Abstract
Antibodies specific for bovine type II collagen (CII) and Fcγ receptors play a major role in collagen-induced arthritis (CIA), a mouse model of rheumatoid arthritis (RA). Our aim was to clarify the mechanism of immune complex-mediated inflammation and modulation of the disease. CII pre-immunized DBA/1 mice were intravenously boosted with extravidin coupled biotinylated monomeric CII-peptide epitope (ARGLTGRPGDA) and its complexes with biotinylated FcγRII/III specific single chain Fv (scFv) fragment. Disease scores were monitored, antibody titers and cytokines were determined by ELISA, and binding of complexes was detected by flow cytometry and immune histochemistry. Cytokine and chemokine secretion was monitored by protein profiler microarray. When intravenously administered into collagen-primed DBA/1 mice, both CII-peptide and its complex with 2.4G2 scFv significantly accelerated CIA and increased the severity of the disease, whereas the monomeric peptide and monomeric 2.4G2 scFv had no effect. FcγRII/III targeted CII-peptide complexes bound to marginal zone macrophages and dendritic cells, and significantly elevated the synthesis of peptide-specific IgG2a. Furthermore, CII-peptide containing complexes augmented the in vivo secretion of cytokines, including IL-10, IL-12, IL-17, IL-23, and chemokines (CXCL13, MIP-1, MIP-2). These data indicate that complexes formed by the CII-peptide epitope aggravate CIA by inducing the secretion of chemokines and the IL-12/23 family of pro-inflammatory cytokines. Taken together, these results suggest that the in vivo emerging immune complexes formed with autoantigen(s) may trigger the IL-12/23 dependent pathways, escalating the inflammation in RA. Thus blockade of these cytokines may be beneficial to downregulate immune complex-induced inflammation in autoimmune arthritis.
Introduction
Due to its similar immunological characteristics involving high levels of auto-antibodies and collagen specific T-cells, collagen-induced arthritis (CIA) obtained by immunizing DBA/1 mice with bovine type II collagen (CII), is a widely used model of human rheumatoid arthritis (RA).Citation1 In this arthritis model anti-CII antibodies are regarded as important factors in the development of arthritis, as the transfer of sera from diseased mice can induce CIA in healthy DBA/1 recipients.Citation2 Furthermore, anti-CII IgG can mediate arthritis by engaging the activating Fc gamma receptors (FcγR), indicating that pathogenic immune complexes play a fundamental role in the onset of disease.Citation3,Citation4
IgG-containing immune complexes that can bind to FcγR are crucial players in the pathogenesis of arthritis, as they have a regulatory role in both the central and the effector phase of CIA.Citation5 In mice, four subtypes of IgG receptors (FcγRI, FcγRIIb, FcγRIII and FcγRIV) have been identified.Citation6,Citation7 Of these FcγRI, FcγRIII, and FcγRIV, expressed on a broad variety of leukocytes including neutrophils, macrophages, NK cells, and dendritic cells, transmit stimulatory signals through an immunoreceptor tyrosine-based activation motif (ITAM).Citation6,Citation8–Citation10 In contrast, FcγRIIb is regarded as an inhibitory receptor and contains an immunoreceptor tyrosine-based inhibition motif (ITIM).Citation11–Citation13 FcγRIIb is expressed on B-cells, macrophages, dendritic cells, and mast cells. FcγRIIb deficiency renders normally resistant strains of mice susceptible to several antibody- or immune complex-dependent models of autoimmunity.Citation14–Citation16 The balance between activating and inhibitory FcγR has a decisive role in the outcome of the disease; FcγRIIb inhibits, while the activating FcγR augments CIA.Citation7,Citation17,Citation18 Moreover, FcRγ chain-deficient mice were completely resistant to antibody-mediated arthritis.Citation3 Although FcγRI and FcγRIII themselves were shown to be dispensable for the development of destructive arthritis, the common FcRγ chain was nevertheless required for the process, indicating that FcγRIV, the new member of the γ chain associated receptor family may play a role in the effector phase of CIA.Citation19
Cytokines may influence the expression of FcγR by several mechanisms; for example IFNγ directly upregulates activating receptor expression on macrophages and polymorphonuclear cells, while IL-17 enhances cartilage destruction by increasing the local amount of FcγR-bearing neutrophils in immune complex-mediated arthritis.Citation20 Subsequently, the crosslinking of activating FcγRIII by immune complexes triggers the release of pro-inflammatory cytokines, TNFα and IL-1 from macrophagesCitation21 may thus establish an amplifying loop, ultimately resulting in severe inflammation and tissue damage in RA.
An arthritis-related immunodominant triple-helical B-cell epitope (between amino acid positions 359–369) in CII has been described that was also recognized by sera from RA patients but not by sera from other donors; moreover, the combined transfer of two mouse monoclonal antibodies specific for the triple helical CII epitope induced arthritis in the non-susceptible BALB/c mice.Citation22,Citation23 We applied the biotinylated CII-peptide corresponding to the monomeric CII sequence (ARGLTGRPGDA) coupled to extravidin. Our aim was to investigate how FcγRII/III targeting of this CII peptide by single chain Fv fragment (scFv) of the monoclonal antibody, 2.4G2 may modulate the immunopathogenesis of CIA. Previously we and others have shown that antibody response against model antigens can be substantially improved by FcγRII/III targeting.Citation24,Citation25 Extravidin-linked molecular constructs of the biotinylated CII-peptide and its complex with mono-biotinylated 2.4G2 scFv were injected intravenously into CII pre-immunized DBA/1 mice, and the in vivo effect on several parameters of CIA, including arthritic score, antibody response to CII and CII-peptide, as well as cytokine/chemokine secretion were monitored. The results indicate that the exposure of collagen preimmunized DBA/1 mice to CII-peptide-containing complexes results in a significant elevation of IL-12/23 family inflammatory mediator secretion, ultimately leading to the acceleration and increased severity of CIA.
Materials and methods
Reagents and antibodies
The 2.4G2 cell clone (IgG2a) was kindly provided by the Department of Immunology, University Hospital Utrecht, The Netherlands. BirA was purchased from Avidity LLC (Aurora, CO), bovine type II collagen and complete Freund adjuvant containing 5 mg/mL heat killed mycobacteria tuberculosis were purchased from Chondrex Inc (Redmond, WA), rat anti-mouse CD45R (B220)–PerCP/Cy5.5 and hamster anti-mouse CD11c–Alexa 647 from AbD Serotec (Oxford, UK). F4/80 rat IgG2b Alexa Fluor 647 conjugated antibodies were from eBioscience Ltd, Hatfield, UK. PE-conjugated rat anti-mouse CR1/2 (7G6 clone) mAb was purchased from Soft Flow Inc (Pécs, Hungary). Marginal zone macrophage marker (macrophage receptor with collagenous structure [MARCO], clone IBL-12Citation26) and the Cy3-labelled goat anti-rat IgG were purified and labelled at the Department of Immunology and Biotechnology, University of Pecs (Pecs, Hungary). Biotinylated CII-peptide (ARGLTGRPGDA) was obtained from Mimotopes Pty Ltd, Melbourne, Australia, and also synthesized by Dr Anna Magyar, Research Group of Peptide Chemistry, Hungarian Academy of Sciences at Eötvös Loránd University of Sciences. For biotinylation, sulpho-NHS-long chain-biotin was used to provide flexibility for the peptide. All other reagents were purchased from Sigma–Aldrich (Budapest, Hungary).
Mice
DBA/1 mice were obtained from Charles River Laboratories (Budapest, Hungary), CD16 and CD64 KO mice were a kind gift from Dr Attila Mocsai (Semmelweis University, Budapest, Hungary).Citation27,Citation28 Mice aged 8–16 weeks were used for all experiments. Animals were kept on a standard diet with tap water ad libitum. All animal studies were approved by the local research ethics committee.
Induction and evaluation of collageninduced arthritis (CIA)
CII was dissolved in 0.1M acetic acid at 2 mg/mL concentration. Six-week old female DBA/1 mice were immunized subcutaneously at the base of the tail with 100 μg CII emulsified in Complete Freund Adjuvant (CFA). At days 30, 45 and 70 after immunization the “complex” group received intravenous injection of preformed complexes of 60 μg biotinylated 2.4G2 scFv, 2.6 μg biotinylated collagen peptide (CII-peptide) and 60 μg extravidin (2:2:1 molar ratio) per animal. The CII-peptide tetramer group was injected with 2.6 μg biotinylated CII-peptide (ARGLTGRPGDA) mixed with 30 μg Extravidin per animal (4:1 molar ratio). The 2.4G2 scFv tetramer group was given 60 μg biotinylated 2.4G2 scFv and 30 μg extravidin per animal (4:1 molar ratio). The tetramer CII-peptide, scFv and the mixed complexes were produced by the stepwise addition of extravidin to achieve the required molar ratio.
Supplementary file 1 shows the composition of the extravidin-bound 2.4G2 scFv, CII-peptide and CII-peptide –2.4G2 scFv mixed complexes resolved by non-reducing sodium dodecyl sulfate polyacrylamide gel electrophoresis. According to the calculated molecular masses, most of the extravidin-bound scFv appeared as dimers and tetramers, while in the extravadin-bound CII-peptide sample we observed a smear between 55–70 kDa, showing three distinct lines, probably corresponding to different numbers of extravidin-bound peptide molecules. The CII-peptide-scFv complexes appeared as smears around 130 kDa (probably corresponding to a mixed complex). Since one molecule of extravidin theoretically binds 4 biotinylated molecules, for simplicity, we call extravidin-linked CII-peptide or scFv constructs “tetramers,” while the mixed constructs are referred to as “complexes.”
The control group of mice received intravenous injections of the buffer only. Blood samples were collected at days 35, 55 and 70 after the CIA induction.
Dates of onset of the disease after immunization were recorded for individual mice. The progress of CIA was evaluated visually, scored on a graded scale of 1–3 for each paw.Citation29 An arthritic index of the disease was calculated, based upon the visual appearance of a paw. Each limb was graded 0–3, representing increased joint swelling, erythema, and visible joint distortion. Changes in the number of affected limbs and the arthritic scores for each limb were recorded twice a week. Arthritic scores were combined to give a global arthritic score of a maximum of 12 for each mouse.
Preparation of the single-chain Fv antibody
2.4G2 scFv was prepared and purified as previously described.Citation24,Citation30 2.4G2 recognizes both FcγRII (CD32) and FcγRIII (CD16).Citation31 Protein constructs also containing a peptide tag recognized by the E. coli biotinyl ligase BirA were biotinylated with the enzyme according to the manufacturer’s instructions.Citation32,Citation33 The functional integrity of 2.4G2 scFv was tested on mouse spleen cells. The scFv bound to B-cellsCitation27 and was able to inhibit Ca2+ mobilization when co-crosslinked with BCRCitation34 (Supplementary file 2).
Flow cytometry
The in vitro binding of biotinylated 2.4G2 scFv or biotinylated CII-peptide attached to extravidin-fluorescein isothiocyanate (FITC), or the extravidin-FITC coupled mixed complexes of the two molecules was analyzed in spleen cell suspension. Spleens from the CIA-induced DBA/1 mice were removed and treated with 2 mg/mL collagenase D (Roche) according to the manufacturer’s instructions: 5 × 105 cells were labeled with preformed complexes of biotinylated 2.4G2 scFv and/or biotinylated CII-peptide and extravidin-FITC for 15 minutes on ice. Cells were simultaneously stained with anti-mouse CD45R (B220)-PerCP/Cy5.5 for detection of B-cells, anti-CD11c hamster IgG labeled with Alexa 647 for dendritic cells, and F4/80 rat IgG2b Alexa 647 for macrophages, respectively. Samples were analyzed by FACS-Calibur flow cytometer (Becton–Dickinson, Franklin Lakes, NJ) and the data were evaluated with FCS Express 3 software (De Novo Software, Los Angeles, CA).
Immunofluorescent detection of the in vivo localization of extravidin-FITC containing complexes
Spleens of mice initially immunized with collagen, then injected intravenously with 2.4G2 scFv-CII-peptide-extravidin-FITC complex, 2.4G2 scFv-extravidin-FITC or CII-peptide-extravidin-FITC were taken out 15 minutes after the intravenous injection and mounted in cryostat-embedding medium (Killik; Bio-Optica, Milan, Italy) and then stored at –80°C until processed. Frozen sections of 8 mm thickness were cut, collected and fixed in ice-cold acetone for 10 minutes and then blocked with 5% bovine serum albumin (BSA) in phosphate buffered saline (PBS) for 30 minutes at room temperature in a wet chamber. Sections were incubated with antibodies specific for the marginal zone macrophage marker [MARCO, clone IBL-12Citation26] and developed using Cy3-labelled goat anti-rat IgG; and with complement receptor (CR1/2) specific antibodies. Fluorescent images were captured using a ColorView CCD camera mounted onto an Olympus BX61 fluorescent microscope. The fluorescent signals were sequentially recorded using a 460–490 nm band-pass excitation filter and 515–550 nm band-pass filter for FITC, and a 530–550 nm band-pass excitation filter with a 590 nm long-pass filter for Cy3, respectively, at the resolution of 300 dpi. After acquisition the two images were merged using Adobe Photoshop with screen mode.
Detection of CII- and CII-peptide specific antibodies
CII- and CII-peptide-specific IgG titer in sera of immunized mice were determined by indirect ELISA. To detect CII specific antibodies, plates were coated with 5 μg/mL bovine collagen, and then blocked with 1% BSA in PBS. Serum samples were diluted 1:200, and then a four-fold dilution series was used for the measurements. For detection of CII-peptide-specific antibodies, biotinylated CII-peptide (1 μg/mL) was added to NeutrAvidin (5 μg/mL) pre-coated plates (Thermo Scientific, Rockford, IL). The plates were blocked with buffer containing 40 mM TRIS-HCl, 150 mM NaCl, 0.5% BSA and 0.1% Tween. Serum samples were used in 1:100 dilutions, and then 5-fold dilution series were used for the measurements. The plates were washed and developed by HRP-conjugated anti-mouse IgG or anti-mouse IgG2a followed by adding TMB peroxidase substrate solution. Finally, the reaction was stopped and the optical density was measured at 450 nm with wavelength correction at 620 nm by ELISA reader (Thermo Electron, Multiscan Ex). Endpoint titers were calculated.
Evaluation of the level of cytokines and chemokines in mouse sera by a protein profiler array
For the detection of cytokines and chemokines in mouse sera, the Mouse Cytokine Array, Panel A (ARY006, R&D Systems, Minneapolis, MN) was used, the estimation of the cytokines and chemokines in sera samples was carried out following the manufacturer’s protocol. Pooled sera samples from each group of mice (collected at day 70, 2 hours after the intravenous injection of 2.4G2 scFv-CII-peptide complexes or tetramer constructs of the scFv and CII-peptide, respectively) were added to the membranes, and after incubation with the detection antibody the membranes were developed with streptavidin-HRP (Thermo Scientific, Rockford, IL), followed by the chemiluminescent reagent, and then exposed to X-ray film. Pixel densities were analyzed in each spot of the array by the GenePix Pro 6.0 program (Molecular Devices, Sunnyvale, CA), and average values of duplicate spots were compared.
Detection of cytokines in the supernatants of cultured spleen cells
In ex vivo experiments the spleen cells of collagen-immunized DBA/1 mice were tested after the animals received two intravenous injections of extravidin plus 2.4G2 scFv or CII-peptide, or the extravidin-coupled complexes of the two molecules, respectively. One week after the last injection mice were sacrificed from each group, and their spleen cells were cultured in the presence of one tenth of the in vivo given quantity of complex, CII-peptide or scFv tetramer. After 24 hours, 48 hours and 72 hours the culture supernatants were tested for cytokine production.
To study the effect of 2.4G2 scFv on cytokine secretion in vitro, spleen cells (2.5 × 106 cells/mL) from C57BL/6 wild type, CD16 KO, or CD64 KO mice were cultured in RPMI-1640 medium or on streptavidin pre-coated, biotinylated 2.4G2 scFv coated immunoplates (Millipore) for 72 hours at 37°C.
The amounts of cytokines in the culture supernatants were measured using the Quantikine ELISA kits according to the manufacturer’s instruction (R&D Systems, Minneapolis, USA). Protein amount was calculated by the formula obtained from standards.
Statistical analysis
Statistical differences between disease scores of various experimental groups of mice were assessed by pairwise comparisons of relevant groups using permutation tests. Briefly, values from the groups to be compared were randomly reassigned to two groups and the difference between the group means was calculated. Distribution of 10000 randomizations was drawn and the two-tailed P value corresponding to the real sample assignments was determined. The arithmetic mean of 50 such P values was accepted as the probability of α-error. Values of P < 0.05 were considered significant and were indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001. We used this test for two reasons: (1) the distribution of the tested variables is neither known nor can be reliably estimated, so a nonparametric test was the choice, and (2) standard nonparametric tests for comparing two groups, such as the Mann–Whitney U test, are less sensitive when the sample number is limited, while permutation tests are robust from this point of view.
Median values of experimental groups in ELISA were compared with a Kruskal–Wallis test. Differences between groups were considered significant for P < 0.05. The data were analysed by using GraphPad Prism version 4.00 for Windows (Graphpad Software Inc, La Jolla CA).
Results
Complexes of 2.4G2 scFv and CII-peptide modulate the kinetics and severity of CIA
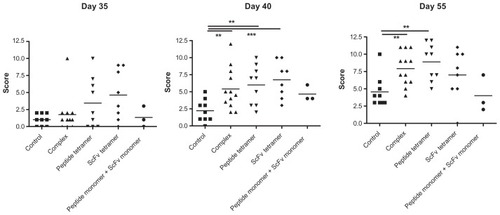
To study the effect of extravidin-coupled complexes of CII-peptide on CIA, DBA/1 mice first received a single subcutaneous injection of bovine type II collagen in CFA on day 0, which was followed by the intravenous injection of extravidin-linked constructs at day 28. Arthritic scores of mice were monitored. Four weeks after the initial immunization with CII in CFA the animals did not show any phenotypic signs of arthritis. Injecting the mice at day 28 with extravidin-linked CII-peptide (peptide tetramer) or 2.4G2 scFv (scFv tetramer) elevated the arthritic score values by five days after the injection, while the scores remained between 0 and 2.5 in the control, untreated group, receiving buffer only. On day 10 (40 days after the initial immunization) we observed a significant difference between the groups receiving buffer and those injected with the extravidin-coupled mixed complexes of CII-peptide and 2.4G2 scFv or with the CII-peptide and the 2.4G2 scFv tetramers, respectively, while mice injected with the monomeric peptide and monomeric 2.4G2 scFv showed similar scores to the control group. At day 55, ten days after the second injection with the same constructs the differences between the control and the complex- or the CII-peptide tetramer-treated groups were still significant, indicating an aggravated disease state ().
Figure 1 Extravidin-linked complexes of biotinylated CII-peptide and biotinylated 2.4G2 scFv, CII-peptide tetramers and 2.4G2 scFv tetramers elevate disease scores in CIA of DBA/1 mice.
Abbreviations: CII, bovine type II collagen; CIA, collagen-induced arthritis; scFv, single chain Fv fragment.
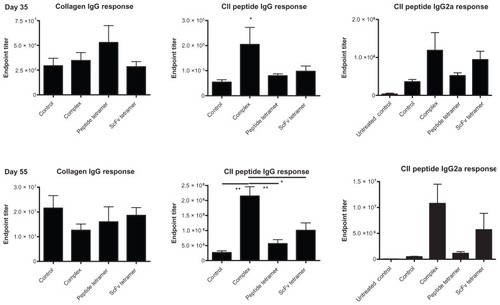
Enhanced CII-peptide-specific antibody titers in sera of collagen immunized and then 2.4G2 scFv-CII-peptide complex-treated mice
We compared the antibody titers in sera of collagen-primed mice 5 days after the first intravenous injection of 2.4G2 scFv-CII-peptide complexes, CII-peptide tetramer and 2.4G2 scFv tetramer, respectively, and then 10 days after the second injection with the same constructs; using either collagen coat or biotinylated CII-peptide as capture antigen. Administration of CII-peptide tetramers slightly elevated the collagen specific antibody titer on day 5 as compared to the non-treated animals, while the other treatments had no effect on anti-collagen IgG production. 10 days after the second injection (55 days after the initial immunization) no significant differences were observed between the anti-collagen titers of different groups (, left panel). On the contrary, the CII-peptide specific IgG titers were significantly higher at both time points in mice treated with FcγRII/III targeted CII-peptide as compared to all other groups. However, CII-peptide specific IgG was also detected in the peptide- or scFv tetramer-treated mice and at lower level in the collagen primed control as well. The CII-peptide specific IgG2a titers showed a similar distribution ( right panels).
Figure 2 Collagen-specific and CII-peptide specific IgG titers in sera of mice treated with 2.4G2 scFv and CII-peptide containing constructs.
Abbreviations: CII, bovine type II collagen; ELISA, enzyme-linked immunosorbent assay; scFv, single chain Fv fragment.
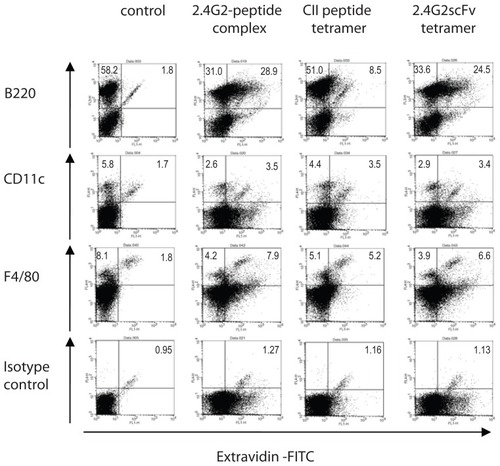
In vitro binding and in vivo localization of 2.4G2 scFv-CII-peptide complexes
To investigate which cell types bind the complexes and the tetramer constructs, flow cytometry and immunohistochemistry experiments were performed. In vitro binding of 2.4G2 scFv in complex with CII-peptide, tetramers of CII-peptide or 2.4G2 scFv (both preformed with extravidin-FITC) to spleen cells of suboptimally collagen immunized mice was tested. Flow cytometric analysis revealed that approximately 47% of B220 positive cells (B-cells), 41% of CD11c positive cells (dendritic cells, DC), and 60% of F4/80 positive cells were also positive for 2.4G2 scFv-CII-peptide complexes. The F4/80 molecule is expressed selectively on subpopulations of myeloid cells, including macrophages and DCs.Citation35 The 2.4G2 scFv tetramer bound at similar ratios to all populations: to 40% of B-cells, 36% of dendritic cells, and 55% of F4/80 positive cells, while the CII-peptide tetramer specifically bound to about 10% of B-cells, and surprisingly, to 30% of dendritic cells (DC), and 40% of macrophages (). Spleen cells of non-immunized mice did not bind CII-peptide tetramers (data not shown). These data indicate that CII-peptide may bind to FcγR on DC and macrophages via peptide-specific IgG.
Figure 3 In vitro binding of 2.4G2 scFv-CII-peptide complexes, CII-peptide and 2.4G2 scFv ‘tetramer’s to spleen cell subsets.
Abbreviations: CII, bovine type II collagen; FITC, fluorescein isothiocyanate; scFv, single chain Fv fragment.
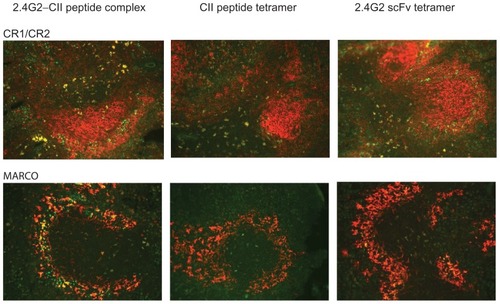
In vivo localization of the intravenously injected extravidin-FITC containing complexes and tetramers in spleen sections of suboptimally collagen immunized mice was visualized by double immunofluorescence. Fifteen minutes after the intravenous injections the 2.4G2 scFv-CII-peptide complexes were observed mainly in the marginal zone (MZ) area in co-localization with the MZ macrophage marker, MARCO. Occasional follicular co-staining was also seen with some of the CR1/CR2 positive, most probably dendritic cells. Co-localization with IgM positive or IgD positive B-cells could not be observed (data not shown). The 2.4G2 scFv tetramers stained fewer cells and showed much less co-staining but a similar distribution, while CII-peptide tetramers stained scattered cells mainly in the T-zone of the spleen (). Collectively these data indicate that the constructs containing 2.4G2 scFv may bind to a fraction of B-cells in the spleen. Shortly after intravenous injection they are mostly located in marginal zone macrophages, and to a lesser extent are associated with dendritic cells in the non-follicular compartment of the white pulp. Binding of CII-peptide tetramers was also detected in this latter population.
Figure 4 In vivo localization of 2.4G2 scFv-CII-peptide complexes, CII-peptide and 2.4G2 scFv tetramers in the spleen of collagen immunized DBA/1 mice.
Abbreviations: CII, bovine type II collagen; FITC, fluorescein isothiocyanate; scFv, single chain Fv fragment.
Induction of cytokine/chemokine secretion by FcγRII/III targeting
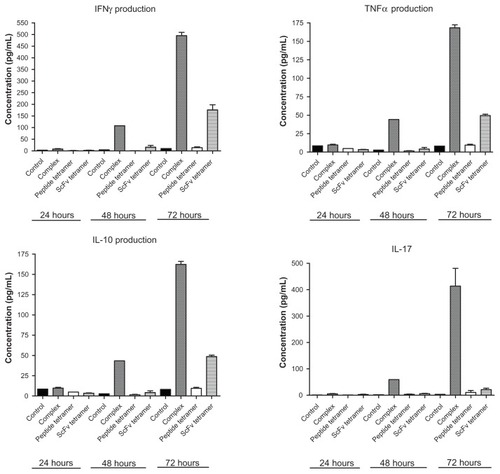
To investigate whether the complexes and tetramers exert their effect on CIA via inducing cytokine/chemokine production, in vitro cultures of spleen cells from collagen immunized and complex- or tetramer-treated mice were tested. Additionally to the in vivo injection of the constructs, spleen cells of mice were cultured in the presence of 2.4G2 scFv-CII-peptide complexes or the tetramer peptide and 2.4G2 scFv, respectively, and then the culture supernatants were tested after 24, 48 and 72 hours. A time-dependent induction of TNFα, IL-17, IFNγ, and IL-10 secretion was detected in supernatants of cells cultured with the 2.4G2 scFv-CII-peptide complexes. In contrast, scFv tetramers triggered a much lower amount of cytokine release, and the CII-peptide tetramers did not induce ex vivo cytokine production (). The TH2 cytokine, IL-4, was not detected in any of the culture supernatants (data not shown). These data indicate that the 2.4G2 scFv-CII-peptide complexes are able to stimulate the release of pro-inflammatory cytokines, including IL-17 that is critical for arthritis; and also suggest that CII-peptide targeted to FcγRII/III is more efficient at stimulating cytokine release as compared to FcγRII/III crosslinking alone.
Figure 5 2.4G2 scFv-CII-peptide complexes induce cytokine production in vitro.
Abbreviations: CII, bovine type II collagen; ELISA, enzyme-linked immunosorbent assay; scFv, single chain Fv fragment.
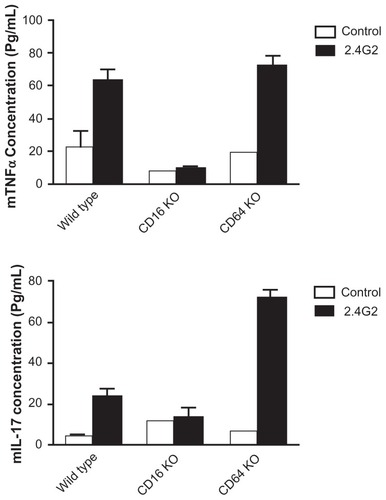
2.4G2 scFv binds to both FcγRII and FcγRIII positive cells. To investigate whether the activating receptor, FcγRIII is indeed responsible for the cytokine release, we compared the 2.4G2 scFv tetramer-induced TNFα and IL-17 production in spleen cell cultures of wild type (C57BL/6), CD16 KO, and CD64 KO mice. The results show that CD64 KO mice were able to produce, while CD16 KO animals failed to secrete these cytokines upon interaction with the 2.4G2 tetramers, indicating that FcγRIII is indispensable for the effect, which cannot be compensated by FcγRI and FcγRII ().
Figure 6 2.4G2 scFv tetramers induce TNFα and IL-17 secretion in murine spleen cell cultures of wild type (C57/Bl6) and C64 KO but not of CD16 KO mice.
Abbreviations: FITC, fluorescein isothiocyanate; ELISA, enzyme-linked immunosorbent assay; scFv, single chain Fv fragment; TNFα, tumor necrosis factor alpha.
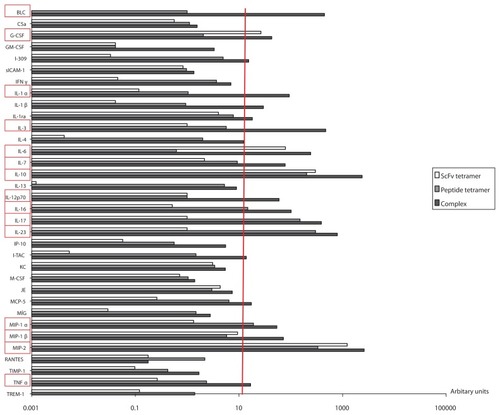
To investigate whether 2.4G2 scFv-CII-peptide complexes and the tetramer constructs are able to induce cytokine/ chemokine synthesis in vivo, collagen pre-immunized mice received a third intravenous injection of complexes or tetramers 70 days post-primary immunization, and then 2 hours after the booster, serum samples were collected. Cytokine/chemokine protein profiler microarray analysis was performed on the pooled sera from each group of mice. The results were normalized to the values of the control group (suboptimally collagen immunized but otherwise untreated mice) (). A highly elevated level of several cytokines and chemokines was detected in the sera of complex-treated mice as compared to the controls. The 2.4G2 scFv-CIIpeptide complexes induced the secretion of B lymphocyte chemoattractant (BLC/CXCL13), granulocyte colony stimulating factor (G-CSF), IL-1α, IL-3, IL-6, IL-7, IL-10, IL-12, IL-16, IL-17, IL-23, macrophage inflammatory protein 1 and 2 (MIP-1, MIP-2) and TNFα. The level of IL-10 and MIP-2 (mouse equivalent of human IL-8) was estimated to be approximately 2500 times higher in complex-treated mice as compared to non-treated, collagen-immunized animals. Peptide tetramers triggered IL-10, IL-17, IL-23 and MIP-2 production, while 2.4G2 scFv tetramers crosslinking FcγRII/III triggered only G-CSF, IL-6, IL-10 and MIP-2 secretion in vivo (). These data indicate that in accordance with their disease amplifying effect, both the FcγRII/III targeted CII peptide and CII-peptide tetramer stimulate the secretion of inflammatory cytokines, playing a crucial role in the initiation and maintenance of CIA.Citation36–Citation39
Figure 7 Chemokine/cytokine profile in sera of mice primed with collagen and then boosted intravenously with 2.4G2 scFv-CII-peptide complexes, or ‘tetramer’ CII-peptide or 2.4G2 scFv.
Abbreviations: CII, bovine type II collagen; scFv, single chain Fv fragment.
Discussion
CIA is a useful model of RA for studying inflammation, autoimmunity and arthritis. Antibodies specific for collagen play a major role in the induction of the disease, and immune complexes formed by the autoantibodies contribute to inflammation and tissue destructionCitation1,Citation2,Citation18,Citation29 However, the mechanism of immune complex-mediated modulation of CIA is not fully understood. Our aim was to investigate the impact of FcγRII/III targeted complexes composed of a collagen epitope peptide and 2.4G2 scFv on the induction and maintenance of CIA.
The CII-peptide was originally described as a triple helical conformational epitope of collagen spanning the 359–369 peptide region and was reported to be target for collagen specific antibodies of mice and for the sera of some RA patients.Citation22,Citation23 We hypothesized that the memory B-cells of collagen primed mice would recognize FcγRII targeted CII-peptide, which in turn might switch off antibody production through the FcγRIIb dependent B-cell inhibition, thus ameliorating disease symptoms. Supplementary file 2 shows that indeed, 2.4G2 scFv is able to inhibit intracellular Ca2+ mobilization in B-cells when it is co-crosslinked with BCR. However, we could not detect the binding of either 2.4G2 scFv or its complexes with CII-peptide to germinal center B-cells in spleen sections when they were applied in vivo.
Comparing CIA scores in the four experimental groups (suboptimally collagen immunized mice treated with 2.4G2 scFv-CII-peptide complexes, CII-peptide tetramers or 2.4G2 scFv tetramers), surprisingly we found that all molecular constructs elevated arthritic scores, thus significantly aggravating disease activity. By comparison, a mixture of monomeric CII-peptide and monomeric scFv did not elevate the arthritic scores as compared to controls. None of the constructs induced any sign of disease when administered into DBA/1 mice that were not immunized previously with collagen (data not shown). We assumed that the elevation of arthritic scores of collagen primed mice by FcγRII/III targeted CII-peptide and 2.4G2 scFv tetramers might be the result of targeting FcγRII/III positive cells,Citation24,Citation25 including dendritic cells (DC) and macrophages.
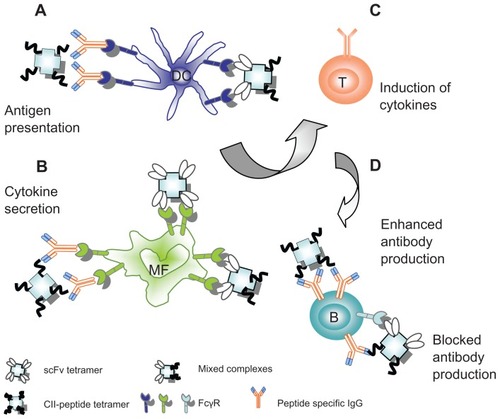
To identify the type of cells that are able to bind the various complexes, we monitored their in vitro and in vivo binding to spleen cell subsets of collagen immunized DBA/1 mice. In vitro assays showed that approximately half of the spleen B-cells from collagen-immunized mice bound 2.4G2 scFv-containing complexes or tetramers. This finding is in line with an earlier observation demonstrating that FcγRIIb expression is markedly downregulated in germinal center B-cells.Citation40 The in situ analysis of the in vivo binding of intravenously injected 2.4G2 scFv-CII-peptide complexes showed that these preferentially bound to MARCO positive marginal zone macrophages, while a weaker reactivity of both the complexes and the peptide tetramers could also be observed with CR1/CR2high cells. The T-zone location of these cells precludes their identification as follicular dendritic cells, but their morphology indicates some relatedness to DC-lineage. Flow cytometric analysis showed that about 40% of dendritic cells and more than half of the F4/80-positive macrophages bound 2.4G2 scFv-containing constructs ex vivo. Since the CII-peptide tetramers, besides binding to a small proportion of B-cells, also bound to macrophages and dendritic cells, we suppose that peptide-specific IgG in the serum of collagen primed mice mediates this binding (). It was reported earlier that antigen complexed with specific IgG can be taken up by DCs in a nondegradative pathway via FcγRIIb and subsequently re-expressed at the DC surface thus promoting B cell proliferation and IgG2a antibody production.Citation41 Thus we assumed that 2.4G2 scFv-CII-peptide complexes binding to FcγRIIb on DC promote the peptide specific IgG2a synthesis in the complex-treated mice.
Figure 8 Possible ways to regulate CIA by complexes targeted to FcγR. (A) On dendritic cells: mixed complexes of 2.4G2 scFv and CII-peptide or tetramers of CII-peptides may directly or indirectly, via peptide-specific IgG, bind to FcγRII/III, thus enhancing the antigen presentation. (B) On macrophages: 2.4G2 scFv tetramers and mixed complexes or CII-peptide tetramers may directly or indirectly, via the bound peptide-specific IgG2a, crosslink FcγRII/III or FcγRIV, thereby inducing cytokine release. (C) Cytokines such as IL-12 or IL-23 produced by DC and macrophages in response to the complexes may induce TH1 cells to produce IFNγ and other cytokines inducing the inflammatory TH1 response. (D) On B-cells: the mixed complexes may simultaneously bind via BCR and the inhibitory FcγRIIb, cross-linking the receptors and block antibody synthesis; alternatively, binding to BCR alone CII-peptide tetramers may facilitate the CII-peptide specific immune response.
To find out if CII-peptide-containing complexes and tetramers induce antibody synthesis, we compared the collagen-specific and the CII-peptide-specific IgG titers in the four experimental groups. Although administration of the various constructs into collagen-pre-immunized mice did not significantly modify the collagen-specific IgG titer, the FcγRII/III targeted CII-peptide complexes significantly elevated the peptide-specific IgG titer as compared to the nontreated and tetramer treated groups. These data indicate that although CII-peptide specific IgG is present in the sera of all groups of collagen pre-immunized mice, FcγRII/III targeted CII-peptide is significantly more efficient in eliciting peptide-specific antibody responses as compared to the CII-peptide alone. Serological data also demonstrated that the FcγRII/III targeted CII-peptide complexes enhanced the synthesis of the inflammatory IgG2a isotype, which is associated with a Th1 response.Citation42 CII-peptide specific IgG2a in the serum would allow CII-peptide-IgG2a complexes to be formed in mice receiving CII-peptide containing constructs. IgG2a and IgG2b bind to stimulatory FcγRIV expressed by macrophages and neutrophils at 10-to-100-fold higher affinities compared to its inhibitory counterpart, FcγRIIb, thus predicting that the production of these IgG subclasses could less effectively be blocked by FcγRIIb-mediated suppression.Citation43 Based on these findings, we suppose that CII-peptide–IgG2a complexes boost the inflammation in collagen primed DBA/1 mice due to their binding to FcγRIV. This is in line with a recent report showing that the murine high-affinity IgG receptor FcγRIV is sufficient for autoantibody-induced arthritis.Citation44
The outcome of immune complex binding to FcγR positive cells depends on the balance between activating and inhibiting FcγR. It was reported earlier that macrophages derived from CIA susceptible mice show a disregulated FcγR expression resulting in a prolonged expression of the activating FcγRI and FcγRIII and down regulation of FcγRIIb.Citation45 Thus FcγRII/III targeted CII-peptide complexes and tetramer 2.4G2 scFv may prominently bind to FcγRIII on macrophages in collagen primed DBA/1 mice, eliciting the production of proinflammatory cytokines such as IL-1 and TNFα.Citation21 Proinflammatory cytokines are important regulators of the synovial inflammation in RA.Citation46 Th1 and Th17 cells producing IFNγ, IL-17 and IL-23 have been shown to aggravate RA,Citation38 moreover, IL-17 together with TNFα was found to be predictive for poor outcome in RA.Citation47 In CIA the IL-23/IL-17 axis is critical for the development of autoimmune arthritis.Citation36 It was also shown that IL-17-deficient mice were resistant to CIA.Citation48 Furthermore, IFNγ and IL-17 amplified FcγR mediated cartilage destruction in murine immune complex-mediated arthritis.Citation20 We found that IFNγ, TNFα, IL-17 and IL-10 were secreted in the in vitro culture of splenocytes of collagen preimmunized mice exposed to FcγRII/III targeted CII-peptide complexes. Further, protein microarray analysis showed a substantial alteration in the secreted cytokine and chemokine profile two hours after injection of the complexes into collagen primed mice. A considerably increased level of IL-1, IL-3, IL-6, IL-7, IL-10, IL-12, IL-17, IL-23 and TNFα was detected and the secretion of chemokines CXCL13, MIP-1, and MIP-2 was also enhanced. CII-peptide tetramers stimulated IL-10, IL-17, IL-23, and MIP-2 production, while 2.4G2 tetramers that may crosslink the activating FcγRIII, enhanced G-CSF, IL-6, IL-10, and MIP-2 secretion.
Macrophage activation is induced by – among others – the action of immune complexes on activating FcγR, triggering the production of a number of inflammatory mediators.Citation49,Citation50 Macrophage inflammatory protein 1α (MIP-1α/CCL3) is produced by RA synovial tissue-lining cells and interstitial macrophages,Citation51,Citation52 and it is induced by TNFα.Citation49 MIP-1α is chemotactic for monocytes, T, B and NK cells, basophils and eosinophils, and abundant MIP-1α was found in RA synovial fluid.Citation53 Expression and contribution of MIP-1α and macrophage inflammatory protein 2 (MIP-2) and also of IL-10 during the evolution of CIA has been described. IL-10 appears to be an important immunomodulator of the pathogenesis of CIA, regulating the expression of MIP-1α and MIP-2.Citation37 IL-23 produced by antigen-stimulated dendritic cells and macrophages is one of the essential factors required for the survival and/or expansion of Th17 cells, which produce IL-17, IL-17F, IL-6, and TNFα. The IL-23/IL-17 axis also plays a key role in the development of autoimmune arthritis in humans.Citation54 IL-23 acts on dendritic cells and macrophages in an autocrine/paracrine manner to stimulate the generation of proinflammatory cytokines, such as IL-1, IL-6, and TNF-α in autoimmune inflammatory diseases.Citation36 A 2-year study on a cohort of RA patients has demonstrated that synovial membrane mRNA levels of IL-1beta, TNF-α, IL-17, and IL-10 were predictive of damage progression. IL-17 was synergistic with TNF-α.Citation47 The synovial milieu in established RA contains various macrophage- and synovial-fibroblast derived cytokines, such as IL-1β, IL-6, IL-7, IL-12, IL-15, IL-18, IL-23p19, and TGFβ that can support the expansion and differentiation of Th1 and/or Th17 cells.Citation49 Bone resorption in RA is induced by osteoclasts, and osteoclast differentiation is achieved by the actions of TNF and IL-1, as well as of IL-17, produced by Th17 cells, and IL-7, produced by synovial fibroblasts. Citation49 These data show that evidence suggesting rheumatoid arthritis as a primarily Th17-/IL-17-dependent autoimmune inflammatory disease is rapidly accumulating.
Taken together, our data suggest that 2.4G2 scFv-CII-peptide complexes and the in vivo formed IgG2a-CII-peptide immune complexes may bind to FcγRIII and/or to FcγRIV, inducing the secretion of IL-23 from dendritic cells and macrophages, which in turn triggers Th17 cells to secrete IL-17, IL-6 and TNFα. Additionally, the enhanced production of further inflammatory cytokines and chemokines was detected after the intravenous injection of the complexes and tetramers. Treatment of collagen preimmunized DBA/1 mice with the FcγRII/III targeted complexes also resulted in increased secretion of IL-3 and G-CSF. Recent data indicate that IL-3 produced by CD4+ T-cells in the presence of CD11b+ macrophages has an important role in the early phase of CIA by activating basophils, thus considerably expanding the range of possible effector cells.Citation55 Furthermore, it was reported that daily injections of M-CSF or G-CSF, 20–24 days after primary immunization with type II collagen, exacerbate disease symptoms in suboptimally immunized DBA/1 mice, showing that M-CSF and G-CSF can be proinflammatory in CIA.Citation56 Among the chemokines induced by the FcγRII/III targeted CII-peptide complexes BLC (CXCL13), MIP1 and MIP-2 are the most important. B lymphocyte chemoattractant cytokine CXCL13 controls follicle formation and may facilitate ectopic lymphoid organogenesis in the synovium and the local production of tissue-specific auto-antibody.Citation57 CXCL13 is expressed in the synovial tissue of RA patients and its blockade reduces the severity of CIA, indicating that CXCL13 plays an important role in the development and pathogenesis of the disease.Citation58
Conclusions
Taken together, these data indicate that the administration of FcγRII/III targeted CII-peptide complexes into collagen primed DBA/1 mice enhances the inflammatory TH1 driven IgG2a response to CII-peptide and stimulates inflammatory cytokine and chemokine production. Boosting the mice with CII-peptide tetramers after primary immunization with collagen may result in the formation of CII-peptide-IgG2a complexes. These complexes may bind to FcγRIV, while 2.4G2 scFv containing complexes bind to FcγRIII; both enhancing the secretion of potent pro-inflammatory mediators such as IL-23 and MIP-2, playing a key role in autoimmune arthritis (). We identified in this study the cytokines and chemokines, the production of which is stimulated by complexes binding to activating FcγR, which might be the targets of future therapies.
Abbreviations
| BLC | = | B lymphocyte chemoattractant |
| CII | = | bovine type II collagen |
| CIA | = | collagen-induced arthritis |
| CR | = | complement receptors |
| FcγR | = | Fcgamma receptors |
| FITC | = | fluorescein isothiocyanate |
| G-CSF | = | granulocyte colony stimulating factor |
| IFN | = | interferon |
| MARCO | = | Macrophage Receptor with Collagenous structure |
| MIP | = | Macrophage Inflammatory Protein |
| RA | = | rheumatoid arthritis |
| scFv | = | single chain Fv fragment |
| TNF | = | tumor necrosis factor |
Acknowledgments
The authors would like to thank Dr Anna Magyar, Research Group of Peptide Chemistry, Office for Research Groups Attached to Universities and Other Institutions of the Hungarian Academy of Sciences, for the synthesis of the biotinylated collagen peptide. The authors thank Arpad Mikesy for taking care of the mice.
Supplementary figures
Figure S1 Composition of the extravidin bound 2.4G2 scFv, CII-peptide and CII-peptide –2.4G2scFv mixed complexes resolved by non-reducing SDS-PAGE.
Notes: Lane 1: 2.4G2 scFv ‘tetramer’: 60 μg biotinylated 2.4G2 scFv and 30 μg extravidin were mixed (4:1 molar ratio), lane 2: ’CII-peptide ‘tetramer’: 2.6 μg biotinylated CII-peptide (ARGLTGRPGDA) was mixed with 30 μg Extravidin (4:1 molar ratio), lane 3: preformed complexes of 60 μg biotinylated 2.4G2 scFv, 2.6 μg biotinylated collagen peptide (CII-peptide) and 60 μg extravidin (2:2:1 molar ratio). Arrows show the position of various size of scFv-extravidin complexes (lane 1) or CII-peptide-extravidin complexes (lane 2), or the mixed complexes (lane 3). The band around 60 kD corresponds to extravidin.
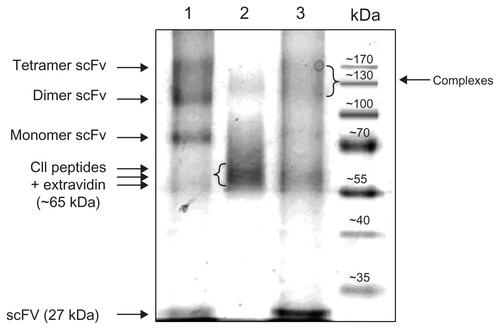
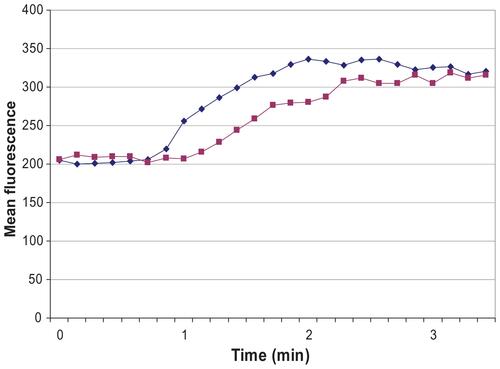
Figure S2 2.4G2 scFv inhibits intracellular rise of free Ca2+ when FcγRIIb and BCR are co-crosslinked.
Notes: Cells were loaded with 5 mM fluo-3/AM indicator and 30 mg/mL Pluronic F-127 for 30 min at 37°C in 1 mL medium. The cells were diluted 10 times and incubated for another 30 min at 37°C, then washed, resuspended and labeled with 7-AAD to exclude the dead cells. All studies were carried out in RPMI 1640 culture medium. Spleen B-cells were treated with: 0.2 μg protein LA + 0.2 μg avidin for BCR crosslinking (blue line), and 10 μg 2.4G2scFv-b + 0.2 μg protein LA + 0.2 μg avidin for BCR-FcγRIIb co-crosslinking (purple line). Protein LA is a hybrid protein recognizing Ig kappa chain, and scFv (Eur J Biochem. December 1, 1998;258(2):890–896.). Kinetics of the change of mean fluorescence are shown, as calculated by Lysys II software (Becton Dickinson, San Jose, CA).
Disclosure
The authors report no conflicts of interest in this work.
Funding
This work was supported by the Hungarian Scientific Research Fund and the National Development Agency (OTKA 60760, OTKA K 68617, OTKA NI 68466, OTKA CK 80689). The European Union and the European Social Fund have provided financial support to the project under the grant agreement no. TÁMOP 4.2.1./B-09/1/KMR-2010-0003. D. Kövesdi currently is a Magyary Zoltan Postdoctoral Fellow; the Fellowship is supported by the EEA Grants and Norway Grants.
References
- CourtenayJSDallmanMJDayanADMartinAMosedaleBImmunisation against heterologous type II collagen induces arthritis in miceNature198028357486666686153460
- StuartJMDixonFJSerum transfer of collagen-induced arthritis in miceJ Exp Med198315823783926886622
- NandakumarKSAndrenMMartinssonPInduction of arthritis by single monoclonal IgG anti-collagen type II antibodies and enhancement of arthritis in mice lacking inhibitory FcgammaRIIBEur J Immunol20033382269227712884302
- NandakumarKSSvenssonLHolmdahlRCollagen type II-specific monoclonal antibody-induced arthritis in mice: description of the disease and the influence of age, sex, and genesAm J Pathol200316351827183714578183
- RowleyMJNandakumarKSHolmdahlRThe role of collagen antibodies in mediating arthritisMod Rheumatol200818542944118521704
- NimmerjahnFBruhnsPHoriuchiKRavetchJVFcgammaRIV: a novel FcR with distinct IgG subclass specificityImmunity2005231415116039578
- RavetchJVBollandSIgG Fc receptorsAnnu Rev Immunol20011927529011244038
- CambierJCAntigen and Fc receptor signaling. The awesome power of the immunoreceptor tyrosine-based activation motif (ITAM)J Immunol19951557328132857561018
- DaeronMStructural bases of Fc gamma R functionsInt Rev Immunol1997161–21279651784
- TakaiTRoles of Fc receptors in autoimmunityNat Rev Immunol20022858059212154377
- DaeronMLatourSMalbecOThe same tyrosine-based inhibition motif, in the intracytoplasmic domain of Fc gamma RIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activationImmunity1995356356467584153
- IsakovNITIMs and ITAMs. The Yin and Yang of antigen and Fc receptor-linked signaling machineryImmunol Res1997161851009048210
- TakaiTMultiple loss of effector cell functions in FcR gamma-deficient miceInt Rev Immunol19961343693818884432
- ClynesRMaizesJSGuinamardROnoMTakaiTRavetchJVModulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptorsJ Exp Med199918911791859874574
- NakamuraAYuasaTUjikeAFcgamma receptor IIB-deficient mice develop Goodpasture’s syndrome upon immunization with type IV collagen: a novel murine model for autoimmune glomerular basement membrane diseaseJ Exp Med2000191589990610704470
- NimmerjahnFRavetchJVFcgamma receptors as regulators of immune responsesNat Rev Immunol200881344718064051
- KleinauSMartinssonPHeymanBInduction and suppression of collagen-induced arthritis is dependent on distinct fcgamma receptorsJ Exp Med200019191611161610790435
- MagnussonSEAndrenMNilssonKESondermannPJacobUKleinauSAmelioration of collagen-induced arthritis by human recombinant soluble FcgammaRIIbClin Immunol2008127222523318346938
- BorossPvan LentPLMartin-RamirezJDestructive arthritis in the absence of both FcgammaRI and FcgammaRIIIJ Immunol200818075083509118354234
- GreversLCvan LentPLKoendersMIDifferent amplifying mechanisms of interleukin-17 and interferon-gamma in Fcgamma receptor-mediated cartilage destruction in murine immune complex-mediated arthritisArthritis Rheum200960239640719180490
- AbrahamsVMCambridgeGLydyardPMEdwardsJCInduction of tumor necrosis factor alpha production by adhered human monocytes: a key role for Fcgamma receptor type IIIa in rheumatoid arthritisArthritis Rheum200043360861610728755
- BurkhardtHKollerTEngstromAEpitope-specific recognition of type II collagen by rheumatoid arthritis antibodies is shared with recognition by antibodies that are arthritogenic in collagen-induced arthritis in the mouseArthritis Rheum20024692339234812355481
- SchulteSUngerCMoJAArthritis-related B cell epitopes in collagen II are conformation-dependent and sterically privileged in accessible sites of cartilage collagen fibrilsJ Biol Chem19982733155115619430695
- AngyalASzekeresZBaloghPCD16/32-specific biotinylated 2.4G2 single-chain Fv complexed with avidin-FITC enhances FITC-specific humoral immune response in vivo in a CD16-dependent mannerInt Immunol2010222718019951957
- SzekeresZHerbathMAngyalAModulation of immune response by combined targeting of complement receptors and low-affinity Fcgamma receptorsImmunol Lett20101301–2667320005256
- KvellKCzompolyTPikkarainenTBaloghPSpecies-specific restriction of cell surface expression of mouse MARCO glycoprotein in murine cell linesBiochem Biophys Res Commun200634141193120216460688
- HazenbosWLGessnerJEHofhuisFMImpaired IgG-dependent anaphylaxis and Arthus reaction in Fc gamma RIII (CD16) deficient miceImmunity1996521811888769481
- Ioan-FacsinayAde KimpeSJHellwigSMFcgammaRI (CD64) contributes substantially to severity of arthritis, hypersensitivity responses, and protection from bacterial infectionImmunity200216339140211911824
- WooleyPHLuthraHSStuartJMDavidCSType II collagen-induced arthritis in mice. I. Major histocompatibility complex (I region) linkage and antibody correlatesJ Exp Med198115436887006792316
- KuruczITitusJAJostCRSegalDMCorrect disulfide pairing and efficient refolding of detergent-solubilized single-chain Fv proteins from bacterial inclusion bodiesMol Immunol19953217–18144314528643113
- UnkelessJCFleitHMellmanISStructural Aspects and Heterogeneity of Immunoglobulin Fc ReceptorsAdv Immunol1981312472707032255
- Chapman-SmithACronanJEJrThe enzymatic biotinylation of proteins: a post-translational modification of exceptional specificityTrends Biochem Sci199924935936310470036
- O’CallaghanCAByfordMFWyerJRBirA enzyme: production and application in the study of membrane receptor-ligand interactions by site-specific biotinylationAnal Biochem199926619159887208
- SvenssonHGHoogenboomHRSjobringUProtein LA, a novel hybrid protein with unique single-chain Fv antibody- and Fab-binding propertiesEur J Biochem199825828908969874260
- van den BergTKKraalGA function for the macrophage F4/80 molecule in tolerance inductionTrends Immunol2005261050650916087400
- IwakuraYIshigameHThe IL-23/IL-17 axis in inflammationJ Clin Invest200611651218122216670765
- KasamaTStrieterRMLukacsNWLincolnPMBurdickMDKunkelSLInterleukin-10 expression and chemokine regulation during the evolution of murine type II collagen-induced arthritisJ Clin Invest1995956286828767769128
- LubbertsETh17 cytokines and arthritisSemin Immunopathol2010321435320127485
- MurphyCALangrishCLChenYDivergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammationJ Exp Med2003198121951195714662908
- RaoSPVoraKAManserTDifferential expression of the inhibitory IgG Fc receptor FcgammaRIIB on germinal center cells: implications for selection of high-affinity B cellsJ Immunol200216941859186812165510
- HauserAEKerfootSMHabermanAMCellular choreography in the germinal center: new visions from in vivo imagingSemin Immunopathol201032323925520614218
- MukherjeePWuBMaytonLKimSHRobbinsPDWooleyPHTNF receptor gene therapy results in suppression of IgG2a anticollagen antibody in collagen induced arthritisAnn Rheum Dis200362870771412860724
- NimmerjahnFRavetchJVFcgamma receptors: old friends and new family membersImmunity2006241192816413920
- MancardiDAJonssonFIannascoliBThe murine high-affinity IgG receptor FcgammaRIV is sufficient for autoantibody-induced arthritisJ Immunol18641899190321248252
- BlomABvan LentPLHolthuysenAEJacobsCvan den BergWBSkewed balance in basal expression and regulation of activating v inhibitory Fcgamma receptors in macrophages of collagen induced arthritis sensitive miceAnn Rheum Dis200362546547112695162
- ArendWPDayerJMInhibition of the production and effects of interleukin-1 and tumor necrosis factor alpha in rheumatoid arthritisArthritis Rheum19953821511607848304
- KirkhamBWLassereMNEdmondsJPSynovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort)Arthritis Rheum20065441122113116572447
- NakaeSNambuASudoKIwakuraYSuppression of immune induction of collagen-induced arthritis in IL-17-deficient miceJ Immunol2003171116173617714634133
- McInnesIBSchettGCytokines in the pathogenesis of rheumatoid arthritisNat Rev Immunol20077642944217525752
- SzekaneczZKimJKochAEChemokines and chemokine receptors in rheumatoid arthritisSemin Immunol2003151152112495637
- KochAEChemokines and their receptors in rheumatoid arthritis: future targets?Arthritis Rheum200552371072115751074
- SzekaneczZKochAEChemokines and angiogenesisCurr Opin Rheumatol200113320220811333349
- KochAEKunkelSLHarlowLAMacrophage inflammatory protein-1 alpha. A novel chemotactic cytokine for macrophages in rheumatoid arthritisJ Clin Invest19949339219288132778
- Paradowska-GoryckaAGrzybowska-KowalczykAWojtecka-LukasikEMaslinskiSIL-23 in the pathogenesis of rheumatoid arthritisScand J Immunol71313414520415779
- BruhlHCihakJNiedermeierMImportant role of interleukin-3 in the early phase of collagen-induced arthritisArthritis Rheum20096051352136119404955
- CampbellIKRichMJBischofRJHamiltonJAThe colony-stimulating factors and collagen-induced arthritis: exacerbation of disease by M-CSF and G-CSF and requirement for endogenous M-CSFJ Leukoc Biol200068114415010914502
- LeglerDFLoetscherMRoosRSClark-LewisIBaggioliniMMoserBB cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5J Exp Med199818746556609463416
- ZhengBOzenZZhangXCXCL13 neutralization reduces the severity of collagen-induced arthritisArthritis Rheum200552262062615692971