Abstract
Purpose
To assess the efficacy of one course of rituximab (two 1-g doses) compared to an alternative tumor necrosis factor-α (TNFα) blocker in rheumatoid arthritis patients who had experienced one previous TNFα blocker failure (eg, etanercept, adalimumab, or infliximab).
Patients and methods
The efficacy of both treatments was studied in this retrospective, multicenter, noninterventional cohort study with 196 patients. All patients had active rheumatoid arthritis defined by a Disease Activity Score-28 of ≥3.2 despite having TNFα blocker therapy, and were followed over 6.6 months on average after switching to rituximab versus a second TNFα blocker (ie, switching to etanercept, adalimumab, or infliximab) at baseline.
Results
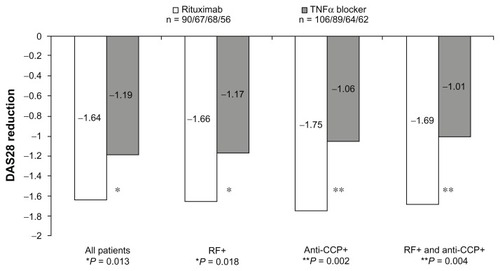
At baseline, both cohorts showed similar demographic and disease-related characteristics (including Disease Activity Score-28). At the end of observation, mean Disease Activity Score-28 was significantly lower after treatment with rituximab than with a second TNFα blocker (−1.64 [95% confidence interval: −1.92; −1.36] versus −1.19 [95% confidence interval: −1.42; −0.96], P = 0.013). This difference between the two groups was even more pronounced when patients were seropositive for rheumatoid factor (−1.66 versus −1.17, P = 0.018) and anti-cyclic citrullinated peptide antibodies (−1.75 versus −1.06, P = 0.002). More rituximab-treated patients achieved good European League Against Rheumatism response than TNFα blocker-treated patients (30% versus 15%), and less patients were nonresponders (22% versus 35%) according to European League Against Rheumatism criteria (P = 0.022, chi-squared test).
Conclusion
Treatment with rituximab was more effective than a second TNFα blocker therapy in rheumatoid arthritis patients after failure of the first TNFα blocker. It was found that anti-cyclic citrullinated peptide antibodies may be a useful predictive biomarker for response to rituximab in patients with TNFα blocker treatment failure.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory systemic disease, preferably affecting the joints, and seriously impairing quality of life of RA patients.Citation1 The ultimate therapeutic goal in treatment of RA is remission, or at least low disease activity, which frequently requires combination therapies of different drugs or sequential adjustment of the treatment strategy. Several treatment options are available, ranging from symptom relief through nonsteroidal anti-inflammatory drugs to disease-modifying antirheumatic drugs (DMARDs) and biologics (antitumor necrosis factor-α [anti- TNFα] blockers, interleukin-1 or −6 inhibitors, B-cell depleting antibodies, selective costimulation inhibitors, and others).Citation2 In case of DMARD failure or loss of efficacy, biological agents offer an option to slow or stop disease activity. Citation3 However, the frequency of patients who do not tolerate or do not respond sufficiently to TNFα blockers within the first year is remarkable: the rate of patients experiencing inadequate treatment response in large randomized clinical trials varies between 21%–58%.Citation4–Citation11
Therapy with rituximab (MabThera®), a chimeric monoclonal antibody specific for the unique cell surface marker CD20 – which is found on B-cells – is one of the treatment options for optimizing RA therapy.Citation5,Citation6,Citation12–Citation14
Since the approval of rituximab, the rheumatologist in daily routine has the option to switch to B-cell-targeted therapy instead of using a second TNFα blocker after treatment failure of the first TNFα-inhibitor. A Swiss cohort study was the first to directly compare rituximab with TNFα blockers in clinical routine.Citation15,Citation16 Its results suggest that in patients with persistent active disease despite anti-TNF therapy, switching to rituximab may be more effective than cycling to an alternative anti-TNF agent.
The present German noninterventional cohort study was designed similarly to the Swiss study.Citation16 However, patients with at least one TNFα blocker failure were included in the Swiss cohort study, while the present study investigated the potential superiority of rituximab after failure of only one TNFα blocker.
Material and methods
This noninterventional study was a multicenter, open-label, retrospective, comparative, postmarketing, observational study in Germany.
Patients
Patients were included after written informed consent was obtained according to the study protocol, which was approved by the Ethics Committee of the Otto-von-Guericke University of Magdeburg (Magdeburg, Germany). Eligible patients had to have an oral dose of corticosteroids up to a methylprednisolone equivalent <20 mg per day. Only patients who received rituximab (two 1-g doses) were included in cohort one (rituximab cohort), and only patients who received a second TNFα blocker (etanercept, adalimumab, or infliximab) were included in cohort two (TNFα blocker cohort). Data of patients who had participated in interventional studies during the recorded period or had received biologic therapies other than TNFα blocker treatment were not included in the documentation within this noninterventional study.
Data from 247 patients in 45 study centers throughout Germany (two to 20 patients per center, median of four patients) were documented for this noninterventional study. Safety data were available for all 247 patients, as all of them received at least one dose of rituximab or TNFα blocker within the documentation period. Evaluable efficacy data were available for 196 patients. In this analysis set, only patients with a baseline Disease Activity Score-28 (DAS28) value of ≥3.2 points, (ie, patients with active RA) were included; they were treated and observed for about 6 months, and had valid DAS28 values at baseline and at the end of observation. Subgroup analyses were performed with patients who were seropositive for rheumatoid factor (RF) or anti-cyclic citrullinated peptide (anti-CCP) antibodies or both biomarkers. Biomarkers were determined using commercially available kits.
Evaluation of clinical outcome
All efficacy, safety, and further variables (eg, demography) were analyzed and interpreted in an exploratory manner. DAS28 was calculated as described by Prevoo et al.Citation17 European League Against Rheumatism (EULAR) response rates were calculated as the proportion of patients who achieved no response (ΔDAS28 ≤0.6 or ΔDAS28 >0.6–1.2 and DAS28 >5.1 at endpoint), moderate response (ΔDAS28 >0.6–1.2 and DAS28 ≤5.1 at endpoint or ΔDAS28 >1.2 and DAS28 >3.2 at endpoint), or good response (ΔDAS28 >1.2 and DAS28 ≤3.2 at endpoint), and compared between cohort one and cohort two.Citation18 Pain was assessed using a visual analog scale ranging from zero to ten. The Health Assessment Questionnaire was used to assess the patients’ physical ability according to eight areas of daily activities.Citation19
Statistical analysis
Patient outcomes (baseline, 3 months, and end of observation) were summarized for continuous variables stating the mean, standard deviation, median, and range, and for categorical variables reporting the total and relative frequency. Pre-post comparisons were calculated for changes from baseline to end of observation as applicable, using 95% confidence intervals. Cohort comparisons were calculated using t-tests for continuous variables and chi-squared tests for categorical variables. DAS28 at 6 months was defined as the primary efficacy endpoint; all other comparisons were interpreted in an exploratory manner.
Results
More female (77.6%) than male (22.4%) patients with a mean age of about 57 years gave their consent to provide their clinical data for this noninterventional study. Ninety patients had been treated with rituximab and 106 with a second TNFα blocker. Comorbidity was present in 76.5% of the total population, most frequently musculoskeletal and cardiovascular disorders. However, the comorbidities, like other patient characteristics, were fairly equally distributed between both cohorts. An overview of the main demographic, clinical, and baseline characteristics is given in .
Table 1 Demographic and disease-related characteristics at start of documentation (baseline)
The median observation plus treatment period was 197 days in the rituximab cohort and 189 days in the TNFα cohort. The most frequent reason for change of the first TNFα therapy was inadequate response in 79.1% of all patients and intolerable side effects in 11.2%.
Almost half of the patients (45.6%) were treated with two rituximab infusions at baseline without a second course during the observation period; 16 patients (17.8%) needed one or two further infusions after 3 months, and 42 patients (46.7%) needed one or two further infusions at 6 months. The majority of patients in the rituximab cohort (83.3%) were treated with rituximab together with methylprednisolone (median of 5 mg in the rituximab cohort and 7.5 mg in the TNFα cohort) plus analgesics plus antihistamines as indicated by the manufacturer. In the TNFα cohort, 47 (44.3%) patients received etanercept (50 mg subcutaneously weekly), 43 (40.6%) patients received adalimumab (40 mg subcutaneously every 2 weeks) and 16 (15.1%) patients were treated with infliximab (intravenous dose of 3 mg/kg at 0 weeks, 2 weeks, and 6 weeks, and thereafter every 8 weeks) as the second TNFα blocker.
Frequency and dosage of DMARD intake fluctuated slightly during the observational period. At baseline, 83.3% of patients treated with rituximab and 82.1% of patients treated with TNFα blockers received DMARDs concomitantly. These numbers decreased to 68.9% and 62.3% after 3 months and increased again to 81.1% and 77.4% after 6 months. Methotrexate was taken by 38%–48% of the patients in both cohorts throughout the observational period. Here, the frequency of patients with concomitant methotrexate therapy also decreased slightly at 3 months and increased again at the end of observation.
Efficacy results
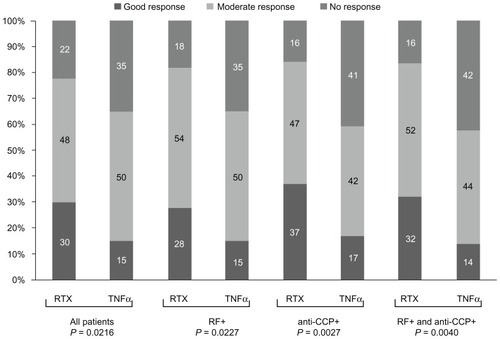
Although improvement was more pronounced under rituximab therapy compared to TNFα treatment in all variables, a statistically significant difference in the total population was only observed in the DAS28 total score (). Interestingly, the higher improvements in the rituximab cohort were only seen in patients seropositive for RF and/or anti-CCP, but not in seronegative patients.
Figure 1 Change in mean disease activity score-28 values between endpoint and baseline in all patients and subgroups.
An additional analysis investigated the influence of the inadequate first TNFα blocker on the outcome of the switch to rituximab or a second TNFα blocker (). If patients were pretreated with etanercept, marked cohort differences were seen in the total cohort as well as in all subgroups according to seropositivity, although the DAS28 changes were at a somewhat lower level compared to the total population (), especially in the TNFα subgroup. Significant cohort differences were already found at 3 months. In contrast, pretreatment with adalimumab was associated with an increased efficacy of rituximab, especially in anti-CCP-positive patients, but had no influence on the efficacy of the second TNFα blocker. The number of patients in the subgroup treated with infliximab was quite small and imbalanced between the two cohorts, and insufficient for subgroup analyses. The available data for all patients show no relevant differences between the two cohorts after pretreatment with infliximab.
Table 2 Efficacy results depending on the first tumor necrosis factor-α inhibitor and seropositivity for rheumatoid factor and anti-cyclic citrullinated peptide: Disease Activity Score-28 change between baseline, 3 months, and 6 months
Comparisons of both cohorts regarding changes in tender joint counts ( and ) between baseline and end of observation revealed differences only in the subgroups of anti-CCP-positive patients (−5.28 [95% confidence interval: −6.78; −3.79] versus −3.06 [95% confidence interval: −4.28; −1.85], P = 0.024) and patients seropositive for both anti-CCP and RF (−5.49 [95% confidence interval: −7.21; −3.77] versus −2.85 [95% confidence interval: −4.05; −1.66], P = 0.013).
Figure 2 The mean absolute values of erythrocyte sedimentation rate, tender joint counts, swollen joint counts, and pain visual analog scale at baseline, 3 months posttreatment, and 6 months posttreatment.
Abbreviations: BL, baseline; ESR, erythrocyte sedimentation rate; M3, 3 months posttreatment; M6, 6 months posttreatment; SJC, swollen joint counts; TJC, tender joint counts; TNFα, tumor necrosis factor-α; VAS, visual analog scale.
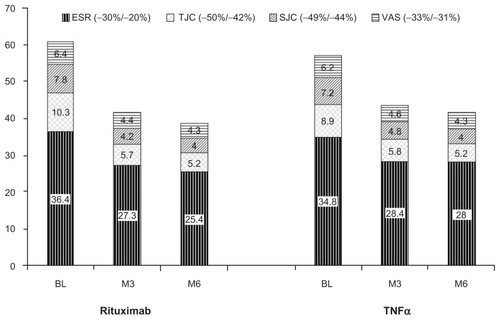
Table 3 Efficacy results: change from baseline to end of observation (196 patients)
For erythrocyte sedimentation rate, cohorts did not differ at the end of observation (); however, at 3 months there was a larger decrease under rituximab compared to the TNFα cohort (−11.2 [95% confidence interval: −15.61; −6.79] versus −4.03 [95% confidence interval: −9.10; 1.05], P = 0.037). A similar difference between both cohorts was observed in the RF-positive subgroup (−12.8 [95% confidence interval: −17.96; −7.64] versus −4.52 [95% confidence interval: −10.48; 1.45], P = 0.038), but not in the anti-CCP-positive subgroup (−9.69 [95% confidence interval: −14.88; −4.49] versus −4.51 [95% confidence interval: −11.71; 2.69], P = 0.239).
No cohort differences were found in other efficacy measures (swollen joint count, pain visual analog scale, C-reactive protein, Health Assessment Questionnaire). Data for swollen joint count and pain visual analog scale are presented in .
In the rituximab cohort, 30.0% (n = 27) of the patients showed good EULAR response, 47.8% (n = 43) of the patients showed moderate response, and 22.2% (n = 20) of the patients showed no response. In the TNFα blocker cohort, the proportion of patients that showed good, moderate, or no response was 15.1% (n = 16), 50.0% (n = 53), and 34.9% (n = 37), respectively. The cohort difference (P = 0.022, chi-squared test) was mainly due to a larger number of patients with good EULAR response (30.0% versus 15.1%) and a lower number of patients with no response in the rituximab cohort (22.2% versus 34.9%) (). For the RF-positive patients, the frequency distribution of response status was different between both cohorts (P = 0.023, chi-squared test). Furthermore, EULAR response was more favorable for rituximab than for the second TNFα blocker in the subgroup of anti-CCP-positive patients (P = 0.003, chi-squared test).
Safety results
Of the 247 patients available for safety analysis, seven (5.6% of 124) patients in the rituximab and five (4.1% of 123) patients in the TNFα blocker cohort suffered from at least one adverse drug reaction during the observation period. In total, 15 adverse drug reactions with a possible or definite relationship to the biologics occurred in the rituximab cohort, and six occurred in the TNFα cohort. With one exception (sinusitis), all adverse drug reactions were reported in female patients. Most frequently, patients developed skin disorders (in three patients of each cohort).
Infusion reactions occurred in three patients treated with rituximab; infusion was interrupted in one patient receiving rituximab (facial hypoesthesia of mild intensity). In another patient, the medication was stopped due to a moderate increase in liver function parameters. The third patient with infusion reactions suffered from infection, generalized pruritus, musculoskeletal pain, urticaria, eye swelling, and fatigue.
The number of adverse drug reactions documented was similar in both cohorts. In general, all were single incidents with no accumulation in a certain class of side effects. Only one patient treated with rituximab experienced an adverse drug reaction of severe intensity (nail dystrophy, dehydration, and anemia). All other reactions were of mild to moderate intensity and no action was taken with respect to the medication. In two patients, the outcome of the adverse drug reaction was reported as “unchanged” (one in either cohort); in all other patients the outcome was reported as “recovered.” No deaths or serious adverse reactions were reported during this observational study.
Discussion
Altogether, the results of the efficacy analysis in RA patients with one TNFα treatment failure were in favor of treatment with rituximab (cohort one) rather than treatment with a second TNFα blocker (cohort two). Significant cohort differences were seen in the improvement of DAS28, the primary efficacy endpoint in this study. This difference between the two groups (rituximab versus TNFα) was even more pronounced when patients were seropositive for anti-CCP antibodies as well as both RF and anti-CCP antibodies. For anti-CCP positive patients, cohort differences could already be seen after 3 months, indicating a faster onset of action of rituximab in this subgroup. Differences in tender joint counts at the end of observation were noted only in the subgroups of anti-CCP-positive patients. According to EULAR criteria, more patients showed good response and less patients showed no response in the rituximab cohort as compared to the TNFα blocker cohort. Again, these cohort differences were more pronounced in the RF-positive patients and especially in the anti-CCP-positive patients.
The results from this noninterventional study investigating RA treatment in routine practice confirm those of placebo-controlled clinical studies that have already proven that rituximab is effective in reducing RA disease activity in patients with prior TNFα blocker failure.Citation5,Citation20 The results from this retrospective investigation extend the experience of the small prospective Swiss cohort study, which showed superior treatment outcome when patients with inadequate response to at least one anti-TNF agent were switched to rituximab compared to an alternative TNFα therapy.Citation15 Changes in DAS28 were of similar magnitude for rituximab in both cohort trials (−1.61 in the Swiss study versus −1.64 in the current study), but slightly better for the second TNFα treatment in the current study (−1.19 versus −0.98 in the Swiss study). As most RA patients in the Swiss study were RF-positive and CCP antibody was not assessed at that time, conclusion on the role of these potential biomarkers could not be made. However, rituximab efficacy could be demonstrated both in patients receiving at least one TNFα-blocker.
A recent study investigating the effects of seropositivity to RF and anti-CCP found reduced response to TNFα blockers in seropositive patients as compared to seronegative for both RF and anti-CCP.Citation21 This result could not be confirmed by the current data (), which show stronger improvements in both cohorts in patients seropositive for RF and especially for anti-CCP compared to seronegative patients; however, the number of the latter subgroup was very small. In addition, the patients in the English study had a more advanced disease. Other studies focusing on the efficacy of rituximab showed that RF positivity, but not anti-CCP positivity, is predictive for rituximab efficacy.Citation22,Citation23 Enhanced efficacy of rituximab in RF-positive versus RF-negative patients could already be shown in the IMAGE (A Study to Evaluate Rituximab in Combination With Methotrexate in Methotrexate-Naïve Patients With Active RA) study.Citation24 CCP antibodies were not addressed in this study. Likewise in the RABBIT (RA – Observation of Biologic Therapy) register, response rates were better in RF-positive patients than in RF-negative patients receiving rituximab; a similar but smaller effect was observed in the anti-CCP-positive versus anti-CCP-negative patients.Citation25 Although RF positivity was predictive for efficacy of rituximab in this trial, the differences between both cohorts were largest in the subgroup of anti-CCP-positive patients. However, there was an overlap in RF and anti-CCP positivity: 62% of the patients in the rituximab cohort were positive for both biomarkers. Combined with the results from the present cohort study, these findings provide clear evidence that treatment with rituximab may be more effective than a second-line biological therapy with the TNFα blockers etanercept, adalimumab, or infliximab. In particular, the results of the anti-CCP-positive subgroup analyses in this study may indicate how to better predict treatment response when selecting the appropriate treatment option in clinical routine, as there still seems to be the need for personalized medicine in RA.Citation26 However, this does not mean that application of rituximab in RF-negative and anti- CCP-negative patients should be avoided. The number of seronegative patients was too small in the current study to compare both cohorts.
Conclusion
The conclusions from this study are limited due to its design as a noninterventional trial. Observational data was collected retrospectively from two cohorts that were treated either with rituximab or a second TNFα blocker. Treatments were not randomized and no information was available as to why patients were treated with either therapy. Therefore, confounding factors cannot be excluded which might have contributed to the differences in efficacy described above. However, there were no structural differences between the two cohorts in patient characteristics and baseline values of efficacy measures. In addition, no such differences could be detected between patients analyzed and those 21.6% of patients who were excluded from the statistical analysis. The consistency between the results of the current study and those of the Swiss cohort trial as well as of those from double-blind trials are considered in favor of valid results of this study.Citation15,Citation5,Citation20
The results of this noninterventional, retrospective cohort study indicate that treatment with rituximab is superior to a second TNFα blocker therapy in RA patients who did not respond to a previous TNFα blocker in terms of clinically significant improvement of disease activity as measured by the DAS28 score. An even stronger difference between cohorts was found in the subgroup of patients seropositive for anti-CCP and for both RF and anti-CCP. Rituximab treatment was compared to the general strategy of cycling to a second TNFα blocker (eg, etanercept, adalimumab, or infliximab); however, rituximab was not compared to every single compound. Other TNFα inhibitors that were not included in this study may show a different outcome. In agreement with previous publications, the current study showed that anti-CCP positivity, in particular, could be a useful predictive biomarker for rituximab in patients with prior TNFα blocker treatment failure. Further attention should be given to concomitant therapies such as other DMARDs potentially influencing the response to biologics in different ways.
Acknowledgments
The authors thank Dr F Meister (ReSearch Pharmaceutical Services Inc, Nuremberg, Germany) for editorial assistance and publication coordination.
Disclosure
This noninterventional study was funded by Roche Pharma AG, Grenzach-Wyhlen, Germany.
References
- StrandVKhannaDThe impact of rheumatoid arthritis and treatment on patients’ livesClin Exp Rheumatol2010283 Suppl 59S32S4020576223
- McInnesIBO’DellJRState-of-the-art: rheumatoid arthritisAnn Rheum Dis201069111898190620959326
- RathTRubbertADrug combinations with methotrexate to treat rheumatoid arthritisClin Exp Rheumatol2010285 Suppl 61S52S5721044434
- RedlichKSchettGSteinerGHayerSWagnerEFSmolenJSRheumatoid arthritis therapy after tumor necrosis factor and interleukin-1 blockadeArthritis Rheum200348123308331914673982
- CohenSBEmeryPGreenwaldMWRituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeksArthritis Rheum20065492793280616947627
- KeystoneEBurmesterGRFurieRImprovement in patient-reported outcomes in a rituximab trial in patients with severe rheumatoid arthritis refractory to anti-tumor necrosis factor therapyArthritis Rheum200859678579318512710
- KeystoneECSchiffMHKremerJMOnce-weekly administration of 50 mg etanercept in patients with active rheumatoid arthritis: results of a multicenter, randomized, double-blind, placebo-controlled trialArthritis Rheum200450235336314872476
- LipskyPEvan der HeijdeDMSt ClairEWInfliximab and methotrexate in the treatment of rheumatoid arthritisN Engl J Med2000343221594160211096166
- SeymourHEWorsleyASmithJMThomasSHAnti-TNF agents for rheumatoid arthritisBr J Clin Pharmacol200151320120811298065
- WeinblattMEKeystoneECFurstDEAdalimumab, a fully human anti-tumor necrosis factor α monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA TrialArthritis Rheum2003481354512528101
- WeinblattMEKremerJMBankhurstADA trial of etanercept, a recombinant tumor necrosis factor receptor: Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexateN Engl J Med199934042532599920948
- EdwardsJCSzczepanskiLSzechinskiJEfficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritisN Engl J Med2004350252572258115201414
- EdwardsJCCambridgeGSustained improvement in rheumatoid arthritis following a protocol designed to deplete B lymphocytesRheumatology (Oxford)200140220521111257159
- EmeryPFleischmannRFilipowicz-SosnowskaAThe efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trialArthritis Rheum20065451390140016649186
- FinckhACiureaABrulhartLB cell depletion may be more effective than switching to an alternative anti-tumor necrosis factor agent in rheumatoid arthritis patients with inadequate response to anti-tumor necrosis factor agentsArthritis Rheum20075651417142317469098
- FinckhACiureaABrulhartLWhich subgroup of patients with rheumatoid arthritis benefits from switching to rituximab versus alternative anti-tumour necrosis factor (TNF) agents after previous failure of an anti-TNF agent?Ann Rheum Dis201069238739319416802
- PrevooMLvan’t HofMAKuperHHvan LeeuwenMAvan der PutteLBvan RielPLModified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritisArthritis Rheum199538144487818570
- FransenJvan RielPLThe Disease Activity Score and the EULAR response criteriaClin Exp Rheumatol2005235 Suppl 39S93S9916273792
- FriesJFSpitzPKrainesRGHolmanHRMeasurement of patient outcome in arthritisArthritis Rheum19802321371457362664
- KeystoneEEmeryPPeterfyCGRituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapiesAnn Rheum Dis200968221622118388156
- PotterCHyrichKLTraceyAAssociation of rheumatoid factor and anti-cyclic citrullinated peptide positivity, but not carriage of shared epitope or PTPN22 susceptibility variants, with anti-tumour necrosis factor response in rheumatoid arthritisAnn Rheum Dis2009681697418375541
- BenucciMManfrediMPuttiniPSAtzeniFPredictive factors of response to rituximab therapy in rheumatoid arthritis: what do we know today?Autoimmun Rev201091280180320656069
- QuartuccioLFabrisMSalvinSRheumatoid factor positivity rather than anti-CCP positivity, a lower disability and a lower number of anti-TNF agents failed are associated with response to rituximab in rheumatoid arthritisRheumatology (Oxford)200948121557155919789202
- TakPPRigbyWFRubbert-RothAInhibition of joint damage and improved clinical outcomes with rituximab plus methotrexate in early active rheumatoid arthritis: the IMAGE trialAnn Rheum Dis2011701394620937671
- StrangfeldAEveslageMKekowJEffectiveness of treatment with rituximab depends on autoantibody status: results from 2 years of experience in the German biologics register RABBIT [Abstract]Arthritis Rheum200960Suppl 101695
- IsaacsJDFerracioliGThe need for personalised medicine for rheumatoid arthritisAnn Rheum Dis20117014721068091