Abstract
Renal cell carcinoma (RCC) is a biologically heterogeneous disease, with many small renal masses (SRMs) exhibiting an indolent natural history, while others progress more rapidly to become life-threatening. Existing multiphase contrast-enhanced imaging methods, such as computed tomography or magnetic resonance imaging, cannot definitively distinguish between benign and malignant solid tumors or identify histologic subtype, and early results of molecular imaging studies (positron emission tomography [PET]) in the evaluation of SRMs have not improved on these established modalities. Alternative molecular markers/agents recognizing aberrant cellular pathways of cellular oxidative metabolism, DNA synthesis, and tumor hypoxia tracers are currently under development and investigation for RCC assessment, but to date none are yet clinically applicable or available. In contrast, immuno-PET offers highly selective binding to cancer-specific antigens, and might identify radiographically recognizable and distinct molecular targets. A phase I proof-of-concept study first demonstrated the ability of immuno-PET to discriminate between clear-cell RCC (ccRCC) and non-ccRCC, utilizing a chimeric monoclonal antibody to carbonic anhydrase IX (cG250, girentuximab) labeled with 124I (124I-girentuximab PET); the study examined patients with renal masses who subsequently underwent standard surgical resection. A follow-up phase III multicenter trial confirmed that 124I-cG250-PET can accurately and noninvasively identify ccRCC with high sensitivity (86%), specificity (87%), and positive predictive value (95%). In the challenge to appropriately match treatment of an incidentally identified SRM to its biological potential, this highly accurate and histologically specific molecular imaging modality demonstrates the ability of imaging to provide clinically important preoperative diagnostic information, which can result in optimal and personalized therapy.
Introduction
The increased routine use of cross-sectional body imaging has resulted in a dramatic rise in the detection of renal tumors over the last 20 years, and in 2012 an estimated 64,000 men and women will be diagnosed with cancer of the kidney or renal pelvis, most commonly renal cell carcinoma (RCC).Citation1 This increased rate of RCC detection has resulted in a stage migration towards localized tumors less than 7 cm in size (stage I),Citation2,Citation3 most notably in elderly patients.Citation4 Furthermore, the malignant potential of small renal masses (SRMs) is heterogenous, ranging from those that are pathologically benign (20%)Citation5 to those that are anticipated to be aggressive RCC (20%–30%),Citation6,Citation7 and more than 20% of kidney cancer patients ultimately will die from their disease.Citation1 However, while the number of treated SRMs has increased to match this increased incidence and rate of kidney tumor detection, overall RCC mortality rates have remained stable. This finding suggests that early detection of RCC has minimally impacted the absolute number of aggressive lesions that have the potential to cause cancer-related death,Citation8 and has raised concerns that we may be overdiagnosing and overtreating indolent disease.Citation9
Surgical excision via radical nephrectomy (RN) or partial nephrectomy (PN) remains the gold-standard therapy for the stage I renal mass,Citation10,Citation11 and a recent analysis of the Surveillance, Epidemiology, and End Results Program registry data suggested that greater than 95% of patients with SRMs undergo some form of intervention.Citation12 While substantial effort has been directed towards increasing the utilization of nephron-sparing surgical techniques,Citation13,Citation14 the potential of overdiagnosis and overtreatment of clinically insignificant RCC remains of significant concern, particularly in the elderly, in whom the risks of intervention may outweigh any predicted survival benefit.Citation15 As a result, investigators have focused attention on defining the natural history of renal tumors managed expectantly,Citation16,Citation17 and close observation with serial reassessment, ie, active surveillance (AS), has become an accepted management strategy. Recently, AS has become incorporated into the current best-practice guideline for select elderly or comorbid patients who are not fit for immediate surgery.Citation10
The ability to match the level of treatment intensity to tumor biology correctly remains elusive. Multiphase contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) currently provides the best pre-operative assessment of a renal tumor, providing anatomic detail, information on bilateral baseline renal function, and clinical staging. While cross-sectional imaging accurately differentiates between solid and cystic lesions, it provides minimal biological information, and poorly predicts for a tumor’s malignant potential or clinical behavior. These existing imaging methods cannot definitively distinguish between benign and malignant solid lesions, the various RCC histologic phenotypes, or indolent versus aggressive tumors. Molecular imaging has the potential to characterize biological processes at the cellular and subcellular level in a noninvasive fashion, as well as provide the macroscopic detail that is obtained from conventional cross-sectional imaging techniques. Compared to 2-deoxy-2-[18F]fluoro-d-glucose positron emission tomography (18F-FDG-PET), which has been shown to have highly variable sensitivity in RCC diagnosis and staging,Citation18 antibody-based molecular imaging (immuno-PET), has demonstrated exciting potential to improve on the existing imaging standard of RCC assessment.Citation19 The most promising results have been reported utilizing 124I-cG250 (girentuximab), a chimeric monoclonal antibody that recognizes carbonic anhydrase (CA) IX,Citation20 which is overexpressed in 93%–97% of clear-cell RCC (ccRCC) tumors.Citation21–Citation23 In this review, we discuss the limitations of conventional imaging, the promise of 124I-cG250 immuno-PET to accurately distinguish ccRCC from non-ccRCC variants in the pretreatment setting, and the potential impact that improved pretreatment tumor characterization and RCC diagnostics may have on contemporary treatment algorithms for SRMs.
Preoperative nonextirpative assessment of malignant potential
Traditionally, contrast enhancement of a solid renal lesion has been considered indicative of malignant pathology. However, a recent systematic review of the literature demonstrated that approximately 15% of SRMs undergoing surgical resection (range 7%–33%) are pathologically benign.Citation24 As a result, a substantial proportion of patients are subject to unneeded risks of morbidity from routine surgeryCitation25,Citation26 and from the possible development of chronic kidney disease.Citation27 Under ideal circumstances, the preoperative tumor clinical assessment would adequately stratify patients into risk categories: patients with potentially dangerous but curable RCC warranting immediate intervention; patients at risk for progression who may benefit from neoadjuvant treatment strategies; and patients with benign tumors or indolent disease best suited for AS. Until this is possible, the predictive utility of nonextirpative management involving repeated radiographic assessments, clinical nomograms, and renal mass biopsy will be the focus of further investigation and refinement.
Considerable effort has been expended to identify additional radiographic characteristics associated with aggressive tumor biology and disease progression following treatment. On conventional CT or MRI, while contrast enhancement of a solid lesion is traditionally considered to indicate a malignant lesion until proven otherwise, recent large series have further demonstrated that increasing tumor size is associated with an increased likelihood of malignancy,Citation28,Citation29 high-grade disease,Citation28–Citation30 clear-cell histology,Citation29,Citation30 and presence of synchronous metastases.Citation31–Citation33 Unfortunately, early efforts to develop quantitative predictive nomograms for malignant pathology and tumor grade using radiographic features (tumor size) and clinical variables such as age, sex, smoking history, and symptomatic presentation have been largely unsuccessful and with limited clinical utility.Citation34,Citation35 However, there is also recent evidence suggesting that more detailed tumor anatomic characteristics, such as tumor location,Citation36,Citation37 may provide insight regarding malignant phenotype. Using the RENAL nephrometry score (NS),Citation38 a standardized, easily reproducible, and validated tumor anatomic classification system, Kutikov et al demonstrated NS can differentiate between high- and low-grade lesions, as well as predict tumor histology. By integrating NS with patient characteristics (age, sex), a clinical tool was developed that can predict for RCC histology and grade, and it has been externally validated as having accuracy rates rivaling those of percutaneous biopsy.Citation39,Citation40
Historically, preoperative determination of an enhancing renal mass pathology has been solely possible by percutaneous biopsy, which has traditionally been used sparingly to confirm a suspected non-RCC diagnosis, such as metastatic disease from another primary malignancy, renal abscess, or renal lymphoma.Citation10 As concerns regarding needle-tract tumor seeding have been largely relegated, the role of tumor biopsy has expanded, and it is now a consideration in most SRM treatment-decision algorithms.Citation41 In a systematic review, Lane et al reported biopsy accuracy rates > 80% in the contemporary era for the prediction of malignant versus benign histology.Citation42 Contemporary series utilizing larger-gauge biopsy needles and improved immunohistological techniques have exceeded these results, estimating biopsy to have accuracy rates of greater than 90%, and to have minimal risk of adverse events.Citation43 However, biopsy has recognized limitations, including increased rates of “ nondiagnostic” biopsies in small tumors,Citation44 as well as the inability to determine tumor grade in >50% of cases.Citation45,Citation46 Although beyond the scope of this review, the association between a number of molecular markers and malignant potential are currently under investigation,Citation47 which may ultimately provide the best means to match treatment to RCC phenotype. Until such markers exist, attention will remain focused on means of improving the quality of information yielded by cross-sectional imaging.
Cross-sectional imaging of the small renal mass
Pretreatment imaging provides important staging and anatomic information to the treating physician. Multiphase contrast-enhanced CT is currently recommended by the American Urological Association,Citation10 European Association of Urology,Citation48 National Comprehensive Cancer Network,Citation49 and American College of RadiologyCitation50 as the imaging modality of choice in the evaluation of renal lesions, with contrast enhancement (attenuation increase of at least 15–20 Hounsfield units from the corresponding noncontrast image) being the most important criterion for differentiating benign from malignant renal lesions. Adequately designed CT protocols should include precontrast and postcontrast imaging at multiple time points, including a nephrographic phase. More complex multiplanar reconstructions and 3-D volume-rendered images are not required in the routine evaluation, but can be helpful for surgical planning.Citation50 CT is highly sensitive even for small renal masses less than 2 cm, demonstrating increased detection rates compared to MRI due to higher spatial resolution. However, while diagnostic accuracy approaches 95% with emerging technologies such as dual-energy CT,Citation51 conventional CT imaging remains limited for differentiating between malignant and benign cystic lesions, and hypovascular lesions such as papillary RCC, which can commonly demonstrate minimal contrast enhancement.
For indeterminate CT results, pregnancy, or contraindication to the administration of intravenous contrast, MRI may be utilized, and can provide additional information regarding anatomic characteristics, local disease extension, and vena cava thrombus involvement. Although the traditional role of MRI has been to better define equivocal CT findings, evolving functional and advanced imaging techniques, including diffusion-weighted and perfusion-weighted imaging, are expanding the role of MRI in the primary evaluation of renal masses.Citation52 MRI can be superior to conventional CT at detecting perirenal fat or venous invasion, and there is emerging evidence to suggest that discrimination between malignant and benign disease and histologic subtypes may be possible with MRI based on degree of enhancement. In 109 renal lesions (64 patients), Taouli et al compared the diagnostic performance of contrast-enhanced MRI with diffusion-weighted MRI, concluding that apparent diffusion coefficient could be used to differentiate between malignant and benign tumors.Citation53 In a single-institution retrospective series of 112 patients undergoing dynamic contrast-enhanced MRI for renal tumors, Sun et al reported that signal-intensity changes on corticomedullary phase images were an effective parameter for preoperatively distinguishing papillary RCC from ccRCC.Citation54 While intriguing, these findings are preliminary, and further study is required to prospectively validate the role of these emerging MRI techniques in the diagnostic evaluation of SRMs.
Molecular imaging of renal tumors
In addition to providing macroscopic detail, molecular imaging has the potential to characterize biological processes at the cellular and subcellular level in a noninvasive fashion. The use of combined anatomic and functional imaging, such as with PET-CT, has received significant attention in the evaluation of presumed malignancy, particularly with RCC. Although PET-CT does not currently have an established role in the staging of RCC,Citation50 functional imaging provides information regarding tumor metabolism, and it has the potential to increase diagnostic accuracy and impact clinical decision-making for newly diagnosed renal lesions. Further, in the targeted therapy era, molecular imaging shows promise for predicting treatment response, as a greater understanding of underlying biological derangements can be expected to lead to recognition of markers that will be discernable on nuclear cross sectional or functional imaging.Citation55
18F-FDG-PET
The use of 18F-FDG to functionally image malignancies is based on the presumably altered glycolytic pathway in malignant cells. A glucose analog, 18F-FDG undergoes phosphorylation in metabolically active tumor cells to form 18F-FDG-6-phosphate, which cannot undergo glycolysis and becomes trapped in the cell in higher concentrations than normal tissue. The resulting elevated concentration of 18F-FDG in cells with abnormally high metabolism produces a higher signal relative to the background on PET. The images can be evaluated visually in a qualitative fashion, and semiquantitatively measured by standard uptake value.Citation56,Citation57 While there was initial enthusiasm for the utilization of 18F-FDG-PET to image RCC, there are significant limitations to its clinical utility.Citation18 In the case of most SRMs, the degree of 18F-FDG uptake and limited spatial resolution of contemporary clinical scanners makes it difficult to differentiate tumor from background uptake present in the normal kidney. Further, the normal excretion and accumulation of 18F-FDG in the urinary collecting system and variable levels of uptake that can occur in benign inflammatory and infectious conditions limit its diagnostic accuracy for larger renal lesions ().Citation55
Figure 1 Computed tomography (CT) appearance of small right posterior renal tumor (A1) and corresponding 18F-fluoro-d-glucose positron emission tomography (18F-FDG-PET) (A2) and fused PET low-dose noncontrast CT (A3) images demonstrating FDG uptake. Please note mild right renal uptake related to tracer excretion in the collecting system. In contrast, a 12.5 cm anterior right renal mass clearly visualized on contrast-enhanced CT (B1) that demonstrated minimal to no FDG uptake and was falsely considered benign on PET and fused images (B2 and B3).
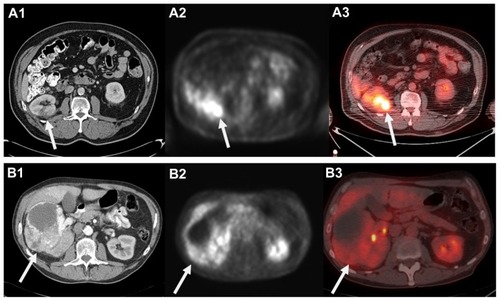
Lawrentschuk et al recently summarized the collective experience using 18F-FDG-PET as a primary imaging modality to diagnose, stage, or restage RCC. These series are predominantly comprised of a number of small retrospective institutional reports (n = 4–66) performed prior to the advent of combination PET-CT scanning. With 18F-FDG-PET alone, the sensitivity for RCC diagnosis and staging ranged from 32% to 100% and 47% to 75%, respectively, with false-negative rates as high as 68% reported.Citation18 In contrast, while sensitivity is still variable and its clinical application for localized RCC remains unproven, the ability of PET to diagnose regional RCC recurrence or bony, nodal, and visceral metastatic RCC appears to be more specific than with CT alone.Citation58 As a result, there was initial enthusiasm for monitoring disease response to systemic targeted therapy with 18F-FDG-PET.Citation59 However, despite improved survival outcomes with targeted therapy, treatment often results in disease stabilization and tumor necrosis rather than actual tumor regression, which makes response assessment by axial imaging criteria challenging. Citation60,Citation61 While molecular imaging with 18F-FDG-PET is still being examined as a determinant of treatment response,Citation62–Citation64 low sensitivity rates even in the setting of advanced disease indicate that it is unlikely that 18F-FDG-PET will influence the selection of candidates with metastatic RCC for up-front cytoreductive nephrectomy or consolidative surgical therapy outside of ongoing clinical trials.Citation65
Emerging molecular agents
To overcome the limitations of 18F-FDG-PET, alternative molecular agents associated with aberrant cellular pathways, such as cellular oxidative metabolism, DNA synthesis, and tumor hypoxia tracers, are currently under development and investigation in RCC. Focus has intensified on the use of radiolabeled amino acids, such as 11C-acetate and fluorine- 18 fluorodeoxyglucose, to exploit their increased role in malignant cell metabolism, survival, and proliferation, but clinical utility of these agents are similarly limited by showing low sensitivity with localized disease and short tracer half-lives.Citation66,Citation67 The DNA analog 18F-fluorothymidine, which is phosphorylated by thymidine kinase in the salvage pathway of DNA synthesis, and the nitromidazole 18F-fluoromisonidazole, whose metabolism and tissue retention are dependent on tissue oxygenation, are also currently in the early phases of investigation in PET imaging in patients with RCC; these also may be more promising in the evaluation of metastatic RCC and in measuring response to systemic therapy.Citation68,Citation69
Antibody-based molecular imaging of renal tumors
The discovery of specific tumor targets involved in proliferation, differentiation, angiogenesis, immune recognition, and metastasis over the past decade has facilitated the development of targeted therapeutic agents including monoclonal antibodies and tyrosine kinase inhibitors (TKIs). PET imaging applying radiolabeled monoclonal antibodies and TKIs has potential to better define the biology and efficacy of targeted agents in individual patients, improve the efficiency of drug development, and identify the patients having the best chance to benefit from specific therapy. The ability of PET to quantitatively image the distribution of the radiolabeled agent makes this technique a valuable tool to assess target expression, and accumulation in both tumor lesions and normal tissues in the pretreatment setting followed by imaging during therapy affords the opportunity to demonstrate that tumor targeting is successful.Citation70 In addition to monitoring of response to therapy, molecular imaging also demonstrates potential to characterize in vivo biology for diagnostic purposes, with potential to characterize tumor aggressiveness and optimally guide treatment planning. Citation55 Currently, a growing number of monoclonal antibodies (U36 [anti-CD44vG], DN30 [anti-cMet], G250 [anti-CA IX], L19-SIP [anti-fibronectin], R1507 [anti-type 1 insulin-like growth factor receptor], J591 and 7E11 [anti-prostate-specific membrane antigen], cetuximab [anti-epidermal growth factor receptor], inbritumumab tiuxetan [anti-CD20], rituximab [anti-CD20], bevacizumab [anti-vascular endothelial growth factor], and trastuzumab [anti-HER2]) have been applied as labeled PET tracers and are currently under investigation in a wide variety of malignancies.Citation70 While a detailed description is beyond the scope of this review, the radiolabeling of small molecules is more challenging and requires a more agent-specific labeling strategy. Although current investigations have been limited to preclinical animal studies, interest in this approach remains high, and a number of protocols have been described that can radiolabel US Food and Drug Administration (FDA)-approved anticancer TKIs.Citation70
To best enable visualization with a PET camera, the agent should be labeled with an inert positron emitter to avoid altering binding properties or pharmacokinetic characteristics. Further, the half-life of the positron emitter should match the agent’s body-residence time as closely as possible. This can range from a few hours for small-molecule agents such as TKIs to several days for monoclonal antibodies.Citation70 The choice of positron emitter is critical to optimize the visualization and quantification of the target of interest, as unbound tracer and other radiolabeled species generated in vivo will increase background activity, reduce the target-background contrast, and decrease overall imaging sensitivity.Citation71 The early development of PET imaging has relied on the use of short-lived nuclides, including 15O, 13N, 11C, and 18F, all with half-lives of a few hours or less. However, recent immuno-PET investigations have utilized antibodies linked to less specific positron-emitting tracers such as iodine-124 (124I) or zirconium-89 (89Zr), which have longer half-lives (days as opposed to hours) that more closely match the pharmacokinetics of intact antibodies than conventional PET radiotracers.Citation18 Disadvantages of the use of monoclonal antibodies for confirmation of target expression include a long body-residence time (which can range from days to weeks) and large molecular size, which can limit tracer diffusion and uptake.Citation70 Evaluation of antibody-based PET probes having faster pharmacokinetics, such as monoclonal antibody fragments and bioengineered proteins, is currently under investigation.Citation72
G250 and carbonic anhydrase IX
First reported in 1986, G250 is a murine monoclonal antibody (mAbG250) developed by immunization of nude mice with human RCC homogenates.Citation73 Subsequently, on isolation and sequential analysis, the G250 antigen was shown to be homologous to the carbonic anhydrase isoenzyme 9 MN/CA IX antigen.Citation74 Early studies investigating the effects of direct mAbG250 treatment in mice with RCC xenografts,Citation75 as well as after vaccination with internal image anti-idiotype antibodies against mAbG250,Citation76,Citation77 resulted in significant antitumor effects, demonstrating promise for CA IX as a therapeutic target. Since administration of the mAbG250 resulted in the formation of human antimouse antibodies,Citation78 a chimeric (cAbG250) immunoglobulin G1 was constructed to reduce its immunogenicity and facilitate repeated injections, with minimal adverse events demonstrated in a dose-escalation trial.Citation79
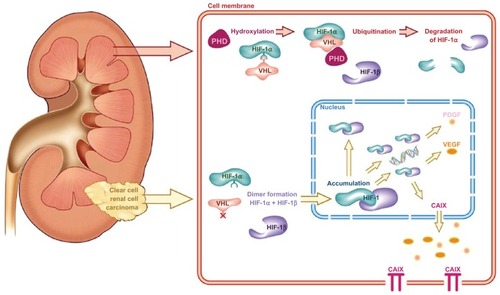
CA IX is a cytosolic and transmembrane enzyme that is active in the adaptation of tumors to hypoxic conditions by regulating the pH of the intracellular and extracellular compartment; CA IX catalyzes the reversible conversion of carbon dioxide and water to carbonic acid. Mediated via hypoxia-inducible factor (HIF)-1α, CA IX overexpression is thought to contribute to an acidic tumor microenvironment to promote cancer progression and metastasis.Citation80,Citation81 Under normal oxygen conditions, HIF-1α is hydroxylated via binding to the Von Hippel–Lindau protein (pVHL), resulting in its ubiquination and controlled degradation. Under hypoxic conditions, pVHL–HIF-1α binding is inhibited, leading to accumulation of HIF-1α and formation of the HIF-1α–HIF-1β complex (). This results in the downstream transcription of a number of hypoxia-inducible factors that promote angiogenesis, including CA IX.Citation82 A mutational loss of pVHL, commonly seen in RCC, results in a cellular microenvironment that mimics hypoxic conditions, which explains the routinely high expression of the CA IX antigen in ccRCC.Citation81 Studies confirm that CA IX expression in normal tissue is minimal, limited to the gastrointestinal tract, gallbladder, and pancreatic ducts, while it is expressed in a number of malignant conditions, including gynecologic, gastrointestinal, breast, skin, and genitourinary cancers.Citation83,Citation84
Figure 2 The Von Hippel–Lindau/hypoxia-inducible factor oxygen-sensing pathway and its relevance in clear-cell renal cell carcinoma.
Abbreviations: PHD, prolyl 4-hydroxylase domain; HIF, hypoxia-inducible factor; VHL, Von Hippel–Lindau; PDGF, platelet-derived growth factor; VEGF, vascular endothelial growth factor; CAIX, carbonic anhydrase IX.
CA IX expression has never been reported in noncancerous renal tissue,Citation85 and a review of the literature suggests overexpression of CA IX in >95% of ccRCC tumors.Citation81 Further, CA IX is rarely expressed in non-ccRCC variants, including papillary, chromophobe, and oncocytic tumors.Citation21 The prognostic value of CA IX expression in ccRCC has been extensively investigated, and although some conflicting evidence exists,Citation23 increased CA IX expression is associated with worse overall survivalCitation21,Citation86,Citation87 and response to interleukin- 2 immunotherapy.Citation22,Citation88 Based on this data, CA IX tumor expression has been incorporated into clinical nomograms to predict disease-free survival for patients with localized and metastatic RCC, albeit with mixed results.Citation89,Citation90 Although further work remains to better define the relationship between pVHL, HIF-1α, and CA IX expression,Citation80 the highly specific overexpression of CA IX in ccRCC makes it an excellent candidate for further evaluation as a prognostic biomarker, therapeutic target, and molecular imaging agent.
RCC-directed imaging with labeled G250
Due to its high affinity for the CA IX antigen (Ka = 4 × 109 M−1), which is not expressed in normal kidney, the utility of G250 for RCC-directed imaging has been investigated in a number of tumor-targeting studies (). In a phase I dose-escalation study of intravenously administered 131I-labeled mAbG250 in 16 presurgical patients with RCC, Oosterwijk et al reported that definitive tumor images were observed in twelve patients with G250-positive tumors and one of three patients with G250-negative tumors using single-photon radioimmunoscintigraphic (RIS) techniques.Citation91 Overall, 90% of primary tumors and metastatic RCC lesions were visualized. A subsequent phase I study investigated a single intravenous administration of 131I-cAbG250 at five escalating dose levels, ranging from 2 to 50 mg, in 16 patients undergoing surgical treatment. This study also reported clear visualization of all primary tumors and documented metastatic lesions,Citation79 and although highly heterogeneous, focal 131I-cAbG250 uptake was as high as 0.52% ID/g in primary tumors, with minimal uptake noted in nontumor tissues.
Table 1 Renal cell carcinoma-directed imaging using radiolabeled G250
In a phase I/II trial designed to determine the maximum tolerated dose and therapeutic potential of 131I-mAbG250, Divgi et al reported visualization of all lesions ≥ 2 cm in diameter in 33 patients with known metastatic RCC, while lesions < 2 cm were poorly visualized. All patients in this trial developed human mouse antibodies after 4 weeks of therapy, precluding the possibility of repeat treatment.Citation78 To compare the efficacy of 131I-cAbG250 RIS and 18F-FDG-PET in detection of metastatic disease, Brouwers et al compared the two nuclear imaging techniques with conventional cross-sectional imaging in 20 patients with metastatic RCC. Of the identified 112 metastatic lesions, 18F-FDG-PET detected 69%, while 131I-cAbG250-RIS detected only 30%.Citation92 In contrast to prior studies, RIS was the least efficient in visualization of metastatic disease sites, and the authors concluded that 131I-cAbG250-RIS may be more appropriate to identify patients who may benefit from targeted radiotherapy rather than as an assessment of metastatic disease extent. However, in subsequent trials, while not all documented sites showed evidence of 131I-cAbG250 uptake, new metastatic lesions were often found that were unrecognized on CT or MRI, indicating a potential role for patients with suspicious but indeterminate lesions that are apparent on conventional imaging.Citation93–Citation95 It is important to place into context that the objective of measuring tumor targeting with G250 in these studies was not solely for diagnostic purposes, but also as a means to guide the development of G250 targeting for therapeutic purposes. Currently, unlabeled and labeled (using a number of different metallic radionuclides) G250 alone, as well as in unfractionated or fractionated form in combination with interleukin-2, interferon-γ, interferon-α-2a, dendritic cells, and radiotherapy, are currently under investigation for treatment of RCC.Citation78,Citation93,Citation94,Citation96–Citation104 With the exception of an early trial showing evidence of transient liver dysfunction,Citation78 these trials have shown minimal toxicity and low levels of cross-reactivity of G250 with normal tissues, suggesting its potential as a therapeutic agent in the adjuvant setting.Citation81
Diagnostic G250 immuno-PET for the SRM
G250 demonstrates a highly accurate ability to target CA IX-expressing ccRCC. While evidence to suggest a therapeutic role for G250, either alone or as combination therapy, is still accumulating, this ability to discriminate between SRM histologic subtypes in a noninvasive fashion has significant clinical implications and is currently being evaluated in phase III trials. Contemporary PET provides specificity and sensitivity not achieved using other imaging techniques, enabling in vivo molecular imaging of suspected malignancy and comparison with normal tissues. However, the historic limitation of PET and other nuclear imaging techniques as stand-alone imaging applications has been poor anatomic resolution, often making exact localization of a lesion difficult. The combined application of PET and cross-sectional imaging has markedly improved both the localization of lesions and the diagnostic accuracy of either modality in patients with a number of solid tumors (including prostate, pancreas, liver, breast, lung, thyroid, among others), when compared as stand-alone imaging studies.Citation105
Early animal models demonstrated that the cG250 antibody can be stably labeled with a number of positron emitters, such as 89Zr and 124I, without causing loss of antigen affinity. Citation106 With a 4-day half-life, 124I facilitates the assessment of long-term antibody pharmacokinetics,Citation107 and is currently approved by the FDA for use in clinical trials. In an open- label, phase I proof- of-concept study designed to evaluate the ability to distinguish between ccRCC and non-ccRCC variants, 26 patients with renal masses scheduled for surgical resection (PN or RN with or without lymph-node dissection) were given a single 185 MBq/10 mg (5 mCi/10 mg) intravenous dose of 124I-cG250 in 50 mL of 5% human serum albumin over 20 minutes, 1 week prior to surgery. For each patient, PET and CT images were obtained preoperatively, on the day before or day of surgery. One patient received immunologically inactive (immunoreactivity < 25%) 124I-cG250 and was excluded from analysis. Of the 16 patients with pathologically confirmed ccRCC, 15 had a positive PET (the 16th had necrotic tumor), for a positive predictive value of 100%, sensitivity of 94%, and specificity of 100%.Citation20 Patients with non-ccRCC histologies demonstrated an absence of antibody accumulation in their tumors, and there was no evidence of immediate or delayed toxicity following 124I-cG250 administration.
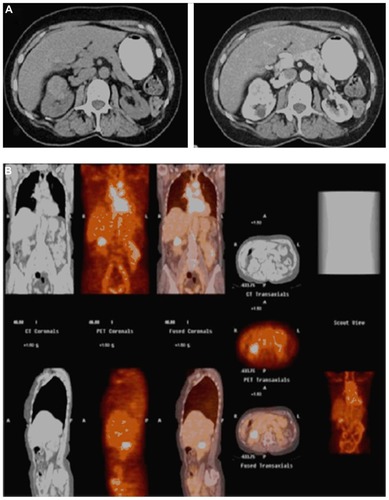
Based on these encouraging findings, a comprehensive, multicenter, open-label phase III comparative study of PET-CT versus diagnostic CT for the detection of ccRCC in the pretreatment setting was undertaken (Redect; NCT00606632). This trial recently completed enrollment, and the initial results were disseminated at the American Urological Association meeting in 2010.Citation108 A total of 226 patients were enrolled, 204 of which underwent 5 mCi/13.7 mg 124I-girentuximab intravenous infusion, 202 underwent surgical resection, and 195 ultimately met final inclusion criteria for data analysis. PET-CT images were obtained 2–6 days following antibody administration and prior to surgical therapy. Multiphase contrast-enhanced abdominal CTs were performed for comparison within 48 hours of 124I-girentuximab PET-CT. 124I-girentuximab PET-CT was able to discriminate ccRCC from non-ccRCC with much higher sensitivity (86%) and specificity (87%) than conventional CT (sensitivity 76%, specificity 47%) and had no associated serious adverse events.Citation108 Moreover, 124I-girentuximab PET-CT exhibited a 95% positive predictive value, indicating that a positive test was highly indicative of ccRCC phenotype. These findings, which currently are in press, have led to considerable enthusiasm regarding the potential of a first-in-class histology-specific diagnostic imaging modality (). In comparison to previous efforts demonstrating only questionable or theoretical utility with molecular imaging to impact treatment, the Redect trial findings demonstrate that immuno-PET results can provide important pretreatment diagnostic information that will directly impact a patient’s subsequent treatment. Future additional potential 124I- girentuximab PET-CT applications include use in the staging of metastatic disease to measure treatment response, confirm ablation success, and to improve on the appropriate selection of candidates for AS protocols.Citation55
Figure 3 A 71-year-old female who presented with an incidentally diagnosed, enhancing 4 cm right renal mass (A), which demonstrated positive uptake on 124I-girentuximab (G250) positron emission tomography/computed tomography (B); pathology following open partial nephrectomy revealed pathologic stage T1b Nx Mx clear cell renal cell carcinoma.
Clinical implications
Localized RCC is a surgically curable disease, with 5-year disease-specific survival rates in excess of 95% for patients having localized tumors treated by RN or PN.Citation109 However, there is increasing evidence to suggest that we are overdiagnosing and overtreating many incidentally detected SRMs, and that a substantial proportion of these likely represent indolent and clinically insignificant tumors. Aggressive surgical resection has remained the gold standard for stage I tumors, in part to ensure treatment of the 20%–30% of small tumors that are estimated to have an aggressive malignant potential. However, reflexive treatment of all SRMs has likely resulted in the overtreatment of many tumors that are not life-threatening, of which at least 15%–20% are pathologically benign.Citation24 This understanding has led to recent interest to characterize the natural history of SRMs that are managed expectantly,Citation16,Citation17 as well as to develop tools that might predict a renal tumor’s malignant potential based on pretreatment patient and imaging characteristics.
To date, currently available molecular imaging has not made a significant impact on the management of localized RCC, largely due to poor sensitivity rates for diagnosing and staging small tumors, compared to existing conventional imaging techniques.Citation18 While there is increased promise for the use of 18F-FDG-PET in the evaluation and monitoring of patients with advanced RCC, it is not recommended in the routine evaluation of the patient presenting with a localized SRM. In contrast, immuno-PET with cG250 offers the ability to discriminate between ccRCC and non-ccRCC phenotypes in the pretreatment setting, with accuracy rates rivaling if not surpassing contemporary percutaneous biopsy series.Citation20,Citation108 Limitations of this emerging imaging technology include greater radiation exposure for the patient, technicians, and family members due to the prolonged half-life of I-124, as well as longer imaging duration compared to more traditional imaging techniques (124I-girentuximab-PET-CT was performed a mean of 5 ± 2 days following tracer injection in the Redect trial).Citation108 Further, concerns regarding how CA IX expression in normal tissues will impact the diagnostic efficacy with 124I-girentuximab PET-CT imagingCitation23 have been largely dismissed. Studies have revealed that in non-ccRCC normal tissues and cell lines, CA IX expression is less homogenous and has lower antigen density per cell when compared to ccRCC tumors.Citation73 This is likely related to functional loss of the VHL gene resulting in CA IX overexpression in ccRCC, in comparison to a locoregional hypoxia mechanism in non-ccRCC tumors and normal tissues.Citation110 The challenge in implementing this novel clinical tool is determining the situation where the result is of maximum benefit. While ccRCC has been shown to have less favorable outcomes compared to papillary and chromophobe variants,Citation111 current guidelines recommend PN for all lesions when possible in appropriate surgical candidates, regardless of tumor histology or grade.Citation10,Citation48 Further, up-front knowledge of histologic type is unlikely to impact treatment decisions in young healthy patients, for whom even a small possibility of metastatic potential would warrant surgical resection, or in elderly or comorbid patients that are not fit to proceed with surgery.
For patients with localized SRMs, immuno-PET is expected to be most useful for patients with poor preoperative renal function, a solitary kidney with a renal mass, bilateral/multifocal disease, or complex cystic disease. Knowledge of histologic type may encourage a nephron-sparing surgical approach compared to radical nephrectomy for complex tumors. Furthermore, the ability to noninvasively determine SRM histology may support a plan of deferring immediate treatment if a non-ccRCC is suggested. While ccRCC is among the most aggressive RCC subtypes and a positive cG250 immuno-PET result might confirm the need for resection, it is important to consider that some immuno-PET-negative patients may still benefit from definitive treatment. Percutaneous biopsy will still play a definitive role in differentiating between benign renal tumors and other more aggressive, non-ccRCC phenotypes, such as papillary type II RCC. For patients presenting with advanced or metastatic RCC, cG250 immuno-PET may play a role in detection of metastases, selecting patients for systemic therapy prior to cytoreductive surgery, guiding selection and optimal duration of systemic therapy, and monitoring treatment response.
Conclusion
Molecular imaging of the SRM provides the potential to characterize biological processes at the cellular level in a noninvasive fashion. Although the poor diagnostic and staging sensitivity of 18F-FDG-PET has dampened enthusiasm for its use in localized RCC, its utility for assessing metastatic tumor burden and efficacy of targeted therapy in advanced disease is currently under investigation. More recently, contemporary studies investigating novel radiopharmacologic and immune- specific agents have demonstrated encouraging potential to improve molecular imaging in RCC. CA IX, which is highly and specifically expressed in ccRCC, has been extensively investigated as both a prognostic marker and therapeutic target. Early clinical experiences demonstrated that cG250 (girentuximab), a monoclonal antibody to CA IX, avidly targets ccRCC, and this affinity has diagnostic and therapeutic applications. Two recent trials have demonstrated that immuno-PET using cG250 (girentuximab) can accurately and reliably discriminate between ccRCC and non-ccRCC tumor phenotypes with higher sensitivity and specificity than current modalities. In contrast to other molecular imaging techniques, cG250 immuno-PET demonstrates the potential to consistently provide critical information that can directly influence the management of patients presenting with localized renal tumors.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRNaishadhamDJemalACancer statistics, 2012CA Cancer J Clin2012621102922237781
- KaneCJMallinKRitcheyJCooperbergMRCarrollPRRenal cell cancer stage migration: analysis of the National Cancer Data BaseCancer20081131788318491376
- CooperbergMRMallinKRitcheyJVillaltaJDCarrollPRKaneCJDecreasing size at diagnosis of stage 1 renal cell carcinoma: analysis from the National Cancer Data Base, 1993 to 2004J Urol200817962131213518423754
- JaysonMSandersHIncreased incidence of serendipitously discovered renal cell carcinomaUrology19985122032059495698
- KutikovAFossettLKRamchandaniPIncidence of benign pathologic findings at partial nephrectomy for solitary renal mass presumed to be renal cell carcinoma on preoperative imagingUrology200668473774017070344
- CrispenPLBoorjianSALohseCMOutcomes following partial nephrectomy by tumor sizeJ Urol200818051912191718801543
- RemziMOzsoyMKlinglerHCAre small renal tumors harmless? Analysis of histopathological features according to tumors 4 cm or less in diameterJ Urol2006176389689916890647
- HollingsworthJMMillerDCDaignaultSHollenbeckBKRising incidence of small renal masses: a need to reassess treatment effectJ Natl Cancer Inst200698181331133416985252
- WelchHGBlackWCOverdiagnosis in cancerJ Natl Cancer Inst2010102960561320413742
- CampbellSCNovickACBelldegrunAGuideline for management of the clinical T1 renal massJ Urol200918241271127919683266
- Van PoppelHDa PozzoLAlbrechtWA prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinomaEur Urol201159454355221186077
- YangGVillaltaJDMengMVWhitsonJMEvolving practice patterns for the management of small renal masses in the USABJU Int201211081156116122372984
- HollenbeckBKTaubDAMillerDCDunnRLWeiJTNational utilization trends of partial nephrectomy for renal cell carcinoma: a case of underutilization?Urology200667225425916442601
- TanHJNortonECYeZHafezKSGoreJLMillerDCLong-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancerJAMA2012307151629163522511691
- SmaldoneMCEglestonBUzzoRDoes partial nephrectomy result in a durable overall survival benefit in the elderly?J Urol20121874e449
- KunkleDAEglestonBLUzzoRGExcise, ablate or observe: the small renal mass dilemma – a meta-analysis and reviewJ Urol2008179412271233 discussion 1233–123418280512
- SmaldoneMCKutikovAEglestonBLSmall renal masses progressing to metastases under active surveillance: a systematic review and pooled analysisCancer20121184997100621766302
- LawrentschukNDavisIDBoltonDMScottAMFunctional imaging of renal cell carcinomaNat Rev Urol20107525826620448659
- LawrentschukNDavisIDBoltonDMScottAMPositron emission tomography (PET), immuno-PET and radioimmunotherapy in renal cell carcinoma: a developing diagnostic and therapeutic relationshipBJU Int200697591692216643471
- DivgiCRPandit-TaskarNJungbluthAAPreoperative characterisation of clear-cell renal carcinoma using iodine-124-labelled antibody chimeric G250 (124I-cG250) and PET in patients with renal masses: a phase I trialLancet Oncol20078430431017395103
- BuiMHSeligsonDHanKRCarbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapyClin Cancer Res20039280281112576453
- AtkinsMReganMMcDermottDCarbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancerClin Cancer Res200511103714372115897568
- LeibovichBCSheininYLohseCMCarbonic anhydrase IX is not an independent predictor of outcome for patients with clear cell renal cell carcinomaJ Clin Oncol200725304757476417947723
- RussoPUzzoRGLowranceWIncidence of benign versus malignant renal tumors in selected studiesJ Clin Oncol201230Suppl 535722203766
- GillISKavoussiLRLaneBRComparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumorsJ Urol20071781414617574056
- SimhanJSmaldoneMCTsaiKJObjective measures of renal mass anatomic complexity predict rates of major complications following partial nephrectomyEur Urol201160472473021621910
- HuangWCLeveyASSerioAMChronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort studyLancet Oncol20067973574016945768
- FrankIBluteMLChevilleJCLohseCMWeaverALZinckeHSolid renal tumors: an analysis of pathological features related to tumor sizeJ Urol20031706 Pt 12217222014634382
- ThompsonRHKurtaJMKaagMTumor size is associated with malignant potential in renal cell carcinoma casesJ Urol200918152033203619286217
- RothmanJEglestonBWongYNIffrigKLebovitchSUzzoRGHistopathological characteristics of localized renal cell carcinoma correlate with tumor size: a SEER analysisJ Urol200918112933 discussion 33–3419012902
- KunkleDACrispenPLLiTTumor size predicts synchronous metastatic renal cell carcinoma: implications for surveillance of small renal massesJ Urol2007177516921696 discussion 169717437785
- NguyenMMGillISEffect of renal cancer size on the prevalence of metastasis at diagnosis and mortalityJ Urol2009181310201027 discussion 102719150563
- ThompsonRHHillJRBabayevYMetastatic renal cell carcinoma risk according to tumor sizeJ Urol20091821414519450840
- JeldresCSunMLibermanDCan renal mass biopsy assessment of tumor grade be safely substituted for by a predictive model?J Urol200918262585258919836799
- LaneBRBabineauDKattanMWA preoperative prognostic nomogram for solid enhancing renal tumors 7 cm or less amenable to partial nephrectomyJ Urol2007178242943417561141
- SchachterLRBachAMSnyderMEKattanMWRussoPThe impact of tumour location on the histological subtype of renal cortical tumoursBJU Int2006981636616831144
- VenkateshRWeldKAmesCDLaparoscopic partial nephrectomy for renal masses: effect of tumor locationUrology200667611691174 discussion 117416765174
- KutikovAUzzoRGThe RENAL nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depthJ Urol2009182384485319616235
- KutikovASmaldoneMCEglestonBLAnatomic features of enhancing renal masses predict malignant and high-grade pathology: a preoperative nomogram using the RENAL Nephrometry scoreEur Urol201160224124821458155
- WangHKZhuYYaoXDExternal validation of a nomogram using RENAL nephrometry score to predict high grade renal cell carcinomaJ Urol201218751555156022425078
- VolpeAFinelliAGillISRationale for percutaneous biopsy and histologic characterisation of renal tumoursEur Urol201262349150422633318
- LaneBRSamplaskiMKHertsBRZhouMNovickACCampbellSCRenal mass biopsy – a renaissance?J Urol20081791202717997455
- WangRWolfJSJrWoodDPJrHigginsEJHafezKSAccuracy of percutaneous core biopsy in management of small renal massesUrology2009733586590 discussion 590–59119118884
- LechevallierEAndreMBarriolDFine-needle percutaneous biopsy of renal masses with helical CT guidanceRadiology2000216250651010924578
- BlumenfeldAJGuruKFuchsGJKimHLPercutaneous biopsy of renal cell carcinoma underestimates nuclear gradeUrology201076361061320163843
- LeveridgeMJFinelliAKachuraJROutcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsyEur Urol201160357858421704449
- PalSKKortylewskiMYuHFiglinRABreaking through a plateau in renal cell carcinoma therapeutics: development and incorporation of biomarkersMol Cancer Ther20109123115312521078774
- LjungbergBCowanNCHanburyDCEAU guidelines on renal cell carcinoma: the 2010 updateEur Urol201058339840620633979
- MotzerRJAgarwalNBeardCNCCN clinical practice guidelines in oncology: kidney cancerJ Natl Compr Canc Netw20097661863019555584
- VikramRCaslinoDDRemerEMACR appropriateness criteria: renal cell carcinoma staging Available from: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/RenalCellCarcinomaStaging.pdf.1995 Last reviewed 2011Accessed October 23, 2012
- GraserABeckerCRStaehlerMSingle-phase dual-energy CT allows for characterization of renal masses as benign or malignantInvest Radiol201045739940520498609
- GiletAGKangSKKimDChandaranaHAdvanced renal mass imaging: diffusion and perfusion MRICurr Urol Rep2012131939822081252
- TaouliBThakurRKMannelliLRenal lesions: characterization with diffusion-weighted imaging versus contrast-enhanced MR imagingRadiology2009251239840719276322
- SunMRNgoLGenegaEMRenal cell carcinoma: dynamic contrast-enhanced MR imaging for differentiation of tumor subtypes – correlation with pathologic findingsRadiology2009250379380219244046
- SmaldoneMCChenDYUzzoRGYuMMolecular imaging of the small renal massUrol Oncol201129658959222078404
- WeberWAGrosuALCzerninJTechnology Insight: advances in molecular imaging and an appraisal of PET/CT scanningNat Clin Pract Oncol20085316017018253106
- HicksRJWareRELauEWPET/CT: will it change the way that we use CT in cancer imaging?Cancer Imaging20066S52S6217114079
- AideNCappeleOBottetPEfficiency of [(18)F]FDG PET in characterising renal cancer and detecting distant metastases: a comparison with CTEur J Nucl Med Mol Imaging20033091236124512845486
- MotzerRJBukowskiRMTargeted therapy for metastatic renal cell carcinomaJ Clin Oncol200624355601560817158546
- EisenhauerEATherassePBogaertsJNew response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)Eur J Cancer200945222824719097774
- WahlRLJaceneHKasamonYLodgeMAFrom RECIST to PERCIST: evolving considerations for PET response criteria in solid tumorsJ Nucl Med200950Suppl 1122S150S19403881
- VercellinoLBousquetGBailletG18F-FDG PET/CT imaging for an early assessment of response to sunitinib in metastatic renal carcinoma: preliminary studyCancer Biother Radiopharm200924113714419243256
- UenoDYaoMTateishiUEarly assessment by FDG-PET/CT of patients with advanced renal cell carcinoma treated with tyrosine kinase inhibitors is predictive of disease courseBMC Cancer201212116222551397
- KayaniIAvrilNBomanjiJSequential FDG-PET/CT as a biomarker of response to Sunitinib in metastatic clear cell renal cancerClin Cancer Res201117186021602821742806
- HarrisonMRGeorgeDJBetter late than early: FDG-PET imaging in metastatic renal cell carcinomaClin Cancer Res201117185841584321926167
- OyamaNOkazawaHKusukawaN11C-Acetate PET imaging for renal cell carcinomaEur J Nucl Med Mol Imaging200936342242719018529
- OzülkerTOzülkerFOzbekEOzpaçaciTA prospective diagnostic accuracy study of F-18 fluorodeoxyglucose-positron emission tomography/computed tomography in the evaluation of indeterminate renal massesNucl Med Commun201132426527221301376
- LawrentschukNPoonAMScottAMFluorine-18 fluorothymidine: a new positron emission radioisotope for renal tumorsClin Nucl Med2006311278878917117073
- LawrentschukNPoonAMFooSSAssessing regional hypoxia in human renal tumours using 18F-fluoromisonidazole positron emission tomographyBJU Int200596454054616104907
- van DongenGAPootAJVugtsDJPET imaging with radiolabeled antibodies and tyrosine kinase inhibitors: immuno-PET and TKI-PETTumour Biol201233360761522270450
- TolmachevVStone-ElanderSRadiolabelled proteins for positron emission tomography: pros and cons of labelling methodsBiochim Biophys Acta20101800548751020153401
- OlafsenTWuAMAntibody vectors for imagingSemin Nucl Med201040316718120350626
- OosterwijkERuiterDJHoedemaekerPJMonoclonal antibody G 250 recognizes a determinant present in renal-cell carcinoma and absent from normal kidneyInt J Cancer19863844894942428759
- OosterwijkEDe WeijertMVan BokhovenABrakenhoffRHPeelenWPDebruyneFMJMolecular characterization of the renal cell carcinoma-associated antigen G250Proc Amer Assoc Cancer Res199637461
- van DijkJUemuraHBeniersAJTherapeutic effects of monoclonal antibody G250, interferons and tumor necrosis factor, in mice with renal-cell carcinoma xenograftsInt J Cancer19945622622688314310
- UemuraHBeniersAJOkajimaEDebruyneFMOosterwijkEVaccination with anti-idiotype antibodies mimicking a renal cell carcinoma-associated antigen induces tumor immunityInt J Cancer19945845555618056452
- UemuraHOkajimaEDebruyneFMOosterwijkEInternal image anti-idiotype antibodies related to renal-cell carcinoma-associated antigen G250Int J Cancer19945646096147509324
- DivgiCRBanderNHScottAMPhase I/II radioimmunotherapy trial with iodine-131-labeled monoclonal antibody G250 in metastatic renal cell carcinomaClin Cancer Res1998411272927399829736
- SteffensMGBoermanOCOosterwijk-WakkaJCTargeting of renal cell carcinoma with iodine-131-labeled chimeric monoclonal antibody G250J Clin Oncol1997154152915379193349
- LamJSPantuckAJBelldegrunASFiglinRAG250: a carbonic anhydrase IX monoclonal antibodyCurr Oncol Rep20057210911515717944
- StillebroerABMuldersPFBoermanOCOyenWJOosterwijkECarbonic anhydrase IX in renal cell carcinoma: implications for prognosis, diagnosis, and therapyEur Urol2010581758320359812
- SmaldoneMCMaranchieJKClinical implications of hypoxia inducible factor in renal cell carcinomaUrol Oncol200927323824519414111
- IvanovSLiaoSYIvanovaAExpression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancerAm J Pathol2001158390591911238039
- PotterCHarrisALHypoxia inducible carbonic anhydrase IX, marker of tumour hypoxia, survival pathway and therapy targetCell Cycle20043216416714712082
- TostainJLiGGentil-PerretAGiganteMCarbonic anhydrase 9 in clear cell renal cell carcinoma: a marker for diagnosis, prognosis and treatmentEur J Cancer201046183141314820709527
- SandlundJOosterwijkEGrankvistKOosterwijk-WakkaJLjungbergBRasmusonTPrognostic impact of carbonic anhydrase IX expression in human renal cell carcinomaBJU Int2007100355656017608827
- PatardJJFergelotPKarakiewiczPILow CAIX expression and absence of VHL gene mutation are associated with tumor aggressiveness and poor survival of clear cell renal cell carcinomaInt J Cancer2008123239540018464292
- de MartinoMKlatteTSeligsonDBCA9 gene: single nucleotide polymorphism predicts metastatic renal cell carcinoma prognosisJ Urol2009182272873419539328
- KimHLSeligsonDLiuXUsing tumor markers to predict the survival of patients with metastatic renal cell carcinomaJ Urol200517351496150115821467
- KimHLSeligsonDLiuXUsing protein expressions to predict survival in clear cell renal carcinomaClin Cancer Res200410165464547115328185
- OosterwijkEBanderNHDivgiCRAntibody localization in human renal cell carcinoma: a phase I study of monoclonal antibody G250J Clin Oncol19931147387508478666
- BrouwersAHDorrULangO131 I-cG250 monoclonal antibody immunoscintigraphy versus [18 F]FDG-PET imaging in patients with metastatic renal cell carcinoma: a comparative studyNucl Med Commun200223322923611891480
- BrouwersAHBuijsWCMuldersPFRadioimmunotherapy with [131I]cG250 in patients with metastasized renal cell cancer: dosimetric analysis and immunologic responseClin Cancer Res20051119 Pt 27178s7186s16203819
- BrouwersAHMuldersPFde MulderPHLack of efficacy of two consecutive treatments of radioimmunotherapy with 131I-cG250 in patients with metastasized clear cell renal cell carcinomaJ Clin Oncol200523276540654816170161
- BrouwersAHBuijsWCOosterwijkETargeting of metastatic renal cell carcinoma with the chimeric monoclonal antibody G250 labeled with (131)I or (111)In: an intrapatient comparisonClin Cancer Res2003910 Pt 23953S3960S14506194
- BleumerIKnuthAOosterwijkEA phase II trial of chimeric monoclonal antibody G250 for advanced renal cell carcinoma patientsBr J Cancer200490598599014997194
- DavisIDWisemanGALeeFTA phase I multiple dose, dose escalation study of cG250 monoclonal antibody in patients with advanced renal cell carcinomaCancer Immun200771317705349
- BleumerIOosterwijkEOosterwijk-WakkaJCA clinical trial with chimeric monoclonal antibody WX-G250 and low dose interleukin- 2 pulsing scheme for advanced renal cell carcinomaJ Urol20061751576216406869
- SteffensMGBoermanOCde MulderPHPhase I radioimmunotherapy of metastatic renal cell carcinoma with 131I-labeled chimeric monoclonal antibody G250Clin Cancer Res19995Suppl 103268s3274s10541374
- DivgiCRO’DonoghueJAWeltSPhase I clinical trial with fractionated radioimmunotherapy using 131I-labeled chimeric G250 in metastatic renal cancerJ Nucl Med20044581412142115299069
- SiebelsMRohrmannKObernederRA clinical phase I/II trial with the monoclonal antibody cG250 (Rencarex) and interferon- alpha-2a in metastatic renal cell carcinoma patientsWorld J Urol201129112112620512580
- BauerSOosterwijk-WakkaJCAdrianNTargeted therapy of renal cell carcinoma: synergistic activity of cG250-TNF and IFNgInt J Cancer2009125111512319384924
- BleumerITiemessenDMOosterwijk-WakkaJCPreliminary analysis of patients with progressive renal cell carcinoma vaccinated with CA9-peptide-pulsed mature dendritic cellsJ Immunother200730111612217198090
- UemuraHFujimotoKTanakaMA phase I trial of vaccination of CA9-derived peptides for HLA-A24-positive patients with cytokine-refractory metastatic renal cell carcinomaClin Cancer Res20061261768177516551861
- PelosiEMessaCSironiSValue of integrated PET/CT for lesion localisation in cancer patients: a comparative studyEur J Nucl Med Mol Imaging200431793293914991245
- BrouwersAVerelIVan EerdJPET radioimmunoscintigraphy of renal cell cancer using 89Zr-labeled cG250 monoclonal antibody in nude ratsCancer Biother Radiopharm200419215516315186595
- VerelIVisserGWBoermanOCLong-lived positron emitters zirconium-89 and iodine-124 for scouting of therapeutic radioimmunoconjugates with PETCancer Biother Radiopharm200318465566114503961
- UzzoRGRussoPChenDThe multicenter phase III Redect trial: a comparative study of 124 I-girentuximab-PET/CT versus diagnostic CT for the pre-operative diagnosis of clear cell renal cell carcinoma (ccRCC) [late-breaking abstract]AUA Annual MeetingMay 29–June 3 2010San Francisco, CA, USA
- RussoPJangTLPettusJASurvival rates after resection for localized kidney cancer: 1989 to 2004Cancer20081131849618470927
- BrouwersAHMuldersPFOyenWJCarbonic anhydrase IX expression in clear cell renal cell carcinoma and normal tissues: experiences from (radio) immunotherapyJ Clin Oncol2008262238083309 author reply 11–1218669472
- BeckSDPatelMISnyderMEEffect of papillary and chromophobe cell type on disease-free survival after nephrectomy for renal cell carcinomaAnn Surg Oncol2004111717714699037