citation31 This study documented an effect of administering a small intrabronchial dose of FVIIa. The effect of intrabronchial lavage with a simple saline solution of FVIIa demonstrated (i) that no patient succumbed after the treatment due to alveolar bleeding, (ii) a significantly improved oxygen gas exchange (P = 0.024), and (iii) a balanced hemostasis (P = 0.031). These findings were subsequently reproduced by three later publications from three independent centers using the identical treatment protocol, however, each study had only a few patients included.Citation32–Citation34 None of the four studies adverse effects (AE) were reported, probably because there was no detectable transmembraneous FVIIa passage from the air side into the blood as evaluated by the prothrombin time. The pathophysiological understanding of the mechanism of action, the marked effect, and the fact that no patients died or were encountering adverse effects as a consequence of the local treatment with FVIIa was most likely the reason for being granted the orphan drug (OD) designation in both Europe (European Medicines Agency [EMEA]), Canary Wharf, London and secondly in the USA (Food and Drug Administration [FDA], Virginia, USA) in spite of the theoretical risk of intra-alveolar thrombotic complications, when treating DAH with FVIIa as a pulmonary deposition.Citation22
However, the systemic administration of FVIIa for off-label use for the treatment of uncontrollable life-threatening hemorrhage has been increasing since the introduction of FVIIa (NovoSeven®, Novo Nordisk A/S, Bagsværd, Denmark),Citation35 but concomitantly a concern for potential thromboembolic complications has equally been increasing, especially after the publication of several meta-analyses recommending caution due to ongoing reports of fatal complications.Citation36,Citation37
A suggestion for the treatment of the three conditions, DAH, pulmonary hemosiderosis and blast lung injury, is shown in Citation31–Citation34 based on published documentation.
Patients suffering from chronic DAH as IPH, often children, are at present generally placed on high-dose steroids, and then weaned to the lowest dose that keeps them from having exacerbations. The use of chronic high dose steroids is problematic because the treatment induces multiple adverse effects both in children but also in the adolescent. The 5-year mortality rate in pediatric patients ranges from 24% to 60%.Citation38 Since no treatment is known at present to be effective, clinical trials with inhaled FVIIa are warranted. It is suggested that the end point could be a fall in numbers of hemosiderin-loaded macrophages in sputum combined with reduced transfusion need. Since FVIIa has a very high degree of efficacy in DAH and is without adverse effects, inhaled FVIIa in a small low daily dose will most likely serve as a prophylactic measure. Such a treatment scheme could make invasive procedures like single-lung transplantation superfluous as this treatment has shown no lasting effect because IPH recurred.Citation38
However, to date no information on treatment of pulmonary hemosiderosis and BLI has been published. In as much as pulmonary hemosiderosis and BLI share the same pathophysiological background alveolar bleeding, treatment suggested in is an extrapolation from the published effective dose in DAH.
Every time the clinician encounters a condition where no documentation exists it should be considered whether the disease is worse than the treatment. In the case of using air side treatment of FVIIa in microalveolar bleeding, inhalation by a simple inhalation device seems to be the treatment of choice, ensuring swift deposition of FVIIa into the alveolar space, without the need for invasive procedures such as bronchoscopy. Using a micropump nebulizer ensures that there will not be any interference with the active site of the FVIIa molecule. The only documented case of inhalation of FVIIa by a micropump where the DAH has been treated with success was a patient with Wegener’s granulomatosis.Citation31 The patient had been extubated a few hours earlier after a bout of hemoptysis and had presented with recurrence of the DAH through bloody tinged expectorate. Instead of reintubation she was treated with an FVIIa dose of 50 μg/kg BWT inhaled via a jet nebulizer. After 5 minutes the patient spontaneous exclaimed that the dyspnea had defervesced, and there were no further episodes of DAH.
Discussion
Biologics are relatively new approaches to treat hitherto untreatable diseases like the DAH syndrome. The immediate and positive effect of local FVIIa administered from the air side is due to the fact that the combined FVIIa and its receptor tissue factor (TF) will bring about a balanced hemostasis, ie, with both durable hemostasis for a remarkably small dose of FVIIa, and an improvement in oxygen transport capacity. These facts also explain the lack of systemic adverse effects ().
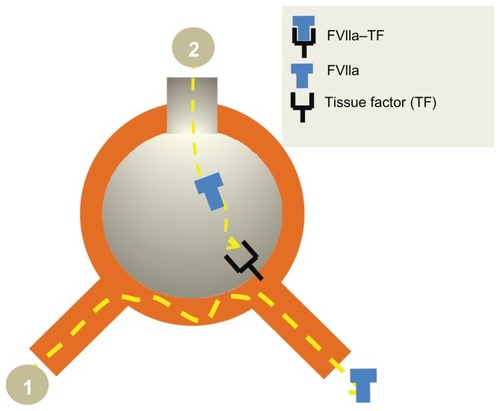
Figure 5 Rationale for local pulmonary treatment of DAH with FVIIa. Intravenous rFVIIa does not reach the alveoli (1) in contrast to the airway route (2) where a direct access to the receptor TF is obtained. The TF-FVIIa complex activates coagulation factor IX and X. Finally the activated TF-FVIIa complex induces a perfect “balanced” hemostasis ie, sufficient fibrin deposition without interference with the oxygen transport. The TFPI are constitutively expressed in the airspace in inflammatory conditions, secondary to alveolar inflammation. The TFPI is usually of less importance as compared to the procoagulatory factors. However, this might be the explanation for the balanced hemostasis.
DAH is a syndrome, not a disease, with three subsets. It is easily conceived that the acute macroscopic hemorrhage has a very high mortality >75%. The microscopic DAH subset has on the contrary a small acute mortality; however, with a severely reduced life expectancy. Lastly the DAH subset associated with blast injuries has anecdotally been successfully treated with inhaled FVIIa (pers comm; Danish Medical Center in Helmand province, Afghanistan and Lars CitationHeslet, 2009).
The clinical picture, “white lungs” on chest film and severely reduced oxygen transport capacity, is a common denominator of the high mortality pulmonary diseases DAH, ARDS and BOOP. Diagnostically they may be separated by a simple algorithm: when an aliquot series of BAL fluid washes shows increasingly bloody return, irrespective of whether it is frons macro- or microscopic bleeding.
Therapy of the DAH syndrome seems to be simple because DAH is the common denominator of multiple underlying diseases and conditions. It is most important to separate the treatment of the alveolar bleeding from the optimization of underlying disease like plasmaferesis, steroids, and antibiotics. Administration of FVIIa into the airways via local administration is suggested as an adjuvant therapy to treatment of the underlying disease (). Taking into consideration the adverse effects of high-dose steroids often used in children with DAH or IPH and further the risk of acquiring irreversible pulmonary fibrosis due to insufficient iron clearance, a trial with inhaled FVIIa seems to be far less risky in spite of such an intervention.Citation34
Conclusions
The documented therapy from four independent centers of instillation of FVIIa or inhalation of the drug into the airways has demonstrated a sustained and immediate hemostatic effect in DAH, however, still only in a small number of patients.
There is an obvious indication for this treatment in patients with the syndrome DAH and with BLI, being the first line intervention to treat the catastrophic high mortality of DAH. This treatment is also safe because the drug does not penetrate from ‘air’ to ‘blood’.
Lastly, patients with confluent opacities on chest film and reduced oxygenation capacity like DAH, ARDS and BOOP, may according to a simple algorithm BALF wash test be categorized into distinctive groups and treated accordingly. It has been estimated that ARDS is likely to be over-diagnosed in 20%, an important issue because the treatments are different.
Larger, however, not necessarily placebo-controlled studies are warranted, taking into account the high mortality when utilizing the above mentioned treatment recommendations and based on the simple diagnostic algorithm to systematically separate the DAH syndrome from the conditions ARDS/ALI and BOOP.
Disclosures
SNC and JDN declare that they have no competing financial or other interests related to the preparation or the content of the manuscript. LH has shares in the pharmacompany Serendex, Copenhagen, Denmark that holds a patent in pulmonary treatment with rFVIIa, but has not received reimbursements, fees, funding, or salary from any organization relating to the content or the preparation of the manuscript. LH declares that he has no other competing interests.
References
- RoseRMKobzikLDushayKThe effect of aerosolized recombinant human granulocyte macrophage colony-stimulating factor on lung leukocytes in nonhuman primatesAm Rev Respir Dis19921465 Pt 1127912861443885
- ChoiGVlaarAPJSchoutenMNatural anticoagulants limit lipopolysaccharide-induced pulmonary coagulation but not inflammationEur Respir J200730342342817537762
- HesletLAndersenJSSengeløvHDahlbäckBDalsgaard-NielsenJInhalation of activated protein C: A possible new adjunctive intervention in acute respiratory distress syndromeBiologics20071446547219707316
- HesletLLook on the “air side” in pneumoniaCrit Care Med200937277477519325384
- AfessaBTefferiALitzowMRDiffuse alveolar hemorrhage in hematopoietic stem cell transplant recipientsAm J Respir Crit Care Med2002166564645
- AgustíCRamirezJPicadoCDiffuse alveolar hemorrhage in allogeneic bone marrow transplantation. A postmortem studyAm J Respir Crit Care Med19951514100610107697223
- AfessaBTefferiALitzowMRPetersSGOutcome of diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipientsAm J Respir Crit Care Med2002166101364136812406834
- MajhailNSParksKDeforTEWeisdorfDJDiffuse alveolar hemorrhage and infection-associated alveolar hemorrhage following hematopoietic stem cell transplantation: related and high-risk clinical syndromesBiol Blood Marrow Transplant200612101038104617067910
- AfessaBTefferiAHoaglandHCLetendreLPetersSGOutcome of recipients of bone marrow transplants who require intensive-care unit supportMayo Clin Proc19926721171221545573
- AfessaBTefferiADunnWFLitzowMRPetersSGIntensive care unit support and Acute Physiology and Chronic Health Evaluation III performance in hematopoietic stem cell transplant recipientsCrit Care Med20033161715172112794410
- JacksonSRTweeddaleMGBarnettMJAdmission of bone marrow transplant recipients to the intensive care unit: outcome, survival and prognostic factorsBone Marrow Transplant19982176977049578310
- CrawfordSWPetersenFBLong-term survival from respiratory failure after marrow transplantation for malignancyAm Rev Respir Dis199214535105141546828
- KhassawnehBYWhitePJrAnaissieEJBarlogieBHillerFCOutcome from mechanical ventilation after autologous peripheral blood stem cell transplantationChest2002121118518811796449
- ScottPHMorganTJDurrantSBootsRJSurvival following mechanical ventilation of recipients of bone marrow transplants and peripheral blood stem cell transplantsAnaesth Intensive Care200230328929412075635
- RubenfeldGDCrawfordSWWithdrawing life support from mechanically ventilated recipients of bone marrow transplants: a case for evidence-based guidelinesAnn Intern Med199612586256338849146
- TorrecillaCCortésJLChamorroCPrognostic assessment of the acute complications of bone marrow transplantation requiring intensive therapyIntensive Care Med19881443933983042827
- CrawfordSWSchwartzDAPetersenFBClarkJGMechanical ventilation after marrow transplantation. Risk factors and clinical outcomeAm Rev Respir Dis198813736826873278664
- FouretPJTouboulJLMayaudCMAkounGMRolandJPulmonary Kaposi’s sarcoma in patients with acquired immune deficiency syndrome: a clinicopathological studyThorax19874242622683616983
- ZamoraMRWarnerMLTuderRSchwarzMIDiffuse alveolar hemorrhage and systemic lupus erythematosus. Clinical presentation, histology, survival, and outcomeMedicine (Baltimore)19977631922029193454
- TeagueCADoakPBSimpsonIJRainerSPHerdsonPBGood-pasture’s syndrome: an analysis of 29 casesKidney Int1978136492504362037
- WojnoKJVogelsangGBBeschornerWESantosGWPulmonary hemorrhage as a cause of death in allogeneic bone marrow recipients with severe acute graft-versus-host diseaseTransplantation199457188928291120
- NewsomeBRMoralesJEDiffuse alveolar hemorrhageSouth Med J2011104426927421606695
- MackenzieIMTunnicliffeBBlast injuries to the lung: epidemiology and managementPhilos Trans R Soc Lond B Biol Sci2011366156229529921149366
- ElsayedNMGorbunovNVKaganVEA proposed biochemical mechanism involving hemoglobin for blast overpressure-induced injuryToxicology1997121181909217317
- GorbunovNVAsherLVAyyagariVAtkinsJLInflammatory leukocytes and iron turnover in experimental hemorrhagic lung traumaExp Mol Pathol2006801112516137675
- ElsayedNMGorbunovNVPulmonary biochemical and histological alterations after repeated low-level blast overpressure exposuresToxicol Sci200795128929617060374
- HenkeDFalkRJGabrielDASuccessful treatment of diffuse alveolar hemorrhage with activated factor VIIAnn Intern Med2004140649349415023729
- DabarGHarmoucheCJammalMEfficacy of recombinant activated factor VII in diffuse alveolar haemorrhageRev Mal Respir2011281106111 [Article in French.]21277485
- HicksKPengDGajewskiJLTreatment of diffuse alveolar hemorrhage after allogeneic bone marrow transplant with recombinant factor VIIaBone Marrow Transplant2002301297597812476294
- PastoresSMPapadopoulosEVoigtLHalpernNADiffuse alveolar hemorrhage after allogeneic hematopoietic stem-cell transplantation: treatment with recombinant factor VIIaChest200312462400240314665531
- HesletLNielsenJDLeviMSengeløvHJohanssonPISuccessful pulmonary administration of activated recombinant factor VII in diffuse alveolar hemorrhageCrit Care2006106R17717184515
- EstellaAJareñoAPerez-Bello FontaiñaLIntrapulmonary administration of recombinant activated factor VII in diffuse alveolar haemorrhage: a report of two case storiesCases J20081115018789132
- GrochovaMKalnasovaBFirmentJPulmonary administration of activated recombinant factor VIIBratisl Lek Listy20111121293321452776
- ColinAAShafieianMAndreanskyMBronchoscopic instillation of activated recombinant factor VII to treat diffuse alveolar hemorrhage in a childPediatr Pulmonol201045441120187104
- LoganACYankVStaffordRSOff-label use of recombinant factor VIIa in US hospitals: analysis of hospital recordsAnn Intern Med2011154851652221502649
- LeviMLevyJHAndersenHFTruloffDSafety of recombinant activated factor VII in randomized clinical trialsN Engl J Med2010363191791180021047223
- StanworthSJBirchallJDoreeCJHydeCRecombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophiliaCochrane Database Syst Rev20072CD00501117443565
- FanLLDeterdingRRLangstonCPediatric interstitial lung disease revisitedPediatr Pulmonol200438536937815376335