Abstract
A growing understanding of the biology of renal cell carcinoma (RCC) has led to the development and US Food and Drug Administration approval of seven new molecular targeted agents over the past 7 years. Axitinib is a potent, selective, second-generation inhibitor of vascular endothelial growth factor receptors and the latest to join the armamentarium of drugs available for the treatment of metastatic RCC. Despite recent advances in the development of molecular targeted agents for metastatic RCC, the ideal sequencing of these agents remains unclear.
Introduction
Kidney cancer is the fourteenth most common cancer worldwide, with an estimated 273,500 new cases diagnosed in 2008.Citation1 Renal cell carcinoma (RCC) accounts for 90% of all kidney cancers with conventional clear-cell carcinoma being the predominant subtype (75%–80%).
Interferon-á (IFN-á) and interleukin-2 (IL-2) treatments have shown poor response rates (<15%), with only modest improvement in overall survival (OS).Citation2 Treatment with IL-2 has shown prolonged remission (arguably, cure) in a small percentage of patients but is associated with considerable treatment-related morbidity and mortality. Highdose IL-2 has shown durable complete remission in approximately 5% of patients but has never demonstrated improvement in OS in a randomized controlled trial.Citation2,Citation3
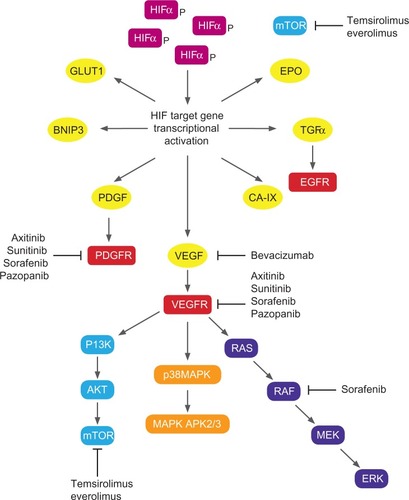
A growing understanding of the biology of RCC has led to the development and US Food and Drug Administration (FDA) approval of seven new agents targeting specific growth pathways. Targets for RCC therapy () include Von Hippel– Lindau (VHL)-mediated pathways – vascular endothelial growth factor (VEGF), epidermal growth factor receptor (EGFR), mammalian target of rapamycin (mTOR), platelet-derived growth factor (PDGF) – and non-VHL mediated pathways, such as Raf-kinase, phosphatidylinositol 3-kinase, and Akt. This review aims to summarize the systemic treatment options for first- and second-line treatment of metastatic renal cell carcinoma (mRCC), with a focus on axitinib, the most recent drug to be approved by the FDA for this indication.
Figure 1 Biological pathways and targets for molecular targeted agents in renal cell carcinoma.
Molecular pathogenesis
Environmental and clinical factors such as smoking, obesity, occupational exposure to toxic compounds (cadmium, asbestos, and petroleum byproducts), and analgesic abuse have been implicated in the etiology of RCC. Several inherited disorders including hereditary papillary renal cancer, VHL disease, and Birt–Hogg–Dubé syndrome predispose to a higher risk of RCC development. VHL disease, an autosomal dominant disorder, is associated with structural abnormalities on chromosome 3p and is characterized by a predisposition to a variety of neoplasms, including RCC. Most patients with primary sporadic clear-cell RCC have either mutations or silencing of the VHL tumor-suppressor gene.Citation4 VHL inactivation simulates hypoxic conditions leading to accumulation of hypoxia-inducible factor (HIF)-1 á. HIF-1 á binds to HIF-1 â, forming a transcriptional factor complex, which translocates to the nucleus and facilitates production of multiple growth factors including VEGF and PDGF. These growth factors subsequently bind to specific tyrosine kinase receptors (vascular endothelial growth factor receptor [VEGFR]-1, VEGFR-2, VEGFR-3, and platelet-derived growth factor receptors [PDGFRs]) resulting in cell migration, endothelial cell growth, and proliferation. mTOR, a serine/threonine-specific kinase is involved in multiple tumor-promoting intracellular signaling pathways in RCC. mTOR inhibition also exhibits antiangiogenesis activity by reducing levels of HIF-1 á and VEGF.Citation5
Current first-line systemic treatment of mRCC
Results of the pivotal Phase III clinical trials of molecular targeted agents that are FDA approved for use in the first-line treatment of patients with mRCC are summarized in .
Table 1 Summary of Phase III clinical trial results for targeted agents approved for first-line treatment of metastatic renal cell carcinoma
Sunitinib
Sunitinib is an orally available multi-kinase inhibitor with potent activity against VEGFRs (types 1-3), PDGFR (á, â), and c-Kit oncogene. In a multicenter Phase III randomized controlled trial of 750 treatment-naïve patients with good/intermediate prognosis mRCC, treatment with sunitinib (50 mg daily for 4 weeks followed by a 2-week break) was associated with an improvement in progression-free survival (PFS) (11 vs 5 months; P < 0.001) and OS (26.4 vs 21.8 months; P = 0.05) when compared with IFN-á.Citation6
Pazopanib
Pazopanib is an oral angiogenesis inhibitor targeting VEGFR PDGFR, and c-Kit oncogene. The pivotal Phase III randomized double-blind placebo-controlled trial evaluated the efficacy and safety of pazopanib in treatment-naïve and cytokine-pretreated patients (n = 435) with advanced RCC. Treatment with pazopanib demonstrated an improved objective response rate (ORR) (30% vs 3%; P < 0.001) and median PFS (9.2 vs 4.2 months; P < 0.0001) when compared to placebo.Citation7
Temsirolimus
Temsirolimus is a parenterally administered rapamycin analog that functions as a competitive inhibitor of mTOR kinase. Temsirolimus was evaluated in a Phase III trial in which 626 previously untreated poor-prognosis patients with metastatic or recurrent RCC were randomly assigned to temsirolimus (25 mg intravenously/week), temsirolimus (15 mg intravenously/week) plus IFN-á (escalated up to 6 million units three times/week as tolerated), or IFN-á monotherapy (escalated up to 18 million units three times/week as tolerated). Temsirolimus significantly prolonged the median PFS (3.1 vs 5.5 months) and median OS (7.3 vs 10.9 months; hazard ratio [HR] 0.73, 95% confidence interval [CI] 0.58–0.92; P = 0.008) when compared with singleagent IFN-á.Citation8
Bevacizumab
Bevacizumab is a monoclonal antibody that binds circulating VEGF and prevents its interaction with VEGFR. Two similarly designed Phase III trials have demonstrated improved PFS with bevacizumab plus IFN-á compared with IFN-á alone. In the avastin and roferon for renal cell carcinoma (AVOREN) trial, 649 treatment-naïve mRCC patients were randomized to IFN-á (9 million units three times/week) plus either bevacizumab (10 mg/kg every 2 weeks) or placebo. Median PFS was significantly improved in the bevacizumab plus IFN-á arm in comparison to the control group (10.2 vs 5.4 months; HR 0.63, 95% CI 0.52–0.75; P = 0.0001).Citation9 Final analysis showed a median OS of 23.3 months with bevacizumab plus IFN-á and 21.3 months with IFN-á plus placebo (HR 0.91, 95% CI 0.76–1.10; P = 0.3360).Citation10 In the Cancer and Leukemia Group B (CALGB) trial 90206, 732 treatment-naïve patients with mRCC were randomly assigned to IFN-á plus bevacizumab or IFN-á plus placebo on schedules similar to those used in the AVOREN trial. There was a statistically significant increase in the ORR (25.5 vs 13.1 percent) and median PFS (8.5 vs 5.2 months; HR 0.71, 95% CI 0.61–0.83) for the bevacizumab plus IFN-á arm.Citation11 The final analysis of this trial revealed a trend toward improved median OS (18.3 vs 17.4 months; HR 0.86; P = 0.07) for the bevacizumab plus IFN-á arm.Citation12
Second-line clinical trials
Sorafenib
Sorafenib is a potent small molecule multi-kinase inhibitor of VEGFR-2, fms-like tyrosine kinase receptor-3, PDGFR, and fibroblast growth factor receptor-1. In the Phase III Treatment Approaches in Renal Cancer Global Evaluation Trial (TARGET), in which 903 patients with advanced RCC who had failed prior standard therapy (IFN-á or IL-2) were randomly assigned to sorafenib (400 mg orally twice daily) or placebo. There was a significant prolongation of median PFS in the sorafenib arm (5.5 vs 2.8 months; HR 0.44, 95% CI 0.35–0.55) but no significant difference in the median OS between the two arms (17.8 vs 15.2 months; HR 0.88, 95% CI 0.74–1.04).Citation13
Everolimus
Everolimus is an orally administered selective inhibitor of mTOR, a key serine-threonine kinase that plays an essential role in downstream protein synthesis of the PI3K/AKT pathway; is dysregulated in many human cancers; and is an essential component of an intracellular signaling pathway regulating cell growth and proliferation, metabolism, and angiogenesis. Everolimus is a derivative of rapamycin and has been in clinical development since 1996 as an immuno-suppressant in solid organ transplantation. The encouraging Phase II clinical trial results in RCC led to the development of the pivotal Phase III clinical trial, RECORD-1.
This trial, a Phase III randomized study, assessed the efficacy of everolimus in patients with mRCC and disease progression on or within 6 months of stopping treatment with sunitinib or sorafenib, or both. A total of 410 patients were randomized in a 2:1 ratio to receive everolimus (10 mg daily) or placebo. The trial was terminated early by the Independent Data Monitoring Committee as an interim analysis demonstrated a significant difference in PFS between the two arms (4.9 vs 1.9 months with everolimus and placebo, respectively; P < 0.001). The improvement in PFS extended to all stratification sets irrespective of risk group, prior treatment status, age, or sex. No complete responses were observed; the partial response rate was 1.8% (n = 5) with everolimus and 0% with placebo. Although Response Evaluation Criteria In Solid Tumors-defined objective responses were rare (<2%) with everolimus, any reduction in tumor measurement from baseline as best percentage change was observed in 47% of patients treated with everolimus versus 10% with placebo. Stable disease by independent central review was noted in 185 (66.8%) of 277 patients in the everolimus arm versus 45 (32.4%) of 139 in the placebo arm. Based on these results, everolimus has been recommended as a second-line treatment option for metastatic clear-cell RCC patients who have failed first-line VEGFR-tyrosine kinase inhibitor (TKI) treatment.Citation14
The median OS was 14.8 months for the everolimus arm versus 14.4 months for the placebo arm (HR 0.87; P = 0.162). The lack of significant difference in survival could be explained by the degree of crossover after the un-blinding of the trial following interim analysis; 76% of patients in the placebo arm had crossed over to everolimus.Citation15 Preplanned, prospective subgroup analysis compared the efficacy of everolimus in patients who received one previous VEGFR-TKI treatment to those who received two or more (26%).Citation16 Of the patients who had received one previous VEGFR-TKI treatment, median PFS was 5.4 months with everolimus and 1.9 months with placebo (HR 0.32, 95% CI: 0.24–0.43; P < 0.001). Of the patients who had received two previous VEGFR-TKI treatments, the median PFS was 4.0 months with everolimus and 1.8 months with placebo (HR 0.32, 95% CI 0.19–0.54; P < 0.001). The authors concluded that everolimus was associated with prolonged PFS relative to placebo in patients who received one or two previous VEGFR-TKI treatments. Patients who had received only one previous VEGFR-TKI treatment had apparently longer PFS with everolimus in comparison with those who had received two previous VEGFR-TKI treatments.
Axitinib
Axitinib (Inlyta®, Pfizer, New York, NY, USA; AG-013736) () is a potent, selective, second-generation inhibitor of VEGFR-1, -2, and -3; PDGF; and colony stimulating factor-1 receptor tyrosine kinases.Citation17,Citation18 Axitinib is 50–450 times more potent than the first-generation VEGFR inhibitors. Based on pharmacokinetic/pharmacodynamic analysis, axitinib appears to be a selective VEGFR-TKI according to current clinical exposure.Citation19
Phase i clinical trials
A Phase I study by Rugo et al investigated the efficacy, safety, and pharmacokinetics of axitinib in advanced cancer.Citation20 Thirty-six patients received axitinib at doses between 5 and 30 mg twice daily. Dose-limiting toxicities included hypertension, hemoptysis, and stomatitis. Axitinib was absorbed rapidly, with plasma peak concentrations noted within 2–6 hours of dosing and steady plasma pharmacokinetic state within 15 days. Three confirmed partial responses were noted. This study recommended the maximum-tolerated dose and recommended Phase II dose of axitinib to be 5 mg twice daily.
Phase II clinical trials
A Phase II multicenter study by Rini et alCitation21 investigated the efficacy and safety of axitinib in patients with sorafenib-refractory metastatic RCC. The starting dose of axitinib was 5 mg twice daily and dose escalation was possible in 53.2% of patients. Of the 62 patients, overall response rate was 22.6%, with a median duration of response of 17.5 months. The median PFS was 7.4 months (95% CI 6.7–11.0) and the median OS was 13.6 months (95% CI 8.4–18.8 months). The most common grade 3/4 adverse events included hand-foot syndrome (16.1%), fatigue (16.1%), hypertension (16.1%), dyspnea (14.5%), and diarrhea (14.5%). Post-hoc analysis of this study by Dutcher et al revealed an ORR of 7.1% for patients with prior treatment with sunitinib and sorafenib, 27.6% for patients with prior cytokines and sorafenib, and 25% for prior treatment with sorafenib alone.Citation22
In a Phase II study by Rixe et al, 52 patients with mRCC who had failed at least one cytokine-based treatment were treated with axitinib 5 mg twice daily.Citation23 The primary endpoint was objective response (complete plus partial response) and secondary endpoints included duration of response, time to progression (TTP), OS, and patient-reported health-related quality of life.Citation24 Results demonstrated an ORR of 44.2% (95% CI 30.5–58.7), with a median response duration of 23.0 months (20.9–not estimable; range 4.2–29.8). Median TTP was 15.7 months (95% CI 8.4–23.4) and the median OS was 29.9 months (95% CI 20.3–not estimable). The updated 5-year survival rate was 20.6% (95% CI 10.9–32.4).Citation22 The most common treatment-related grade 3/4 toxicities included fatigue (21.2%), diarrhea (19.2%), and hypertension (15.4%).
The results of these Phase II trials () suggested that axitinib could be an effective second-line treatment in patients with advanced RCC and led to the development of the pivotal Phase III trial.
Table 2 Summary of second-line Phase II clinical trials of Axitinib in metastatic renal cell carcinoma
Phase III clinical trial
Axitinib versus sorafenib in advanced renal cell carcinoma (AXIS), a multicenter Phase III randomized controlled trial, which compared axitinib to sorafenib in patients with mRCC who had progressed despite first-line therapy containing sunitinib, bevacizumab plus IFN-á, temsirolimus, or cytokines.Citation25 A total of 723 patients with metastatic clear-cell RCC were randomized 1:1 to either axitinib (5 mg twice daily) or sorafenib (400 mg twice daily). Dose increments of axitinib to 7 mg and then to 10 mg were allowed for patients without hypertension or adverse reactions above grade 2. The median duration of treatment was 6.4 months (range 0.03–22) in the axitinib arm and 5.0 months (range 0.03–20) in the sorafenib arm. The median PFS was 6.7 months (95% CI 6.3–8.6) for axitinib versus 4.7 months (95% CI 4.6–5.6) for sorafenib (HR 0.665, 95% CI 0.544–0.812; P < 0.0001). In cytokine-pretreated patients, the median PFS was 12.1 months for axitinib versus 6.5 months for sorafenib (HR 0.464, 95% CI 0.318–0.676; P < 0.0001). In sunitinib-pretreated patients, the median PFS was 4.8 months for axitinib and 3.4 months for sorafenib (HR 0.741, 95% CI 0.573–0.958; P = 0.0107). In the overall patient population and the two main subgroups (prior sunitinib treatment and prior cytokine treatment), there was a statistically significant advantage for axitinib over sorafenib for the primary endpoint of PFS.
The median OS was 20.1 months (95% CI 16.7–23.4) for axitinib versus 19.2 months (95% CI 17.5–22.3) for sorafenib. In the sunitinib-pretreated patients, the median OS was 15.2 months (95% CI 12.8–18.3) in the axitinib arm and 7.7 months (95% CI 4.4–12.4) in the sorafenib arm. In the cytokine-pretreated patients, the median OS was 29.4 months (95% CI 24.5–not reached) in the axitinib arm and 27.8 months (95% CI 23.1–34.5) in the sorafenib arm. There were no statistically significant differences between the arms in terms of OS in the overall population or in the subgroups by prior therapy.
The ORR assessed by blinded independent radiology review was 19.4% for axitinib and 9.4% for sorafenib (P = 0.0001). The objective response to axitinib in the sunitinib-pretreated patients was 11.3% (95% CI 7.2–16.7) and in the cytokine-pretreated patients was 32.5% (95% CI 24.5–41.5). The median duration of response was 11 months (95% CI 7.4–not reached) for axitinib and 10.6 months for sorafenib (8.8–11.5). The open-label trial design and the potential for dose escalation in the axitinib arm have been criticized. However, it is still unclear if there is any additional benefit of axitinib dose escalation and this is the subject of an ongoing Phase II trial.Citation26
Secondary end points of the trial included kidney-specific symptoms, function assessments, and heath-related quality of life. The composite end point of time to deterioration using patient questionnaires (Functional Assessment of Cancer Therapy – Kidney Cancer Symposium Index [FKSI-15]) demonstrated a statistically significant advantage for axitinib when compared with sorafenib (HR 0.829, 95% CI 0.707–0.993; P = 0.020).Citation27 The authors concluded that treatment with axitinib led to a statistically significant improvement in PFS along with a delay in worsening of composite time-to-deterioration end points when compared with sorafenib in treatment-refractory RCC.
Discussion
Axitinib is a potent and selective VEGFR-TKI, which has demonstrated significant antitumor activity in patients with mRCC. Data from the pivotal AXIS trial makes a compelling case for its use as second-line treatment following failure of previous IFN-á or TKI therapy.Citation25 Based on the results of this trial, the FDA (January 2012) and European Medicines Agency (September 2012) approved the use of axitinib for the treatment of advanced RCC after failure of one prior systemic therapy. AXIS is the first trial to have demonstrated the superiority of one targeted agent over another in mRCC in the second-line setting following failure of one prior systemic therapy. The efficacy of axitinib versus sorafenib in the first-line setting is being investigated in a Phase III trial of patients with mRCC who are either treatment naïve or have progressed after one therapy.Citation28 Results from the TARGET trial are unlikely to influence management in the current era, as the standard first-line treatment on the trial was either IFN-á or IL-2. Results from the nexavar versus torisel as second-line therapy for advanced renal cell carcinoma (INTORSECT) trial, which compared the efficacy of temsirolimus versus sorafenib as second-line treatment in patients with mRCC were recently presented at the Thirty-Seventh Congress of the European Society of Medical Oncology in October 2012.Citation29 Results from this trial showed no significant difference in median PFS (4.28 vs 3.91 months) or OS (12.27 vs 16.64 months) for patients treated with temsirolimus and sorafenib, respectively.
The drug choice in the second-line setting remains unclear, as results from the RECORD-1, AXIS, and INTORSECT trials leave many questions unanswered. In the AXIS trial, the improvement in median PFS was less pronounced in patients receiving first-line sunitinib (4.8 vs 3.4 months; HR 0.741; P = 0.010) when compared with patients receiving first-line cytokines (12.1 vs 6.5 months; HR 0.464; P < 0.0001). In the real-world scenario, the post-cytokine cohort is dwindling rapidly, as targeted agents are becoming increasingly available worldwide, either as standard treatment or as part of clinical trials. RECORD-1 and AXIS are two very different clinical trials and it would be difficult to directly compare outcomes.
The rapidity of drug development in this setting makes it difficult to choose the ideal comparator for randomized clinical trials for patients with mRCC. The ideal comparator for the AXIS trial would have been everolimus, but it was not FDA approved until May 2009. Both everolimus and axitinib have demonstrated significant benefit in patients with mRCC who have progressed after at least one line of systemic therapy, albeit in a different patient population. Patients in the AXIS trial had only failed first-line therapy whereas nearly 80% of patients in the RECORD-1 trial were heavily pretreated. In addition to VEGFR inhibitors, patients in the everolimus arm were previously treated with immunotherapy (65%), chemotherapy (13%), and hormonal therapy (2%). The ideal sequencing of axitinib and everolimus following failure of one TKI remains a challenge and perhaps could only be addressed by a randomized Phase III trial.
Conclusion
The FDA has approved seven new drugs over the past 7 years, heralding the unprecedented drug development now being undertaken for the management of RCC. Axitinib is the most recent drug to have received FDA approval and is indicated for the treatment of advanced RCC after failure of one prior systemic therapy. Although the AXIS trial met its primary end point of improved PFS, the magnitude of median PFS improvement was more pronounced in the prior cytokine-treatment group when compared with the prior sunitinib-treatment group. There were no statistically significant differences between the treatment arms in terms of OS in the overall population or in the subgroups by prior therapy. Indirect comparisons between the efficacy of everolimus and axitinib are unlikely to be helpful, given the differences in the trial populations. Both everolimus and axitinib are potent drugs in this patient population and the ideal sequencing of drugs in the second-line setting can only be determined by a head-to-head comparison between these agents.
Disclosure
The authors declare no conflicts of interest in this work.
References
- FerlayJShinHRBrayFFormanDMathersCParkinDMEstimates of worldwide burden of cancer in 2008: GLOBOCAN 2008Int J Cancer2010127122893291721351269
- WirthM PImmunotherapy for metastatic renal cell carcinomaUrol Clin North Am19932022832957684167
- FyfeGFisherRIRosenbergSASznolMParkinsonDRLouieACResults of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapyJ Clin Oncol19951336886967884429
- CliffordSCProwseAHAffaraNABuysCHMaherERInactivation of the von Hippel-Lindau (VHL) tumour suppressor gene and allelic losses at chromosome arm 3p in primary renal cell carcinoma: evidence for a VHL-independent pathway in clear cell renal tumourigenesisGenes Chromosomes Cancer19982232002099624531
- LandSCTeeARHypoxia-inducible factor 1alpha is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motifJ Biol Chem200728228205342054317502379
- MotzerRJHutsonTETomczakPOverall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinomaJ Clin Oncol200927223584359019487381
- SternbergCNDavisIDMardiakJPazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trialJ Clin Oncol20102861061106820100962
- HudesGCarducciMTomczakPGlobal ARCC Trial. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinomaN Engl J Med2007356222271228117538086
- EscudierBPluzanskaAKoralewskiPAVOREN Trial investigatorsBevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trialLancet200737096052103211118156031
- EscudierBBellmuntJNégrierSPhase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survivalJ Clin Oncol201028132144215020368553
- RiniBIHalabiSRosenbergJEBevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with meta-static renal cell carcinoma: CALGB 90206J Clin Oncol200826335422542818936475
- RiniBIHalabiSRosenbergJEPhase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206J Clin Oncol201028132137214320368558
- EscudierBEisenTStadlerWMSorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trialJ Clin Oncol200927203312331819451442
- MotzerRJEscudierBOudardSRECORD-1 Study GroupEfficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trialLancet2008372963744945618653228
- MotzerRJEscudierBOudardSRECORD-1 Study GroupPhase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factorsCancer2010116184256426520549832
- CalvoEEscudierBMotzerRJEverolimus in metastatic renal cell carcinoma: Subgroup analysis of patients with 1 or 2 previous vascular endothelial growth factor receptor-tyrosine kinase inhibitor therapies enrolled in the phase III RECORD-1 studyEur J Cancer201248333333922209391
- ChoueiriTKAxitinib, a novel anti-angiogenic drug with promising activity in various solid tumorsCurr Opin Investig Drugs200896658671
- KellyRJRixeOAxitinib – a selective inhibitor of the vascular endothelial growth factor (VEGF) receptorTarget Oncol20094429730519876699
- Hu-LoweDDZouHYGrazziniMLNonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3Clin Cancer Res200814227272728319010843
- RugoHSHerbstRSLiuGPhase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: pharmacokinetic and clinical resultsJ Clin Oncol200523245474548316027439
- RiniBPhase 2 study of Axitinib in sorafenib-refractory metastatic renal cell carcinomaJ Clin Oncol2009274462446819652060
- DutcherJPWildingGRHudesWMSequential axitinib (AG-013736) therapy of patients (pts) with metastatic clear cell renal cell cancer (RCC) refractory to sunitinib and sorafenib, cytokines and sorafenib, or sorafenib aloneJ Clin Oncol200826SupplAbstr 5127
- RixeOBukowskiRMMichaelsonMDAxitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II studyLancet Oncol200781197598417959415
- MotzerRJde LaMotteRougeTHarzstarkALAxitinib second-line therapy for metastatic renal cell carcinoma (mRCC): five-year (yr) overall survival (OS) data from a phase II trialJ Clin Oncol201129SupplAbstr 4547
- RiniBIEscudierBTomczakPComparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trialLancet201137898071931193922056247
- PfizerAxitinib (AG-013736) with or without dose titration (increase) in patients with kidney cancerClinicalTrialsgov [website on the Internet]Bethseda, MDUS National Library of Medicine2009 [updated November 9, 2012]. Available from: http://clinicaltrials.gov/ct2/show/NCT00835978NLM identifier: NCT00835978. Accessed November 23, 2012
- CellaDEscudierBRiniBIPatient-reported outcomes (PROs) in a phase III AXIS trial of axitinib versus sorafenib as second-line therapy for metastatic renal cell carcinoma (mRCC)J Clin Oncol201129SupplAbstr 4504
- PfizerAxitinib (AG-013736) for the treatment of metastatic renal cell cancerClinicalTrialsgov [website on the Internet]Bethseda, MDUS National Library of Medicine2009 [updated November 19, 2012]. Available from: http://clinicaltrials.gov/ct2/show/NCT00920816NLM identifier: NCT00920816. Accessed November 23, 2012
- HutsonTEscudierBEstebanETemsirolimus vs Sorafenib as Second Line Therapy in Metastatic Renal Cell Carcinoma: Results From the INTORSECT TrialPresented at the 37th Congress of the European Society for Medical Oncology (ESMO)Vienna, AustriaSeptember 28–October 2, 2012 Abstract LBA22