Abstract
Asthma is a chronic disease characterized by airway inflammation, bronchial hyperresponsiveness, and recurrent episodes of reversible airway obstruction. The disease is very heterogeneous in onset, course, and response to treatment, and seems to encompass a broad collection of heterogeneous disease subtypes with different underlying pathophysiological mechanisms. There is a strong need for easily interpreted clinical biomarkers to assess the nature and severity of the disease. Currently available biomarkers for clinical practice – for example markers in bronchial lavage, bronchial biopsies, sputum, or fraction of exhaled nitric oxide (FeNO) – are limited due to invasiveness or lack of specificity. The assessment of markers in peripheral blood might be a good alternative to study airway inflammation more specifically, compared to FeNO, and in a less invasive manner, compared to bronchoalveolar lavage, biopsies, or sputum induction. In addition, promising novel biomarkers are discovered in the field of breath metabolomics (eg, volatile organic compounds) and (pharmaco)genomics. Biomarker research in asthma is increasingly shifting from the assessment of the value of single biomarkers to multidimensional approaches in which the clinical value of a combination of various markers is studied. This could eventually lead to the development of a clinically applicable algorithm composed of various markers and clinical features to phenotype asthma and improve diagnosis and asthma management.
Introduction to the pathophysiology of asthma
Asthma affects over 300 million individuals worldwide,Citation1 making it one of the most prevalent common chronic diseases. Although the respiratory disease is rarely fatal, the economic burden is extensive due to direct and indirect medical expenses, including prescription drug costs, health care costs, and productivity losses.Citation2
The disease is characterized by airway inflammation, bronchial hyperresponsiveness, and recurrent episodes of reversible airway obstruction. Asthma can be classified as “atopic” or “nonatopic” based on the presence (atopic) or absence (nonatopic) of specific immunoglobulin (Ig)E antibodies to common environmental allergens. Atopic asthma is the most common form of asthma. In allergen-sensitized patients with atopic asthma, re-exposure to an aeroallergen will lead to an IgE-mediated inflammatory cascade in the airways. Airway resident cells (ie, macrophages and mast cells), newly mobilized immune cells (ie, eosinophils and neutrophils), and epithelial cells play an important role in this inflammatory cascade.Citation3 In allergic inflammation, there seems to be a disturbed balance in T helper (Th)1-type and Th2-type cytokines – with dominance towards Th2 cytokines.Citation4 Th2 cells produce cytokines such as interleukin (IL)-4 and IL-13, which induce a class-switch in B-cells to the production of IgE. Th2 cells also produce IL-5, which recruits eosinophils to the lung, and IL-9, which stimulates mast cell proliferation. Upon activation, mast cells start to produce histamine, cysteinyl-leukotrienes, and prostaglandin D2, which in its turn will lead to the additional recruitment of eosinophils, Th2 cells, and basophils to the tissue.Citation5
Parallel to the allergic asthma model with airway epithelial cells and the adaptive immune response as important pillars, an additional nonallergic asthma paradigm has been proposed. In the nonallergic asthma model, the innate immune system responds to constantly invading respiratory viruses and bacteria. This systemic innate response is driven by sentinel cells such as macrophages, dendritic cells, granulocytes, and innate lymphoid cells. A recent review by Holtzman provides a comprehensive overview of both the allergic and nonallergic immune response in asthma.Citation6
A prolonged presence of activated inflammatory cells in the airways leads to chronic inflammation and induces tissue alterations in composition, content, and organization of the airways (“airway remodeling”). Important cytokines released by epithelial cells and associated with remodeling are IL-25, thymic stromal lymphopoietin, and IL-33. The remodeling response is characterized by subepithelial basement membrane thickening, epithelial cell disruption, neoangiogenesis, goblet cell metaplasia, enlarged submucosal glands, and airway smooth muscle hyperplasia.Citation7 This airway remodeling is regarded as a continuous process, while the number of inflammatory cells infiltrated in the respiratory tract can vary over time. This latter process is evoked by stimuli such as allergens, climate, or respiratory tract infections. However, the observation of airway remodeling in young asthma patients suggests that the process may even precede airway inflammation.Citation8
Asthma biomarkers for diagnosis, phenotyping, and treatment efficacy
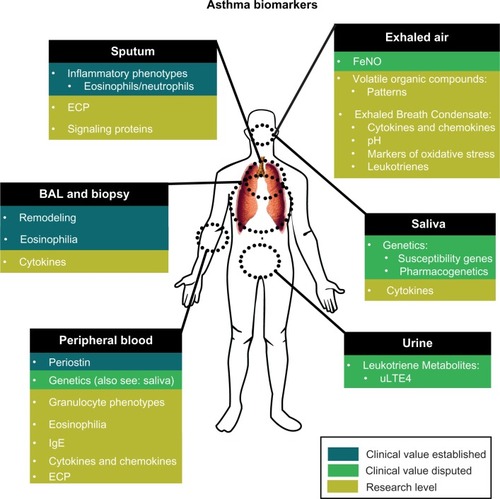
Asthma diagnosis and management is generally based on reported asthma symptoms, often combined with lung function tests to assess reversible airway obstruction and airway hyperresponsiveness. However, symptoms and lung function measurements may not reflect underlying airway inflammation. Bronchoscopy with biopsies and bronchoalveolar lavage (BAL) are considered the gold standard to assess airway inflammation, but are too invasive for general application in clinical practice.Citation9 In addition, asthma seems to encompass a broad collection of heterogeneous disease subtypes with different underlying pathophysiological mechanisms.Citation10 There is a need for asthma biomarkers to identify clinical relevant asthma phenotypes, optimize diagnosis, and guide treatment. In this paper, we will provide an overview of asthma biomarkers already available for clinical practice and promising biomarkers currently under development (). In addition, we will address the promises and barriers of the implementation of asthma biomarkers into clinical practice.
Clinically available biomarkers
Sputum induction, bronchoscopy/biopsy, and bronchoalveolar lavage
Tissue-specific diagnostic methods such as bronchoalveolar lavage, bronchoscopy, or bronchial biopsy, are used to measure airway inflammation and remodeling, and provide reliable and detailed clinical information of asthmatic patients. Airway remodeling has been observed in bronchial biopsies of both adults and children with asthma.Citation11 BAL fluid of asthmatic patients shows elevated levels of Th2 cytokines compared to healthy individuals.Citation12 In difficult-to-treat asthma in children, BAL and endobronchial biopsy should be considered to objectify the presence of airway eosinophilia and other typical pathological features of asthma.Citation13 Thus, invasive and tissue-specific diagnostic methods are valuable in certain patient populations and clinical research settings. However, the invasiveness of these diagnostic procedures limits the use of these methods for daily clinical routine in most asthma patients. Even sputum induction, a diagnostic technique in which the patient inhales nebulized saline solution in increasing concentrations to liquefy sputum, is regarded as too invasive, technically complex, and too variable for daily clinical routine. This allocates the procedure to specialized medical centers.Citation14 There is a strong correlation between cellular components present in airway fluid obtained by BAL and cells present in airway fluid obtained by sputum induction.Citation15,Citation16 Therefore, compared to BAL, sputum induction is the preferred method to diagnose the inflammatory phenotype of asthma classically based on the presence of different types of granulocytes. Recent studies indicate that the performance of this technique increases when combined with the analysis of other cellular components such as exosomes and signaling proteins.Citation17
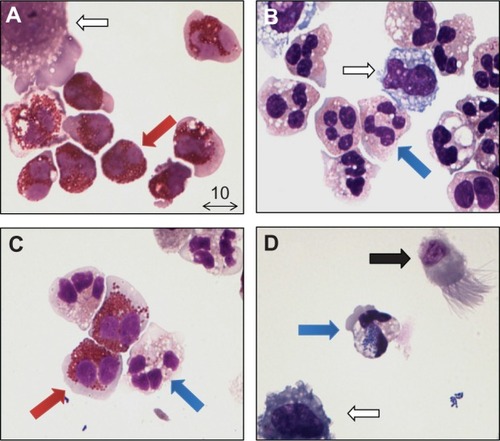
Distinct inflammatory patterns have been established in the sputum of asthmatic adults and asthmatic children based on eosinophil and neutrophil percentages of total nonsquamous cells in the sputum. Currently, four inflammatory phenotypes have been identified based on analysis of sputum: eosinophilic; neutrophilic; mixed; and paucigranulocytic types ().Citation18 It has been suggested that higher levels of sputum eosinophils are associated with a better response to corticosteroids,Citation19–Citation21 but results remain inconsistent.Citation22–Citation24 Furthermore, the pattern of inflammatory sputum phenotypes seems to be different for adult patients and pediatric patients, and the reproducibility of sputum induction measurements over time has been a point of scientific debate since the introduction of this technique.Citation18,Citation25,Citation26
Figure 2 Inflammatory phenotypes of adult asthma patients obtained by sputum induction. (A) Eosinophilic type; marked by the presence of eosinophils ≥3% (red arrow). The hollow arrow indicates an alveolar macrophage. (B) Neutrophilic type; marked by the presence of neutrophils (blue arrow) ≥61%. The hollow arrow indicates an alveolar macrophage. (C) Mixed type; marked by the presence of both eosinophils (red arrow) ≥3% and neutrophils (blue arrow) ≥61%. (D) Paucigranulocytic type; marked by a lack of eosinophils (<3%) and neutrophils (<61%). The arrow shows a ciliated pseudostratified columnar airway epithelial cell (black arrow), a neutrophil with phagocytosed bacteria inside (blue arrow) and an alveolar macrophage (hollow arrow). May-Grünwald/Giemsa staining, photograph at 100× magnification, courtesy of Dr JAM van der Linden (UMC Utrecht, The Netherlands).
Other sputum and BAL markers that have been investigated include soluble mediators such as eosinophil cationic protein (ECP), hypoxia inducible factor-1α (HIF-1α), and vascular endothelial growth factor (VEGF).Citation27 ECP is released during degranulation of eosinophils and can be measured in sputum, BAL fluid, and in serum. It is considered to be a nonspecific marker for inflammation and therefore lacks the specificity for diagnosing asthma. Meijer et al showed that sputum ECP has no predictive value for clinical response to corticosteroids in asthmatic patients.Citation28 Its added value as a diagnostic tool would be in the measurement of the extent of inflammation and severity of asthma; eg, moderate versus severe asthma.Citation29 HIF-1α and VEGF protein levels have shown to be upregulated in lung specimens from allergen-challenged asthma patients obtained by BAL and endobronchial biopsies.Citation30
Nitric oxide in exhaled breath
Almost a decade ago, the first reports emerged of increased levels of nitric oxide in exhaled breath (FeNO) in patients with asthma.Citation31,Citation32 Since then, a high number of studies have assessed the clinical value of exhaled nitric oxide in asthma management. Several FeNO analyzers became commercially available, and international guidelines on FeNO measurement were published.Citation33,Citation34
Nitric oxide is produced when the amino acid L-arginine is catalyzed by nitric oxide synthases (NOS) into the amino acid L-citrulline. There are three known isoforms of NOS, but in particular, inducible NOS seems to play a role in the elevated levels of NO in the exhaled breath of asthmatics. The expression of the enzyme is upregulated by a wide range of inflammatory cytokines. It remains unclear which cells are responsible for the increased NO production, but airway epithelial cells and eosinophils are thought to be the most important candidates.Citation35 It is thought that inflamed airways will produce increased levels of NO. High FeNO is thought to be a surrogate marker of ongoing eosinophilic airway inflammation, and may reflect uncontrolled asthma and predict asthma exacerbations.Citation36
Despite the initial enthusiasm about FeNO as a new and noninvasive marker of airway inflammation, the clinical usefulness of FeNO to measure asthma control is still debated. Studies that have investigated the association between asthma control and FeNO provide inconsistent results (), and studies assessing the relationship between FeNO and other airway inflammation markers, such as sputum eosinophilia or the presence of eosinophils in bronchial specimens, remain inconclusive.Citation37,Citation38 This may be partly caused by a non-overlap in asthma symptoms and airway inflammation. Furthermore, this relationship is complicated due to various other factors that seem to influence FeNO levels, including age, atopy, medication use, therapy adherence, and airway infections.Citation36 In addition, tailoring asthma treatment based on FeNO measurements did not decrease asthma exacerbations or lead to better asthma control according to a meta-analysis performed by Petsky et al.Citation39 FeNO might, nevertheless, still be a valuable marker in asthma management. Zacharasiewicz et al showed that the combination of increased levels of FeNO and the percentage of sputum eosinophils were significant predictors of exacerbation upon steroid reduction in children with stable asthma.Citation40 Studies by Szefler et alCitation41 and Knuffman et alCitation42 showed that pediatric asthma patients with elevated FeNO levels were more likely to respond to corticosteroids compared to montelukast.
Table 1 Studies that assessed the association between fraction of FeNO and asthma control
Reports on the relationship between FeNO and treatment response remain inconsistent, though there is a suggestion that higher baseline FeNO is associated with a better response to treatment.Citation43 Although the clinical value of a single FeNO measurement is limited, combining this measure with other markers of airway inflammation may lead to a more accurate assessment of underlying disease state.
Biomarkers under development
Blood
Peripheral blood is easy to obtain, and the procedure itself is less invasive than sputum induction and bronchoscopy. Since inflamed tissue releases chemoattractants and cytokines, which recruit activated immune cells from the peripheral blood, the dynamic process of immune cells entering and leaving the blood stream can be used as an indirect readout of the state of disease.
Peripheral blood eosinophilia has been described extensively as a potential asthma biomarker.Citation44 Blood eosinophilia correlates with bronchial hyperresponsiveness and asthma-related inflammation.Citation45 The specificity of using peripheral blood eosinophilia to diagnose asthma is, however, rather low, as allergies, autoimmune disease, and parasitic infections cause blood eosinophilia as well. Therefore, its role as a diagnostic measurement remains limited. The same applies to total and allergen-specific IgE levels in serum.Citation46 Several studies have evaluated whether the presence of inflammatory soluble mediators such as chemokines and cytokines were applicable as biomarkers for type and extent of asthma phenotypes.Citation47 Recent studies utilized multiplex analysis, allowing the parallel analysis of multiple cytokines within one serum/plasma sample.Citation48,Citation49 Unfortunately, these studies have led to neither a clinically useful diagnostic tool to identify distinct disease phenotypes, nor to a tool to assess disease severity. A weakness of studies assessing inflammatory chemokine and cytokine profiles lies in the fact that the choice of mediators to be studied determines the (lack of) success of this approach, and that several inflammatory mediators may still be unidentified. Anti-inflammatory mediators (such as receptor antagonists) are often neglected. In addition, little consideration has been given to the complex interaction between inflammatory mediators.Citation50
A different approach is to examine shifts in activation profiles of inflammatory cells in peripheral blood and attempt to link these shifts to clinical phenotypes. These inflammatory cells will integrate all pro- and anti-inflammatory signals and change their phenotypes accordingly. Studies on the activation status of peripheral blood cells have provided some insight into the systemic innate immune response in allergic asthma. Many studies have shown that inflammatory cells such as monocytes and granulocytes respond with upregulation of several activation markers in response to inflammatory signals.Citation51–Citation53 Many of these markers, such as CD11b/CD18 (Mac-1), CD63, CD66 and CD67, are typically found in granules that fuse with the plasma membrane upon activation of the cells with inflammatory mediators.Citation54 Unfortunately, studiesCitation55,Citation56 that compared the presence of the markers on blood cells and tissue cells obtained from sputum and BAL did not take into account that cells homing to the tissue under homeostatic conditions exhibit the same phenotype.Citation57 The process of homing of the cells towards the tissue compartment is already sufficient to activate the cells both in homeostasis as well as in disease. The expression of these markers in the peripheral blood has not led to a clear link between expression profiles of granulocytes and type of asthma.
Elegant work by Johansson et al has shown that eosinophils change their activation status of membrane-bound integrins rather than overall expression in response to inflammatory signals.Citation58 The application of antibodies specifically recognizing activated states of integrins provided solid data that show that blood eosinophils in poorly controlled asthma are characterized by activated integrins. This situation is consistent with the hypothesis that these cells are primed and prepared to leave the peripheral blood for the tissues. We have obtained similar data by the application of antibodies recognizing activated FcγRs.Citation51,Citation59 These data demonstrated that eosinophils become first activated in the peripheral blood and subsequently home for the tissue, leaving behind unprimed cells.Citation60 These studies have indicated that changes in the phenotype of inflammatory cells can aid in the diagnosis of the type and extent of severity of allergic asthma. But they also show that the differences are very subtle and not applicable yet in the clinical routine.
Closer to clinical implementation might be the biomarker periostin. Periostin is a recently discovered matricellular protein that is secreted by bronchial epithelial cells under the influence of IL-13. The presence of periostin in serum correlates strongly with sputum eosinophilia.Citation61 A study by Corren et al showed that patients with high levels of serum periostin responded better to lebrikizumab (anti-IL-13 therapy) compared to patients with low levels of periostin.Citation62
Air
The measurement of volatile organic compounds (VOCs) in exhaled breath is a novel metabolomic approach to study molecular signatures of respiratory disease. Exhaled breath contains a complex mixture of potentially thousands of VOCs. These compounds are produced due to metabolic processes in the airways, and the presence and/or concentrations of the different compounds are likely influenced by the presence of airway inflammation. There exist different methods to assess VOCs; one can assess profiles of VOCs (“breathprints”) present in exhaled breath using polymer-based gas sensor arrays (“electronic nose”),Citation63 or identify individual molecular components using gas chromatography-mass spectrometry (GC-MS).Citation64 Asthma patients can be differentiated from healthy controls based on their breathprints,Citation65 as can asthmatic patients from COPD patients.Citation66 However, the method was less successful in distinguishing mild asthmatics from severe asthmatics.Citation65 Breathprints of COPD patients do correlate with the presence of eosinophils and neutrophils in induced sputum, as well as with levels of ECP and myeloperoxidase in induced sputum, suggesting that the electronic nose might be capable of assessing distinct types of underlying airway inflammation.Citation67
Using the other approach, GC-MS, Dallinga et al showed that the measurement of a limited set of VOCs in exhaled air could differentiate asthmatic children from controls with high sensitivity (95%) and high specificity (89%).Citation64 A study by Ibrahim et al showed that a set of 15 VOCs could discriminate asthmatic patients from controls, and also could classify patients according to inflammatory sputum phenotype and asthma control (based on the Asthma Control Questionnaire).Citation68
The assessment of VOC in exhaled breath seems to be a very promising approach, especially when knowledge of clinically relevant VOCs is integrated into a user-friendly handheld device such as an electronic nose. However, validation of clinical relevant VOC patterns in a large population of asthmatic patients is necessary, as well as longitudinal assessment of VOC patterns, the assessment of the influence of asthma treatment, and emergence of international guidelines on VOC measurement. A large Europe-wide study to assess the clinical utility of VOCs in asthma in-depth is currently taking place.Citation69
Biomarkers in breath can also be measured in exhaled breath condensate (EBC). When exhaled breath is cooled, a liquid phase can be obtained, which contains condensed water vapor as well as nonvolatile substances. Various markers in EBC have been found to be elevated in asthmatics when compared to healthy individuals, including adenosine concentration,Citation70 markers of oxidative stress (ie, hydrogen peroxide),Citation71 cytokines and chemokines,Citation72 nitric oxide-related products,Citation73 isoprostanes,Citation74 and leukotrienes.Citation74 Furthermore, the pH of EBC has been reported to be decreased in acute asthmatics and poorly controlled asthmatics.Citation75,Citation76
In spite of these results, the measurement of markers in EBC is still in its research phase, and several important methodological problems complicate the clinical utility of EBC.Citation77 A standardized methodology for EBC collection is lacking, as are established reference values. Various factors such as the type of condenser equipment used, cooling temperature, condenser tube coating, cleaning procedures, breathing patterns, ambient air pollution, or concentrations of relevant cytokines too low for reliable determination influence the measurement and compromise reproducibility.
Urine: leukotriene metabolites
Cysteinyl leukotrienes (LTs) C4 and D4 are lipid mediators, which are thought to play a role in asthma pathogenesis. They can be released from various cells, including eosinophils, neutrophils, and mast cells. LTC4 and LTD4 in the plasma are rapidly converted into the less active LTE4 metabolite. A fraction of LTE4 is excreted in urine. The urinary LTE4 (uLTE4) concentration is used as a marker of total body LT production.Citation78 Studies by Szefler et al and Cai et al showed that asthmatic patients with higher levels of uLTE4 were more likely to respond to leukotriene antagonists (LTRA) when compared to asthmatic patients with lower uLTE4 levels.Citation41,Citation79
(Pharmaco)genetics
Twin studies have shown that asthma contains a considerable genetic component.Citation80 Genome-wide association studies have identified several loci to be associated with asthma risk, including the ORMDL3 locus, ADAM33, and various cytokines and cytokine receptor genes (IL18R1, IL33, IL2RB, IL10, TGFB1, and IL6R).Citation81–Citation84
A recent review by Dijk et al provides a thorough overview of asthma susceptibility genes that have been found by genome-wide association studies.Citation85 Nevertheless, effect sizes are small, and the identified genetic variants can only explain a small part of the asthma heritability. This could be due to the heterogeneity in asthma phenotypes and the underestimated influence of environmental–gene interactions. For example, recent work by Ierodiakonou et al showed an interaction between variation in TGFB1 and smoking on asthma severity.Citation86 Carrying a G-allele of rs6957 in TGFB1 was associated with higher submucosal eosinophils and basement membrane thickness, but only in current or ex-smoking asthmatics.
A more promising genetic approach for clinical asthma practice might be pharmacogenomics: the association of genomic variations and medication response. Variation in genes coding for proteins involved in the drug metabolism pathway may influence drug concentration and efficacy. Observational studies have found genetic variation to be associated with persistent symptoms as well as with lung function in steroid-treated asthmatics.Citation87–Citation90 A study by Hawkins et al found a positive correlation with variations in STIP1, coding for an adaptor protein in the glucocorticoid receptor complex, and baseline lung function and improvement in lung function upon corticosteroid treatment in 382 adults with asthma.Citation89 A study by Tantisira et al showed that asthma patients with a variant in the GLCCI1 have less improvement in lung function upon inhaled corticosteroids (ICS) treatment.Citation90GLCCI1 encodes Glucocorticoid Induced Transcript 1, a protein of unknown function. Furthermore, a single-nucleotide polymorphism in the FCER2 gene, coding for a low-affinity IgE receptor, has been associated with an increased risk of asthma-related hospital visits, uncontrolled asthma, and higher daily steroid dosages.Citation87,Citation88 Variation in TBX21 (encoding transcription factor T-bet) has been related to improved airway responsiveness in childhood asthma upon treatment with ICS.Citation91 T-bet is thought to be an important regulator of the Th1/Th2 balance.Citation92
Pharmacogenomic studies on response to LTRA have found most association with ALOX5,Citation93,Citation94 a 5-lipoxygenase, and LTC4S, a glutathione S-transferase.Citation95,Citation96 However, a step closer to clinical implementation is the assessment of the beta-adrenergic receptor gene (ADRB2) in order to determine response to β2-agonists, for which randomized clinical trial (RCT) data are available.Citation97–Citation99 The beta-adrenergic receptor is a G-protein coupled receptor that is expressed in smooth muscle in the airways; activation induces bronchial relaxation. β2-agonists are the most frequently prescribed drugs to relieve airway obstruction, and act through the beta-adrenergic receptor. Evidence suggests that genetic variations in the gene are associated with an altered treatment response. Recently, a small RCTCitation97 based on prospective testing of genetic variation in the ADRB2 gene (alteration in amino acid at position 16; Arg16Gly) showed encouraging results in 62 children with persistent asthma. Asthmatic children homozygous for the variant genotype were randomized to a long-acting β2 agonist (LABA) plus ICS or to LTRA plus ICS. The group treated with ICS and LTRA scored better on asthma symptoms and quality of life, used less rescue medication, and were fewer days absent from school compared to the children treated with LABA plus ICS,Citation97 suggesting that asthmatic children homozygous for ADRB2 Arg16Gly substitution (B16 Arg/Arg) benefit more from LTRA compared to LABA as add-on treatment to ICS. Yet there was no difference in lung function improvement.
On the other hand, RCTs performed in adults found no effect. A post hoc pharmacogenetic analysis of two large RCTs in which asthmatic patients were treated with LABA only or LABA combined with ICS found no differences in exacerbations, use of rescue medication, night awakenings, and lung function when patients were stratified according to differences in ADRB2 Arg16Gly genotype.Citation100 In a crossover RCT, asthmatic patients with the B16 Arg/Arg (homozygote for the risk allele) or B16 Gly/Gly (homozygote for the wild-type allele) were randomized to LABA plus ICS or placebo plus ICS. There was no difference in lung function improvement between the groups when ICS was added. Remarkably, airway responsiveness in the patients with B16 Gly/Gly did improve significantly when ICS was added to the treatment, while it did not in the B16 Arg/Arg group.Citation98 Airway responsiveness was measured as methacholine PC20 doubling dose: the dose of methacholine that provokes a 20% drop in the volume of exhaled air during the first second of a forced expiratory maneuver.
So far, pharmacogenetic studies have been limited by small sample sizes, heterogeneous populations, and lack of replication. However, the emergence of new sequencing technologies and innovative strategies of analyses, as well as the increase in international research consortia, may lead to the identification and replication of clinical relevant associations in the near future. In addition, the development of innovative – though expensive – targeted treatment strategies (such as omalizumab [anti-IgE], mepolizumab [anti-IL5], and lebrikizumab [anti-IL13]) may provide a novel clinical context for pharmacogenetics in order to identify subgroups of asthma patients that will benefit the most from these treatments.
Ease of biomarker detection and current limitations
Progressive insight into medical biology leads to a layered profile of studying disease mechanisms. Asthma research is shifting from a broad perspective (studying symptom expression, lung function, and response to medication) to a more narrow focus: cellular profiles, protein analysis, and genetic markers, possibly combined with clinical measures. These biological parameters can be measured in different body compartments, and build up to a complexity that has not yet been fully understood. From a biological point of view, there are an almost indefinite number of possible biomarkers that can be measured in the context of asthma. Yet the clinical applicability (eg, clinical added value, specificity, sensitivity, and invasiveness) limits the number of appropriate clinical usable biomarkers. Noninvasive, reliable, and easily interpreted biomarkers would ideally be standard in daily clinical routine, but are currently unavailable.
Conclusion and future directions
Single biomarker approaches to phenotype asthma are increasingly regarded to be inaccurate and outdated. In diagnosing the presence of eosinophilic inflammation for example, FeNO is a very sensitive biomarker, but not very specific. Intuitively, combining FeNO with markers of eosinophilic inflammation (such as the percentage of eosinophils in peripheral blood or eosinophil receptor expression) or other biomarkers would increase specificity. To test this hypothesis, studies combining multiple known biomarkers should be performed. Currently, research consortia like U-BIOPRED (Unbiased Biomarkers in Prediction of Respiratory Disease Outcomes, http://www.ubiopred.european-lung-foundation.org/) and SARP (Severe Asthma Research Program, http://www.severeasthma.org) aim to integrate the process of data collection and multidimensional approaches to phenotype asthma.
Single biomarker approaches remain important in the process of biomarker discovery, as newly identified biomarkers can be integrated in a multidimensional approach to strengthen the diagnostic ability of a clinically applicable algorithm to phenotype asthma. Only then will personalized asthma treatment be in reach.
Disclosure
Susanne JH Vijverberg has been paid by an unrestricted grant from GlaxoSmithKline (GSK). Bart Hilvering has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. Jan AM Raaijmakers is a part-time professor at the Utrecht University, Vice-President External Scientifc Collaborations for GSK in Europe, and holds stock in GSK. Anke-Hilse Maitland-van der Zee received an unrestricted grant from GSK. Furthermore, the department of Pharmacoepidemiology and Clinical Pharmacology, Utrecht Institute for Pharmaceutical Sciences, which employs authors Susanne JH Vijverberg, Jan AM Raaijmakers, and Anke-Hilse Maitland-van der Zee, has received unrestricted research funding from the Netherlands Organisation for Health Research and Development, the Dutch Health Care Insurance Board, the Royal Dutch Pharmacists Association, the private public-funded Top Institute Pharma, including co-funding from universities, government, the EU Innovative Medicines Initiative, EU 7th Framework Program, the Dutch Medicines Evaluation Board, the Dutch Ministry of Health, and industry (including GSK, Pfizer, and others). Jan-Willem Lammers and Leo Koenderman are full professors in the Department of Respiratory Medicine at the University Medical Centre Utrecht. Both collaborated in a TI-Pharma–funded project. TI-Pharma is a public private partnership between the Universities of Utrecht, Groningen, Maastricht, the Dutch government, GSK, Nycomed, and Danone.
References
- global strategy for asthma management and preventionGlobal Initiative for Asthma (GINA)2012 Available from: http://www.ginasthma.org/documents/4Accessed June 20, 2013
- MasoliMFabianDHoltSBeasleyRglobal Initiative for Asthma (GINA) ProgramThe global burden of asthma: executive summary of the GINA dissemination committee reportAllergy200459546947815080825
- BusseWWLemanskeRFAsthmaN Engl J Med2001344535036211172168
- HwangSSKimYULeeSTranscription factor YY1 is essential for regulation of the Th2 cytokine locus and for Th2 cell differentiationProc Natl Acad Sci U S A2013110127628123248301
- BarnesPJThe cytokine network in asthma and chronic obstructive pulmonary diseaseJ Clin Invest2008118113546355618982161
- HoltzmanMJAsthma as a chronic disease of the innate and adaptive immune systems responding to viruses and allergensJ Clin Invest201212282741274822850884
- MauadTBelEHSterkPJAsthma therapy and airway remodelingJ Allergy Clin Immunol20071205997100917681364
- Baena-CagnaniCARossiGACanonicaGWAirway remodeling in children: When does it start?Curr Opin Allergy Clin Immunol20077219620017351476
- ConnettGJBronchoalveolar lavagePaediatr Respir Rev200011525616263445
- HaldarPPavordIDShawDECluster analysis and clinical asthma phenotypesAm J Respir Crit Care Med2008178321822418480428
- BossleyCJFlemingLGuptaAPediatric severe asthma is characterized by eosinophilia and remodeling without TH2 cytokinesJ Allergy Clin Immunol20121294974982 e1322385633
- BrightlingCESymonFABirringSSBraddingPPavordIDWardlawAJTH2 cytokine expression in bronchoalveolar lavage fluid T lymphocytes and bronchial submucosa is a feature of asthma and eosinophilic bronchitisJ Allergy Clin Immunol2002110689990512464957
- PayneDMcKenzieSAStaceySMisraDHaxbyEBushASafety and ethics of bronchoscopy and endobronchial biopsy in difficult asthmaArch Dis Child200184542342611316690
- PetskyHLKynastonJATurnerCTailored interventions based on sputum eosinophils versus clinical symptoms for asthma in children and adultsCochrane Database Syst Rev20072CD00560317443604
- FahyJVEosinophilic and neutrophilic inflammation in asthma: insights from clinical studiesProc Am Thorac Soc20096325625919387026
- MacedoPHewMTorregoAInflammatory biomarkers in airways of patients with severe asthma compared with non-severe asthmaClin Exp Allergy200939111668167619622091
- HouCZhaoHLiWIncreased heat shock protein 70 levels in induced sputum and plasma correlate with severity of asthma patientsCell Stress Chaperones201116666367121643870
- SimpsonJLScottRBoyleMJGibsonPGInflammatory subtypes in asthma: Assessment and identification using induced sputumRespirology2006111546116423202
- PavordIDBrightlingCEWoltmannGWardlawAJNon-eosinophilic corticosteroid unresponsive asthmaLancet199935391712213221410392993
- BacciECianchettiSBartoliMLow sputum eosinophils predict the lack of response to beclomethasone in symptomatic asthmatic patientsChest2006129356557216537853
- GreenRHBrightlingCEMcKennaSAsthma exacerbations and sputum eosinophil counts: A randomised controlled trialLancet200236093471715172112480423
- MartinRJSzeflerSJKingTSThe Predicting Response to Inhaled Corticosteroid Efficacy (PRICE) trialJ Allergy Clin Immunol20071191738017208587
- LexCJenkinsGWilsonNMDoes sputum eosinophilia predict the response to systemic corticosteroids in children with difficult asthma?Pediatr Pulmonol200742329830317262857
- WenzelSEEosinophils in asthma – closing the loop or opening the door?N Engl J Med2009360101026102819264692
- WangFHeXYBainesKJDifferent inflammatory phenotypes in adults and children with acute asthmaEur Respir J201138356757421233265
- FlemingLWilsonNRegameyNBushAAre inflammatory phenotypes in children with severe asthma stable?Eur Respir J200730Suppl 51483S
- FahyJVLiuJWongHBousheyHAAnalysis of cellular and biochemical constituents of induced sputum after allergen challenge: a method for studying allergic airway inflammationJ Allergy Clin Immunol1994936103110398006308
- MeijerRJPostmaDSKauffmanHFArendsLRKoëterGHKerstjensHAAccuracy of eosinophils and eosinophil cationic protein to predict steroid improvement in asthmaClin Exp Allergy20023271096110312100060
- KohGCShekLPGohDYVan BeverHKohDSEosinophil cationic protein: is it useful in asthma? A systematic reviewRespir Med2007101469670517034998
- Huerta-YepezSBaay-GuzmanGJBebenekIGHypoxia inducible factor promotes murine allergic airway inflammation and is increased in asthma and rhinitisAllergy201166790991821517900
- KharitonovSAYatesDRobbinsRALogan-SinclairRShinebourneEABarnesPJIncreased nitric oxide in exhaled air of asthmatic patientsLancet199434388901331357904001
- AlvingKWeitzbergELundbergJMIncreased amount of nitric oxide in exhaled air of asthmaticsEur Respir J199369136813707507065
- DweikRABoggsPBErzurumSCAn official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FeNO) for clinical applicationsAm J Respir Crit Care Med2011184560261521885636
- KharitonovSAlvingKBarnesPJExhaled and nasal nitric oxide measurements: recommendations. The European Respiratory Society Task ForceEur Respir J1997107168316939230267
- YatesDHRole of exhaled nitric oxide in asthmaImmunol Cell Biol200179217819011264714
- PijnenburgMWJongsteJCExhaled nitric oxide in childhood asthma: a reviewClin Exp Allergy200838224625918076708
- PayneDNAdcockIMWilsonNMOatesTScallanMBushARelationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisoloneAm J Respir Crit Care Med20011648 Pt 11376138111704581
- LemièreCErnstPOlivensteinRAirway inflammation assessed by invasive and noninvasive means in severe asthma: eosinophilic and noneosinophilic phenotypesJ Allergy Clin Immunol200611851033103917088126
- PetskyHLCatesCJLiAKynastonJATurnerCChangABTailored interventions based on exhaled nitric oxide versus clinical symptoms for asthma in children and adultsCochrane Database Syst Rev20094CD00634019821360
- ZacharasiewiczAWilsonNLexCClinical use of noninvasive measurements of airway inflammation in steroid reduction in childrenAm J Respir Crit Care Med2005171101077108215709050
- SzeflerSJPhillipsBRMartinezFDCharacterization of within-subject responses to fluticasone and montelukast in childhood asthmaJ Allergy Clin Immunol2005115223324215696076
- KnuffmanJESorknessCALemanskeRFJrPhenotypic predictors of long-term response to inhaled corticosteroid and leukotriene modifier therapies in pediatric asthmaJ Allergy Clin Immunol2009123241141619121860
- VijverbergSJKoendermanLKosterESvan der EntCKRaaijmakersJAMaitland-van der ZeeAHBiomarkers of therapy responsiveness in asthma: Pitfalls and promisesClin Exp Allergy201141561562921488995
- MassanariMHolgateSTBusseWWJimenezPKianifardFZeldinREffect of omalizumab on peripheral blood eosinophilia in allergic asthmaRespir Med2010104218819619846286
- JansenDFRijckenBSchoutenJPThe relationship of skin test positivity, high serum total IgE levels, and peripheral blood eosinophilia to symptomatic and asymptomatic airway hyperresponsivenessAm J Respir Crit Care Med1999159392493110051274
- Platts-MillsTAThe role of immunoglobulin E in allergy and asthmaAm J Respir Crit Care Med20011648 Pt 2S1S511704610
- BhaktaNRWoodruffPGHuman asthma phenotypes: from the clinic, to cytokines, and back againImmunol Rev2011242122023221682748
- KatoMYamadaYMaruyamaKHayashiYSerum eosinophil cationic protein and 27 Cytokines/Chemokines in acute exacerbation of childhood asthmaInt Arch Allergy Immunol2010152Suppl 1626620523065
- PatilSPWisniveskyJPBussePJHalmEALiXMDetection of immunological biomarkers correlated with asthma control and quality of life measurements in sera from chronic asthmatic patientsAnn Allergy Asthma Immunol2011106320521321354022
- LangereisJDSchweizerRCLammersJWKoendermanLUlfmanLHA unique protein profile of peripheral neutrophils from COPD patients does not reflect cytokine-induced protein profiles of neutrophils in vitroBMC Pulm Med2011114421896197
- KantersDten HoveWLuijkBExpression of activated FcγRII discriminates between multiple granulocyte-priming phenotypes in peripheral blood of allergic asthmatic subjectsJ Allergy Clin Immunol200712051073108117697704
- JohanssonMWKellyEABusseWWJarjourNNMosherDFUp-regulation and activation of eosinophil integrins in blood and airway after segmental lung antigen challengeJ Immunol2008180117622763518490765
- PillayJKampVMvan HoffenEA subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1J Clin Invest2012122132733622156198
- FaurschouMBorregaardNNeutrophil granules and secretory vesicles in inflammationMicrobes Infect20035141317132714613775
- MengelersHJMaikoeTBrinkmanLHooibrinkBLammersJWKoendermanLImmunophenotyping of eosinophils recovered from blood and BAL of allergic asthmaticsAm J Respir Crit Care Med199414923453518306028
- KellyEAKoziol-WhiteCJClayKJPotential contribution of IL-7 to allergen-induced eosinophilic airway inflammation in asthmaJ Immunol200918231404141019155487
- FortunatiEKazemierKMGruttersJCKoendermanLVa n den BoschVJHuman neutrophils switch to an activated phenotype after homing to the lung irrespective of inflammatory diseaseClin Exp Immunol2009155355956619077082
- JohanssonMWBarthelSRSwensonCAEosinophil β1 integrin activation state correlates with asthma activity in a blind study of inhaled corticosteroid withdrawalJ Allergy Clin Immunol200611761502150416751021
- LuijkBLindemansCAKantersDGradual increase in priming of human eosinophils during extravasation from peripheral blood to the airways in response to allergen challengeJ Allergy Clin Immunol20051155997100315867857
- KoendermanLvan der LindenJUlfmanLCofferPEosinophilsRogersDFDonnellyLEHuman Airway Inflammation56Totowa (NJ)Humana Press2001217226
- JiaGEricksonRWChoyDFPeriostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patientsJ Allergy Clin Immunol20121303647654 e1022857879
- CorrenJLemanskeRFHananiaNALebrikizumab treatment in adults with asthmaN Engl J Med2011365121088109821812663
- WilsonADBaiettoMAdvances in electronic-nose technologies developed for biomedical applicationsSensors20111111105117622346620
- DallingaJWRobroeksCMvan BerkelJJVolatile organic compounds in exhaled breath as a diagnostic tool for asthma in childrenClin Exp Allergy2010401687619793086
- DragonieriSSchotRMertensBJAn electronic nose in the discrimination of patients with asthma and controlsJ Allergy Clin Immunol2007120485686217658592
- FensNZwindermanAHvan der ScheeMPExhaled breath profiling enables discrimination of chronic obstructive pulmonary disease and asthmaAm J Respir Crit Care Med2009180111076108219713445
- FensNde NijsSBPetersSExhaled air molecular profiling in relation to inflammatory subtype and activity in COPDEur Respir J20113861301130921700610
- IbrahimBBasantaMCaddenPNon-invasive phenotyping using exhaled volatile organic compounds in asthmaThorax201166980480921749985
- U-BIOPRED [webpage on the Internet]European Lung Foundation Available from: http://www.ubiopred.european-lung-foundation.orgAccessed May 2, 2013
- LázárZCervenakLOroszMAdenosine triphosphate concentration of exhaled breath condensate in asthmaChest2010138353654220382721
- LoukidesSBourosDPapatheodorouGPanagouPSiafakasNMThe relationships among hydrogen peroxide in expired breath condensate, airway inflammation, and asthma severityChest2002121233834611834641
- RobroeksCMRijkersGTJöbsisQIncreased cytokines, chemokines and soluble adhesion molecules in exhaled breath condensate of asthmatic childrenClin Exp Allergy2010401778420205697
- DonnellyLEExhaled breath condensate: Nitric oxide-related compoundsHorvathIde JongsteJCEuropean Respiratory Monograph (Exhaled Biomakers)2010207216
- MontuschiPExhaled breath condensate: 8-isoprostane and eicosanoidsHorvathIde JongsteJCEuropean respiratory monograph: exhaled biomarkers49Sheffield, UKEuropean Respiratory Society Journals Ltd2010196206
- TseliouEBessaVHillasGExhaled nitric oxide and exhaled breath condensate pH in severe refractory asthmaChest2010138110711320173051
- BikovAAntusBLosonczyGHorvathIExhaled breath condensate pHHorvathIde JongsteJCEuropean Respiratory Monograph (Exhaled Biomarkers)2010173182
- HorvathIHuntJBarnesPJExhaled breath condensate: Methodological recommendations and unresolved questionsEur Respir J200526352354816135737
- RabinovitchNUrinary leukotriene E4 as a biomarker of exposure, susceptibility and risk in asthmaImmunol Allergy Clin North Am201232343344522877620
- CaiCYangJHuSZhouMGuoWRelationship between urinary cysteinyl leukotriene E4 levels and clinical response to antileukotriene treatment in patients with asthmaLung2007185210511217393242
- LosHPostmusPEBoomsmaDIAsthma genetics and intermediate phenotypes: A review from twin studiesTwin Res200142819311665340
- FerreiraMAMathesonMCDuffyDLIdentification of IL6R and chromosome 11q13.5 as risk loci for asthmaLancet201137897951006101421907864
- MoffattMFGutIGDemenaisFA large-scale, consortium-based genomewide association study of asthmaN Engl J Med2010363131211122120860503
- HimesBEHunninghakeGMBaurleyJWGenome-wide association analysis identifies PDE4D as an asthma-susceptibility geneAm J Hum Genet200984558159319426955
- HobbsKNegriJKlinnertMRosenwasserLBorishLInterleukin-10 and transforming growth factor-beta promoter polymorphisms in allergies and asthmaAm J Respir Crit Care Med19981586195819629847292
- DijkFNde JongsteJCPostmaDSKoppelmanGHGenetics of onset of asthmaCurr Opin Allergy Clin Immunol201313219320223407123
- IerodiakonouDPostmaDSKoppelmanGHTGF-β1 polymorphisms and asthma severity, airway inflammation, and remodelingJ Allergy Clin Immunol2013131258258523111237
- TantisiraKGSilvermanESMarianiTJFCER2: A pharmacogenetic basis for severe exacerbations in children with asthmaJ Allergy Clin Immunol200712061285129117980418
- KosterESMaitland-van der ZeeAHTavendaleRFCER2 T2206C variant associated with chronic symptoms and exacerbations in steroid-treated asthmatic childrenAllergy201166121546155221958076
- HawkinsGALazarusRSmithRSThe glucocorticoid receptor heterocomplex gene STIP1 is associated with improved lung function in asthmatic subjects treated with inhaled corticosteroidsJ Allergy Clin Immunol2009123613761383 e719254810
- TantisiraKGLasky-SuJHaradaMGenomewide association between GLCCI1 and response to glucocorticoid therapy in asthmaN Engl J Med2011365131173118321991891
- TantisiraKGHwangESRabyBATBX21: A functional variant predicts improvement in asthma with the use of inhaled corticosteroidsProc Natl Acad Sci U S A200410152180991810415604153
- LiJRLiJGDengGHA common promoter variant of TBX21 is associated with allele specific binding to yin-yang 1 and reduced gene expressionScand J Immunol201173544945821272048
- TelleriaJJBlanco-QuirosAVarillasDALOX5 promoter genotype and response to montelukast in moderate persistent asthmaRespir Med2008102685786118339529
- DrazenJMYandavaCNDubeLPharmacogenetic association between ALOX5 promoter genotype and the response to anti-asthma treatmentNat Genet199922216817010369259
- SampsonAPSiddiquiSBuchananDVariant LTC4 synthase allele modifies cysteinyl leukotriene synthesis in eosinophils and predicts clinical response to zafirlukastThorax200055Suppl 2S28S3110992553
- KangMJKwonJWKimBJPolymorphisms of the PTGDR and LTC4S influence responsiveness to leukotriene receptor antagonists in Korean children with asthmaJ Hum Genet201156428428921307858
- LipworthBJBasuKDonaldHPTailored second-line therapy in asthmatic children with the arg(16) genotypeClin Sci (Lond)2013124852152823126384
- WechslerMEKunselmanSJChinchilliVMEffect of [beta]2-adrenergic receptor polymorphism on response to longacting [beta]2 agonist in asthma (LARGE trial): A genotype-stratified, randomised, placebo-controlled, crossover trialLancet200937497031754176419932356
- BleeckerERNelsonHSKraftMβ2-receptor polymorphisms in patients receiving salmeterol with or without fluticasone propionateAm J Respir Crit Care Med2010181767668719910613
- BleeckerERPostmaDSLawranceRMMeyersDAAmbroseHJGoldmanMEffect of ADRB2 polymorphisms on response to longacting β2-agonist therapy: A pharmacogenetic analysis of two randomised studiesLancet200737096052118212518156033
- VijverbergSJHKosterESKoendermanLExhaled NO is a poor marker of asthma control in children with a reported use of asthma medication: A pharmacy-based studyPediatr Allergy Immunol201223652953622624949
- MahutBTrinquartLLe BourgeoisMMulticentre trial evaluating alveolar NO fraction as a marker of asthma control and severityAllerg y2010655636644
- Sardón-PradoOKorta-MuruaJValverde-MolinaJAssociation among lung function, exhaled nitric oxide, and the CAN questionnaire to assess asthma control in childrenPediatr Pulmonol201045543443920425850
- OzierAGirodetPBaraITunon de LaraJMarthanRBergerPControl maintenance can be predicted by exhaled NO monitoring in asthmatic patientsRespir Med2011105798999621292461
- Perez-de-LlanoLACarballadaFCastro AnonOExhaled nitric oxide predicts control in patients with difficult-to-treat asthmaEur Resp J201035612211227
- ShiraiTFuruhashiKSudaTChidaKRelationship of the asthma control test with pulmonary function and exhaled nitric oxideAnn Allergy Asthma Immunol2008101660861319119704
- KhaliliBBoggsPBShiRBahnaSLDiscrepancy between clinical asthma control assessment tools and fractional exhaled nitric oxideAnn Allergy Asthma Immunol2008101212412918727466
- MichilsABaldassarreSVan MuylemAExhaled nitric oxide and asthma control: a longitudinal study in unselected patientsEur Respir J200831353954618057062
- SennaGPassalacquaGSchiappoliMLombardiCWilcockLCorrelation among FEV1, nitric oxide and asthma control test in newly diagnosed asthmaAllergy200762220720817298431
- RobroeksCMvan de KantKDJöbsisQExhaled nitric oxide and biomarkers in exhaled breath condensate indicate the presence, severity and control of childhood asthmaClin Exp Allergy20073791303131117845410
- RosiasPPDompelingEDentenerMAChildhood asthma: exhaled markers of airway inflammation, asthma control score, and lung function testsPediatr Pulmonol200438210711415211692
- StrunkRCSzeflerSJPhillipsBRRelationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in childrenJ Allergy Clin Immunol2003112588389214610474
- FranklinPJTurnerSWLe SouëfPNStickSMExhaled nitric oxide and asthma: Complex interactions between atopy, airway responsiveness, and symptoms in a community population of childrenThorax200358121048105214645971
- JonesSLKittelsonJCowanJOThe predictive value of exhaled nitric oxide measurements in assessing changes in asthma controlAm J Respir Crit Care Med2001164573874311549525
- SippelJMHoldenWETillesSAExhaled nitric oxide levels correlate with measures of disease control in asthmaJ Allergy Clin Immunol2000106464565011031334