Abstract
Current standard immunomodulatory therapy with interferons (IFNs) for relapsing–remitting multiple sclerosis (MS) exhibits proven, but limited, efficacy and increased side effects due to the need of frequent application of the drug. Therefore, there is a need for more effective and tolerable drugs. Due to their small size, optimization of therapy with IFNs in MS by PEGylation is feasible. PEGylation of an IFN means that at least one molecule of polyethylene glycol (PEG) is covalently added. This modification is a standard procedure to increase the stability, solubility, half-life, and efficacy of a drug, and is applied in several drugs and diseases. Currently, a therapy regimen applying PEG-IFN beta-1a in MS is being developed to achieve an optimized relationship between therapy-related side effects and pharmacokinetic/pharmacodynamic efficacy. Phase I studies demonstrated that subcutaneous PEG-IFN beta-1a at a dose of 125 μg every 2 or 4 weeks might be at least as efficient and safe as the current standard therapy with IFN beta-1a. A global Phase III clinical study is investigating the efficacy of PEG-IFN beta-1a in terms of reduction of the relapse rate in relapsing–remitting MS patients. The latest primary safety and efficacy analysis after 1 year has revealed a favorable risk–benefit profile with no significant difference between dosing regimens. Compared to placebo, the annualized relapse rate was reduced by about one-third and new or newly enlarging T2 brain lesions were reduced by about one-third when dosing every 4 weeks or by two-thirds when dosing every 2 weeks. This presents a significant effect of the dosing interval, favoring administration every 2 weeks. Chronic administration of PEGylated proteins mostly at toxic concentrations causes vacuolation of renal epithelium in animals, which – along with the issue of occurrence of anti-PEG antibodies – has to be addressed by Phase IV studies.
Interferon beta-1a (IFN beta-1a) in the management of multiple sclerosis (MS)
Taking into account premature retirement in young adults, MS is the most prevalent neurological disease. At the onset of natural history, the relapsing–remitting disease course prevails. Intravenous methylprednisolone pulse therapy is the standard treatment for relapse.Citation1 After licensing, according to the results of randomized and placebo-controlled clinical trials,Citation2,Citation3 type I IFNs – beta-1a (Rebif®; Merck Serono, Geneva, Switzerland; Avonex®; Biogen Idec, Weston, MA, USA) and beta-1b – as well as glatiramer acetate currently represent the standard immunomodulatory long-term treatment for relapsing–remitting MS. Part of therapeutic efficacy seems to be modulation of the signal transduction pathways.Citation4 Efficacy of these drugs has been proven, but is limited, as relapse rate and disease progression are reduced by approximately one-third.Citation2,Citation3 Avonex significantly reduces the increase in brain MRI T2 lesion load by approximately more than two-thirds after 1 year, but less so after 2 years.Citation3 The REGARD (Rebif Versus Glatiramer Acetate in Relapsing MS Disease) and BEYOND (Betaferon Efficacy Yielding Outcomes of a New Dose) studies didn’t show a difference in the effect of the respective high dose and high frequency IFN compared to glatiramer acetate.Citation5,Citation6 For that reason, both types of IFNs can be considered equal. Rebif (IFN beta-1a 22 μg or 44 μg subcutaneously [SC] every other day) is licensed for relapsing forms of MS. Safety and efficacy in patients with chronic progressive MS have not been established for Avonex (IFN beta-1a 30 μg intramuscularly [IM] once weekly), but it is licensed for patients who have experienced a first clinical episode and present magnetic resonance imaging (MRI) features that are consistent with MS.Citation7 The INCOMIN (Independent Comparison of IFNs) study seems to show that high and frequent dosage of IFN is more effective,Citation8 but there might be a ceiling effect.Citation6
All these currently available immunomodulatory therapy regimens are safe. Yet, there are increased side effects due to the need of frequent, at least weekly, application of the drug, depending on the specific type of drug. Overall, these include injection site skin reactions, influenza-like symptoms, depression, hepatic injury with elevation of liver enzymes, hematologic abnormalities and anxiety. Especially in the case of glatiramer acetate, a postinjection reaction might occur.
In cases of disease progression or initial high disease activity, an escalation to another therapy regimen is possible, eg, chemotherapy with mitoxantrone or immunosuppression with natalizumab.
Pharmacology of IFN beta-1a conjugated to polyethylene glycol (PEG)
There is clearly a need for more effective and tolerable drugs. IFN beta-1a can be conjugated to PEG. PEGylation of an IFN (PEG-IFN; ) means that at least one molecule of PEG is covalently added. This modification is a standard procedure to increase the stability, solubility, half-life, and efficacy of a drug, and is applied in several drugs and diseases.Citation9
Figure 1 Pegylated interferon: a ribbon protein with a polymer linked by a bridge between two multicolored cysteine residues.
Except for PEGylation and glycosylation, other compounds are analyzed for polymeric conjugation and delivery of proteins such as polyamino acid polymers and hybrid-modified PEG polymers.Citation10 A polymer made of glutamic acid and vitamin E can serve as an amphiphilic vehicle which forms nanoparticles in water.Citation11 The administration of IFN beta-1a with this “Medusa® polymer” (Flamel Technologies, Venissieux, France) might produce a longer duration of the drug effect.
The PEGylation method
In general, various factors such as molecular weight, size, availability of surface groups to link, and the number of chains per molecule play an important role for the choice of the kind of covalent modification that is used in PEGylation. The method of PEGylation might be preferred to other techniques if the protein is small and therefore renal clearance high. The cutoff molecular weight regarding glomerular filtration of proteins is about 60 kDa and low molecular weight proteins benefit the most from reduced renal clearance and improved half-life that is achieved by PEGylation. If the protein is large, unstable, or requires extensive macromolecular interactions to gain its biological activity, PEGylation can lead to loss of activity. Efficiency of PEGylation is reduced if it leads to a drastic decrease in activity. PEGylation can lead to heterogeneity in the conjugation; therefore, proteins containing more conjugation sites on the surface may be difficult to handle with regard to fluctuations in product quality. Molecular modeling is used to analyze possible conjugation sites.Citation10
Results on PEGylation were first reported in the 1970s and have been extensively discussed. Here, a short summary is given.Citation10 PEG portions are repeating units of ethylene glycol that are inert and amphiphilic. PEG is synthesized by anionic ring opening polymerization of ethylene oxide initiated by nucleophilic attack of a hydroxide ion on the epoxide ring. Several derivatives of PEG molecules are available that vary in molecular weight and structure (eg, linear or branched). The most common amino acids used for covalent bonding of activated PEG polymers to the drug of interest are lysine and the N-terminal amino group. Several processes affect the pharmacological behavior of the conjugates: the increased size and molecular weight of a molecule, changes in conformation, steric interference of intermolecular interactions, increased hydrophilicity, and changes in electrostatic binding properties.Citation12 Yet, the most prominent effect of PEGylation is a prolonged circulation time of conjugated therapeutic substances resulting from a decreased rate of renal clearance or a reduction of proteolysis and opsonization.Citation9 PEG molecules are highly hydrated, which increases the hydrodynamic radius of the conjugate approximately five- to ten-fold and improves solubility and decreases the rate of glomerular filtration. The protective shell formed by PEGylation also prevents uptake and clearance by reticuloendothelial cells, decreases the formation of neutralizing antibodies against the protein by masking antigenic sites, and offers protection from proteolytic enzymes.Citation13 In addition, PEGylation increases the absorption half-life of SC administered agents and is associated with a decreased volume of distribution.Citation14 Due to steric interference of PEGylation, even with smaller proteins, some reduction in activity and binding affinity is frequent. However, this reduction in binding may be offset by increased systemic exposure.Citation10
Although not frequently observed, anti-PEG antibodies may form, usually when repeated chronic doses of PEGylated proteins are given. These anti-PEG antibodies may potentially increase the clearance of proteins attached to PEG.Citation15 On the other hand, increases in protein stability have been reported as a result of PEG masking hydrophobic sites on the protein surface involved in noncovalent interactions that would lead to subsequent aggregation, loss of activity, or increased immunogenicity.Citation16
Nonspecific PEGylation usually results in heterogeneously PEGylated conjugates, whereas site-specific PEGylation of specific functional groups enables more precise control. For example, nonspecific PEGylation of tumor necrosis factor-alpha (TNF-alpha) resulted in heterogeneous conjugates with decreased bioactivity but site specific PEGylation of mono-PEGylated TNF-alpha showed a higher degree of molecular uniformity as well as a higher bioactivity in vitro and a greater antitumor therapeutic potency than random mono-PEGylation.Citation17 N-terminal site-specific PEGylation of recombinant human granulocyte colony stimulating factor resulted in higher molecular weight of 30 kDa, longer in vivo half-life, and 60% higher drug bioavailability.Citation18
PEGylated drugs
The first PEGylated drug was approved in 1990. Currently, around ten products consisting of PEGylated proteins are marketed, including PEG-IFN alpha-2a and -2b for the treatment of hepatitis C and PEG-certolizumab PEGol with parent drug anti-TNF Fab for the treatment of rheumatoid arthritis and Crohn’s disease. Especially for IFN alpha-2a, branched PEG conjugate reduced renal clearance 100-fold and increased the half-life from 9 hours to 77 hours, and the safety profile was comparable.Citation19,Citation20 PEG-IFN alpha-2b SC administered once weekly or IFN alpha-2b administered three times a week in chronic hepatitis C for 24 weeks produced dose-related reductions in white blood cells, neutrophils, and platelets but dose-related increases in serum neopterin, serum oligoadenylate synthetase activity, and oral temperature, which were qualitatively similar for both types of IFNs. The same was observed for the reported adverse events such as flu-like symptoms.Citation21
As of now, MS is not addressed by marketed products. Yet, a PEGylated form of IFN beta-1a was tested together with or without ribavirin in hepatitis C and achieved an improvement in liver histology compared to the situation before treatment.Citation22
PEG-IFNs for the treatment of MS
Distributed Avonex and Rebif contain a relatively small 166 amino acid single-chain glycoprotein of 22.5 kDa, which offers a relatively small half-life of 10 hours. For that reason, in the optimization of therapy with IFNs for MS, PEGylation is an eligible method.
In rodents, Basu et al evaluated a large range of more than 20 mono- or multi-PEG-IFN beta-1b with different sizes and attachment sites with regard to pharmacokinetic and pharmacodynamic properties, including immunogenicity and aggregation.Citation23 The functional activities of the constructs were maintained, while with regard to unfavorable aggregates, an ameliorated result was obtained. Selected compounds demonstrated diminished immunoglobulin responses. For analyses in primates, they chose a branched 40 kDa PEG-IFN beta-1b, but the results are not yet published. Mager et al analyzed a SC administered 40 kDa PEG-IFN beta-1a in monkeys and found enhanced drug exposure and similar pharmacodynamic properties compared to the non-PEG-IFN, eg, a typical dose-dependent biphasic pattern of neopterin.Citation24 Clinical data is not available.
Currently developed PEG-IFNs for MS
Several PEG-IFN beta drugs are currently developed including ARX-424 by Merck Serono together with Ambrx Inc (La Jolla, CA, USA) (Phase I). BaroFold Inc (Aurora, CO, USA) has successfully completed Phase I clinical trials on recombinant human IFN beta BaroFeron™, demonstrating acceptable levels of safety and tolerability compared to the marketed product. Now its partner, Nuron Biotech (Exton, PA, USA), has developed PEG-IFN beta-1b NU400 derived from aggregate-free IFN NU100 (identical to BaroFeron™) and is planning to enter Phase I clinical studies in 2013. SC administered AZ01 (Allozyne Inc, Seattle, WA, USA) is a site-specific PEGylated form of human IFN beta-1b that has been tested in a double-blind and placebo-controlled multiple ascending dose Phase Ib clinical trial of a 14- or 28-day dosing interval. Overall, the substance is reported to be well tolerated and presents the typical side effects of IFNs.
PEG-IFN beta-1a for MS
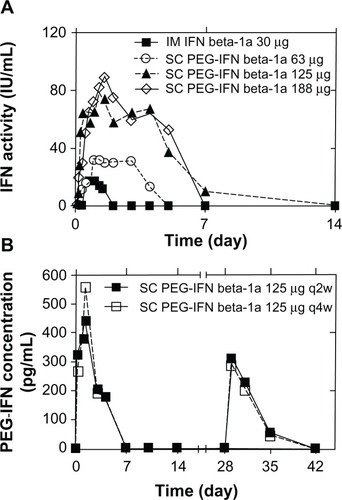
PEG-IFN beta-1a (developed by Biogen Idec) is the only substance in a Phase III clinical study. It is produced by adding methoxy-PEG-O-2-methylpropionaldehyde of 20 kDa to the alpha amino group of the IFN beta-1a N-terminus.Citation25 This is feasible as the latter is not needed to attach to the IFN receptor. This PEG-IFN retained approximately half of in vitro activity compared to IFN in antiviral and anti-proliferative assays, but in vivo activity was enhanced in a mouse tumor model and pharmacokinetic properties in rats were improved.Citation26 In healthy volunteers, two randomized and blinded Phase I clinical studies were conducted: (1) a single-dose study (n = 60) comparing SC or IM PEG-IFN beta-1a at a dose of 63, 125, or 188 μg with IM standard IFN beta-1a 30 μg () and (2) a placebo-controlled multidose study (n = 69) comparing SC PEG-IFN beta-1a administered at a dose of 63, 125, or 188 μg once every 2 weeks or 4 weeks with placebo ().Citation25
Figure 2 Pharmacokinetics for PEG-IFN beta-1a. (A) Pharmacokinetic effects from the cytopathic effect assay in the single-dose study. (B) Pharmacokinetic profiles from the enzyme-linked immunosorbent assay in the multidose study.
Abbreviations: SC, subcutaneous; IFN, interferon; IM, intramuscular; PEG-IFN, PEGylated interferon; PEG, polyethylene glycol; q2w, once every 2 weeks; q4w, once every 4 weeks.
Efficacy
A dose proportional increase in PEG-IFN beta-1a exposure, with four-fold greater exposure at 63 μg than with the unmodified IFN beta-1a (), and dose-dependently greater increases in neopterin concentration and dose-dependently greater changes in T-helper cell pathway gene expression and lymphocyte subsets compared to the standard IFN were observed in the Phase I clinical study mentioned above.Citation25 PEG-IFN beta-1a leads to a delay in achieving peak pharmacological effects (12 versus 6 hours), longer detectability (≤7 versus 2 days)Citation25 (measured by a specific enzyme-linked immunosorbent assay and a cell-based antiviral cytopathic effect bioassay), and sustained responses (≤15 versus 4 days).Citation27 This supports a less frequent dosing of PEG-IFN beta-1a compared to the current standard therapies and supports the notion of the effect of dosing. Yet, a clear limitation of these studies is the fact that PEGylation was not tested with the standard dosage of IFN beta-1a. For that reason, comparability between the unmodified substance and the PEGylated forms is limited when taking into account possible dosage effects.Citation8
Safety
In both studies, PEG-IFN beta was safe and well tolerated and no serious adverse event or discontinuation of the study occurred. The percentage of participants with adverse events was similar in the IFN beta-1a (92%) and the PEG-IFN beta-1a (88%–100%) group.Citation25 In both studies and all cohorts, flu-like symptoms were the most commonly reported adverse event and considered treatment-related, with a possible PEG-IFN dose dependency declining after repeated dosing. At a dosage of 188 μg PEG-IFN beta-1a every 4 weeks, there was no reduction in the incidence of flu-like symptoms after repeated dosing. There was a frequency-dependent transient reduction in absolute neutrophil count: it was more frequent in patients receiving PEG-IFN compared to those receiving the standard IFN and those who received it every 2 weeks exhibited a value below 1500 cells/mm3 more often than those who received it every 4 weeks. Shifts below 1500 cells/mm3 resolved within 2 weeks after dosing. The alanine aminotransferase or aspartate aminotransferase shifts in the two studies were mostly below three times the upper limit of normal, while the median was 1.2–1.3 of the upper limit of normal for alanine aminotransferase and aspartate aminotransferase.
First, a screening assay was performed to detect binding anti-IFN beta-1a antibodies, then positive samples were characterized using a cell-based neutralizing antibody assay. To detect binding anti-PEG antibodies, a screening assay was applied and then positive samples were characterized using a titration assay. No participants developed binding or neutralizing antibodies to IFN beta-1a or the respective part of the combined PEGylated molecule. In both studies, six patients in total were positive for anti-PEG antibodies prior to dosing, but titers remained stable during the study. Five patients developed anti-PEG antibodies in the course of the study. Their effect was estimated to be unclear due to the low incidence.Citation25 Thus, developing antibodies might play a more important and possibly pharmacodynamic role in PEG-IFN beta-1a compared to the slightly immunogenic standard IFN beta-1a. This issue has to be addressed by further long-term studies, especially as occurrence of antibodies takes time.
Results
SC and IM administration produced similar results in the single-dose study. Taking into account more convenient SC administration, this regimen was chosen for the multidose study and the Phase III study. Interestingly, 125 μg PEG-IFN provided 50% less exposure than 188 μg, but the pharmacological response measured by neopterin concentration was only slightly lower while significantly exceeding the pharmacological effect of the 63 μg dose. On the other hand, the 125 μg PEG-IFN dose is better tolerated in regards to the occurrence of flu-like symptoms, reduction in absolute neutrophil count, and severity of injection site reactions compared with the 188 μg dose. For that reason, the 125 μg dose was chosen for evaluation in the Phase III clinical study. SC 125 μg PEG-IFN beta-1a offers a 5.4-fold higher cumulative area under the curve when administered every 2 weeks and a 2.7-fold higher area under the curve when administered every 4 weeks compared with the standard IM 30 μg dose of IFN beta-1a once weekly.Citation25
Efficacy in Phase III studies
IFN alpha
Intention-to-treat and per-protocol analysis did not reveal a significant difference in patients suffering from hepatitis C treated with PEG-IFN alpha-2b or consensus IFN and ribavirin more frequently, with response rates around 40%. Both therapy regimens proved to be safe with similar tolerability.Citation28
Cost-effectiveness of PEG-IFN alpha
In chronic hepatitis C, a sustained viral response was achieved; in patients treated with PEG-IFN alpha-2b and ribavirin, an increase of 4% in quality-adjusted life-years compared to the same regimen without PEG-IFN was demonstrated using a Markov model. The regimen with PEG-IFN was shown to be cost-effective in about 90% of cases compared to the non-PEG-IFN regimen. Patients on PEG-IFN presented lower costs in all health states.Citation29
IFN beta
The ongoing 2-year ADVANCE (Efficacy and Safety Study of BIIB017) clinical trial is the first Phase III study to investigate the efficacy of PEG-IFN beta-1a.Citation30 It is a global, multicenter, randomized, placebo-controlled, double-blinded (for the first year) and dose-blinded (for the second year), parallel-group study in 1516 randomized patients who are administered PEG-IFN beta-1a at a dose of 125 μg every 2 weeks or 4 weeks after an initial titration phase starting at 63 μg. The drug is supplied as a liquid in prefilled syringes to deliver 0.5 mL of 0.25 mg/mL. Inclusion criteria involve patients aged 18–65 years suffering from relapsing–remitting MS with an Expanded Disability Status Scale score of ≤5.0 and presenting at least two relapses within the preceding 3 years and at least one relapse within 1 year prior to randomization. Patients who receive the placebo will be randomized a second time after 1 year to either receive the low or high frequency PEG-IFN beta-1a. The aim is to investigate the efficacy of the substance measured by its ability to reduce the relapse rate after 1 year. Like other studies on MS, secondary aim is to investigate the efficacy by determining the course of other variables such as the total number of new or newly enlarging MRI T2 lesions, the proportion of patients with relapses, the quality of life by means of the MS Impact Scale physical score, and the Expanded Disability Status Scale score (depending on the initial score). After 2 years, patients have the option of enrolling in the ATTAIN (Long-Term Safety and Efficacy Study of BIIB017) open-label, dose-frequency blinded extension study, with a treatment period of 2 years.Citation27
The ADVANCE study has included more than 1500 relapsing–remitting MS patients.Citation30 The primary efficacy analysis after 1 year (Biogen Idec) reveals that the primary endpoint, the annualized relapse rate at 1 year, was met for both dosing intervals. Compared to placebo, the annualized relapse rate reduction was 35.6% (P < 0.001) in the every 2 weeks dosing regimen and 27.5% (P < 0.02) in the every 4 weeks dosing regimen. Additionally, compared to placebo, all secondary endpoints were met. In both therapy groups, the risk of a 12-week confirmed disability progression – measured by the Expanded Disability Status Scale – was reduced by 38% (P < 0.04). The proportion of patients who relapsed was reduced by 39% (P < 0.001) in the every 2 weeks dosing regimen and 26% (P < 0.03) in the every 4 weeks dosing regimen. The number of new or newly enlarging brain MRI hyperintense T2 lesions was reduced by 67% (P < 0.001) in the every 2 weeks dosing regimen and 28% (P < 0.001) in the every 4 weeks dosing regimen.
Altogether, both dosing regimens showed favorable safety and tolerability data. The number of adverse events leading to discontinuation of the study as well as serious adverse events was similar in both dosing groups. The most common adverse events in the treatment groups were redness at the injection site and influenza-like illness. The most common serious adverse event was infection, occurring ≤ 1% in each treatment group.
Long-term safety, tolerability and quality of life
Animal studies
Chronic intravenous administration of PEGylated proteins caused some unintended consequences such as vacuolation of renal cortical tubular epithelium in male and female Sprague Dawley rats at daily intravenous doses of 10, 20, or 40 mg/kg PEG-linked TNF-binding protein for 3 months.Citation31 After 2 months of recovery, these lesions were only partly reversible, but there were no changes in variables such as creatinine. Interestingly, equivalent doses of PEG alone or TNF-binding protein alone did not cause microscopic changes. Treatment with another PEG-linked protein of similar weight produced similar changes. Lesions were more severe if the PEG-linked protein was of lower weight, most likely due to filtration characteristics at the glomerulus. However, most of these incidents of tubular vacuolation have occurred in animals exposed to high or toxic doses several orders of magnitude higher than administered in the ADVANCE study.Citation32 A diet of 4% triethylene glycol for 2 years did not induce toxicity in rats.Citation33 In mice, no significant embryo toxicity or teratogenicity was observable.
Studies on IFN alpha
There are no long-term safety studies on PEG-IFN beta in humans; thus, this section focuses on IFN alpha. Patients (38 men and 20 women) with chronic hepatitis C received 0.035–2 μg/kg PEG-IFN alpha-2b SC weekly or 2 million IU SC IFN alpha-2b three times a week for 24 weeks in an open-label randomized study.Citation21 Blood pressure, pulse rate, electrocardiograms, and physical examinations did not change significantly. Adverse events were qualitatively similar in both groups during the treatment and in the following 4 weeks. Flu-like syndromes peaked at 12 hours after the first dose and disappeared 24 hours after despite elevated concentrations of PEG-IFN during the whole time interval. A dose effect for adverse events was recognized. Repeated dosing reduced these responses. Yet, as usual, microscopic studies were not performed. Four weeks after the end of therapy, blood cell, neutrophil, and platelet counts returned to baseline. Altogether, safety in IFN alpha-2b and PEG-IFN alpha-2b was comparable. Hence, a similar result seems to be achievable with PEG-IFN beta.
Quality of life in patients treated with PEG-IFN alpha
Due to the development history, there are only published studies on PEG-IFN alpha. Ninety-nine patients with chronic hepatitis C and 91 healthy control persons showing better Short Form-36 (SF-36) scores at baseline were analyzed for health-related quality of life by completing the SF-36 quality of life questionnaire.Citation34 SF-36 comprises 36 questions addressing body pain, emotional role, general health, mental health, physical function, physical role, social function, and vitality; higher scores represent better health status. Interestingly, in the middle of therapy with self-administered PEG-IFN alpha-2a or -2b and ribavirin for 24 or 48 months, values in all SF-36 scales were below the baseline level, returned to baseline at the end of therapy, and were elevated in almost all scores 6 months after the end of therapy. There was a positive correlation between age and mental health. As expected from other studies,Citation35 nonresponders presented lower scores in almost all areas, meaning a worse outcome. However, this result was not significant due to the great percentage of patients with a sustained virological response.
Discussion and conclusion
Current standard immunomodulatory therapy with IFNs for relapsing–remitting MS exhibits proven, but limited, efficacy and increased side effects due to the need of frequent, at least weekly, application of the drug. Therefore, there is a need for more effective and tolerable drugs. Distributed Avonex and Rebif contain a relatively small 166 amino acid single-chain glycoprotein of 22.5 kDa, which offers a relatively small half-life of 10 hours. For that reason, optimization of therapy with IFNs in MS by PEGylation is feasible besides possible therapy regimens with other compounds.
Currently, a therapy regimen applying PEG-IFN beta-1a in MS is being developed to achieve an optimized relationship between the inconvenience of current therapies (eg, the necessity to self-inject relatively frequently) and therapy-related side effects on the one hand and pharmacokinetic and pharmacological efficacy on the other hand. In general, better tolerability might even allow a dose escalation that is known to be beneficial in MS or combination therapy with other drugs.Citation36
Phase I studies showed evidence that SC PEG-IFN beta-1a at a dose of 125 μg every 2 weeks or 4 weeks might be at least as safe and efficient as the current standard therapy with IM IFN beta-1a.Citation25 This issue is currently being addressed by the global ADVANCE Phase III clinical study investigating the efficacy of PEG-IFN beta-1a in terms of reduction of the relapse rate in patients suffering from relapsing–remitting MS.Citation30 In the primary efficacy and safety analysis released by Biogen Idec after 1 year, the most common adverse events in the treatment groups were redness at the injection site and influenza-like illness. The most common serious adverse event was infection, occurring ≤ 1% in each treatment group. Altogether, safety and tolerability data in both dosing regimens were favorable. Compared to placebo, the annualized relapse rate reduction was 35.6% in the every 2 weeks dosing regimen and 27.5% in the every 4 weeks dosing regimen. Additionally, compared to placebo, all secondary endpoints were met. The number of new or newly enlarging brain MRI hyperintense T2 lesions was reduced by 67% (P < 0.001) in the every 2 weeks dosing regimen and 28% (P < 0.001) in the every 4 weeks dosing regimen, demonstrating a clear subclinical dosing effect that exceeds the clinical dosing effect. This seems to favor the 2-week interval and still doubles the dosing interval of the standard IM IFN, which exhibits a similar reduction rate of relapse and reduction rate of new or newly enlarging brain MRI hyperintense T2 lesions. This is supported by the safety data as the number of adverse events leading to discontinuation of the study and serious adverse events were similar in both dosing groups. Altogether, SC PEG-IFN beta-1a at a dose of 125 μg seems to achieve similar results to standard Avonex, with a doubled dosing interval and a similar rate of side effects.
Chronic administration of PEGylated proteins mostly at toxic concentrations causes vacuolation of renal epithelium in animals, which has to be addressed by Phase IV studies; the same applies to the relevance of anti-PEG antibodies.
Due to its effects on the outcome, in hepatitis C stratification of patient populations (eg, virus genotype 1 or 2) before initiation of therapy with PEG-IFN alpha is essential.Citation37 For that reason, a certain degree of stratification in MS, which is already partly demonstrated by license criteria of current standard therapeutic regimens, may be advisable.
In chronic hepatitis C, IFN alpha-2b and ribavirin showed an increase of 4% in quality-adjusted life-years and cost-effectiveness compared to the same nonPEGylated regimen. In MS, this might apply to PEG-IFN beta versus the nonPEGylated form as well. Yet, additional clinical studies that address the arising detailed questions, including health economic issues, need to be performed.
Disclosure
The author reports no conflicts of interest in this work.
References
- ReussRRetzlaffKVogelSFrankeFEOschmannPAutoimmune hepatitis after high-dose intravenous methylprednisolone pulse in RR-MSCent Eur J Med200723356359
- PRISMS Study GroupRandomized double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosisLancet19983529139149815049820297
- JacobsLDCookfairDLRudickRAMultiple Sclerosis Collaborative Research GroupIntramuscular interferon beta-1a for disease progression in relapsing multiple sclerosisAnn Neurol19963932852948602746
- ReussRPohleSRetzlaffKHembergerJOschmannPInterferon beta-1a induces tumor necrosis factor receptor 1 but decreases tumor necrosis factor receptor 2 leukocyte mRNA levels in relapsing–remitting multiple sclerosisNeuroimmunomodulation200916317117619246939
- MikolDDBarkhofFChangPComparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trialLancet Neurol200871090391418789766
- O’ConnorPFilippiMArnasonB250 microg or 500 microg interferon beta-1b versus 20 mg glatiramer acetate in relapsing–remitting multiple sclerosis: a prospective, randomised, multicentre studyLancet Neurol200981088989719729344
- GalettaSLThe controlled high risk Avonex multiple sclerosis trial (CHAMPS study)J Neuroophthalmol200121429229511756862
- DurelliLVerdunEBarberoPEvery-other-day interferon beta-1b versus once-weekly interferon beta-1b for multiple sclerosis: results of a 2-year prospective randomised multicentre study (INCOMIN)Lancet200235993161453146011988242
- BailonPBertholdWPolyethylene glycol-conjugated pharmaceutical proteinsPharm Sci Technolo Today199818352356
- PisalDSKosloskiMPBalu-IyerSVDelivery of therapeutic proteinsJ Pharm Sci20109962557257520049941
- ChanYPMeyrueixRKravtzoffRNicolasFLundstromKReview on Medusa: a polymer-based sustained release technology for protein and peptide drugsExpert Opin Drug Deliv20074444145117683256
- HarrisJMChessRBEffect of pegylation on pharmaceuticalsNat Rev Drug Discov20032321422112612647
- DelgadoCFrancisGEFisherDThe uses and properties of PEG-linked proteinsCrit Rev Ther Drug Carrier Syst199293–42493041458545
- CalicetiPVeroneseFMPharmacokinetic and biodistribution properties of poly(ethylene glycol)-protein conjugatesAdv Drug Deliv Rev200355101261127714499706
- WangXIshidaTKiwadaHAnti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomesJ Control Release2007119223624417399838
- RajanRSLiTArasMModulation of protein aggregation by polyethylene glycol conjugation: GCSF as a case studyProtein Sci20061551063107516597829
- YamamotoYTsutsumiYYoshiokaYSite-specific PEGylation of a lysine-deficient TNF-alpha with full bioactivityNat Biotechnol200321554655212665803
- ZhaiYZhaoYLeiJSuZMaGEnhanced circulation half-life of site-specific PEGylated rhG-CSF: optimization of PEG molecular weightJ Biotechnol20091423–425926619497340
- HershfieldMSPEG-ADA replacement therapy for adenosine deaminase deficiency: an update after 8.5 yearsClin Immunol Immunopathol1995763 Pt 2S228S2327554473
- ReddyKRWrightTLPockrosPJEfficacy and safety of pegylated (40-kd) interferon alpha-2a in noncirrhotic patients with chronic hepatitis CHepatology200133243343811172346
- GluePFangJWRouzier-PanisRHepatitis C Intervention Therapy GroupPegylated interferon alpha-2b: pharmacokinetics, safety, and preliminary efficacy dataClin Pharmacol Ther200068555656711103758
- RaoHYLiJZhangLF[Effect of pegylated interferon beta-1-a therapy on the liver fibrosis in chronic hepatitis C: a semi-quantitative analysis.]Zhonghua Yi Xue Za Zhi200888296100 Chinese.18353212
- BasuAYangKWangMStructure-function engineering of interferon beta-1b for improving stability, solubility, potency, immunogenicity, and pharmacokinetic properties by site-selective mono-PEGylationBioconjug Chem200617361863016704199
- MagerDENeuteboomBJuskoWJPharmacokinetics and pharmacodynamics of pegylated IFN-beta 1a following subcutaneous administration in monkeysPharm Res2005221586115771230
- HuXMillerLRichmanSA novel PEGylated interferon beta-1a for multiple sclerosis: safety, pharmacology, and biologyJ Clin Pharmacol201252679880821680782
- BakerDPLinEYLinKN-terminally PEGylated human interferon-beta-1a with improved pharmacokinetic properties and in vivo efficacy in a melanoma angiogenesis modelBioconjug Chem200617117918816417267
- KieseierBCCalabresiPAPegylation of interferon-beta-1a: a promising strategy in multiple sclerosisCNS Drugs201226320521422201341
- SjogrenMHSjogrenRJrLyonsMFAntiviral response of HCV genotype 1 to consenus interferon and ribavirin versus pegylated interferon and ribavirinDig Dis Sci20075261540154717406822
- FonsecaMCBranco de AraujoGTAraujoDVCost effectiveness of peginterferon alfa-2B combined with ribavirin for the treatment of chronic hepatitis C in BrazilBraz J Infect Dis200913319119920191195
- Clinicaltrials.gov [homepage on the Internet]. Efficacy and Safety Study of BIIB017 (ADVANCE). Available from: http://www.clinicaltrials.gov/ct2/show/NCT00906399?term=nct00906399&rank=1. Accessed May 13, 2013.
- BendeleASeelyJRicheyCSennelloGShoppGRenal tubular vacuolation in animals treated with polyethylene-glycol-conjugated proteinsToxicol Sci19984221521579579027
- YoungMAMalavalliAWinslowNVandegriffKDWinslowRMToxicity and hemodynamic effects after single dose administration of MalPEG-hemoglobin (MP4) in rhesus monkeysTransl Res2007149633334217543852
- Final report on the safety assessment of triethylene glycol and PEG-4Int J Toxicol200625Suppl 212113817090481
- SinakosEGigiELallaTHealth-related quality of life in Greek chronic hepatitis C patients during pegylated interferon and ribavirin treatmentHippokratia201014212212520596269
- BonkovskyHLWoolleyJMConsensus Interferon Study GroupReduction of health-related quality of life in chronic hepatitis C and improvement with interferon therapyHepatology19992912642709862876
- BukowskiRTendlerCCutlerDRoseELaughlinMMStatkevichPTreating cancer with PEG intron: pharmacokinetic profile and dosing guidelines for an improved interferon-alpha-2b formulationCancer200295238939612124839
- MaruDSBruceRDBasuSAlticeFLClinical outcomes of hepatitis C treatment in a prison setting: feasibility and effectiveness for challenging treatment populationsClin Infect Dis200847795296118715156