Abstract
Erythropoietin (Epo) is an essential hormone that binds and activates the Epo receptor (EpoR) resident on the surface of erythroid progenitor cells, thereby promoting erythropoiesis. Recombinant human erythropoietin has been used successfully for over 20 years to treat anemia in millions of patients. In addition to erythropoiesis, Epo has also been reported to have other effects, such as tissue protection and promotion of tumor cell growth or survival. This became of significant concern in 2003, when some clinical trials in cancer patients reported increased tumor progression and worse survival outcomes in patients treated with erythropoiesis-stimulating agents (ESAs). One of the potential mechanisms proffered to explain the observed safety issues was that functional EpoR was expressed in tumors and/or endothelial cells, and that ESAs directly stimulated tumor growth and/or antagonized tumor ablative therapies. Since then, numerous groups have performed further research evaluating this potential mechanism with conflicting data and conclusions. Here, we review the biology of endogenous Epo and EpoR expression and function in erythropoiesis, and evaluate the evidence pertaining to the expression of EpoR on normal nonhematopoietic and tumor cells.
Introduction
Erythropoietin (Epo) is a hormone, so named because of early studies demonstrating that Epo had a singular effect on stimulation of erythropoiesis, the formation of red blood cells.Citation1 Epo functions by binding to and activating the Epo receptor (EpoR) expressed on the surface of committed erythroid progenitor cells. This in turn induces erythroid progenitor cell survival, proliferation, and differentiation into circulating enucleated hemoglobin-containing red blood cells (RBCs), which are critical for oxygen transport.
The cloning of the EPO gene in the early 1980s allowed for the development of recombinant erythropoietins and analogs (erythropoiesis-stimulating agents [ESAs]), offering an alternative to transfusion as a method of raising hemoglobin levels in patients with anemia. However, in some clinical trials, the treatment of cancer patients with recombinant human Epo (rHuEpo) or other ESAs has been associated with decreased locoregional control of tumor growth and/or decreased survival.Citation2,Citation3 Some investigators have reported that ESAs may have nonhematopoietic effects via direct activation of EpoR on nonhematopoietic cells, including tumor cells. This hypothesis was used as one possible explanation for the decreased locoregional control of tumor and decreased survival reported in some ESA clinical trials in anemic cancer patients.
In this review, we examine the mechanisms by which ESAs stimulate the formation of normal erythroid cells, and explore the hypothesis that ESAs can stimulate growth or survival of other nonhematopoietic cell types, including tumor cells.
Erythropoiesis
Maturing erythroid progenitor cells expand in number and decrease in size as they progress through a series of differentiation stages (). The first committed erythroid cell type forms characteristic “burst” colonies in semisolid medium, and was therefore called a burst-forming unit-erythroid cell (BFU-E). BFU-E cells are present at 40–120 cells per 105 bone marrow cells,Citation4 and further differentiate into colony-forming unit-erythroid (CFU-E) cells. CFU-E cells, present at concentrations of 200–600 cells per 105 bone marrow cells,Citation4 begin synthesis of hemoglobin and differentiate into erythroblasts. Erythroblasts enucleate forming reticulocytes, so named because of the “reticulin” associated with the residual ribosomal RNA detectable with dyes such as methylene blue. After several days, mitochondria are degraded, reticulin declines, and the cells become mature RBCs. RBCs lack DNA, and therefore can neither divide nor alter gene expression in response to stimuli.Citation5
Figure 1 Erythropoiesis and the expression of stage-specific markers.
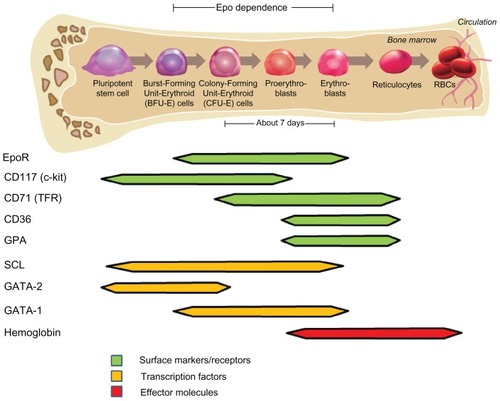
Erythropoiesis occurs in specialized niches in the bone marrow, encompassing a macrophage surrounded by maturing erythroid cells.Citation6 In healthy humans, 2 × 1011 RBCs are generated per day and constitute 99% of circulating cells and approximately 40%–45% of the blood volume. To sustain this level of RBC production, a substantial fraction (25%) of the cells in a normal bone marrow smear are erythroid precursors.Citation7 However, erythroid precursors in the “liquid” portion of bone marrow represent a smaller proportion (0.01%–1%).Citation8–Citation11 RBCs have a lifespan of 3–4 months under normal conditions in humans,Citation12 but can be decreased in such disease states as renal failure.Citation13
Erythropoietin
Erythropoiesis is stimulated when Epo, a glycoprotein hormone expressed primarily in the kidney, binds and activates the EpoR expressed on the surface of erythroid progenitor cells. HuEpo is encoded by a single gene on chromosome 7Citation14 (mouse chromosome 5) that is transcribed into a 1.6–2.0 kb mRNACitation15 and translated into a 193 amino acid (aa) precursor protein. During transit through the secretory apparatus, the 27 aa signal peptide and C-terminal arginine are removed, carbohydrate chains are added (3 N-linked and 1 O-linked) and the ~30-kDa glycoprotein is released into the surrounding fluids. This process occurs rapidly, and Epo does not typically accumulate intracellularly.Citation16
The normal level of circulating Epo in humans is approximately 5 pM (~20 mU/mL; 100 pg/mL), substantially below the Kd of the Epo–EpoR interaction (~100 pM), indicating that only a fraction of the EpoR is Epo bound under normal conditions. However, this level of binding is sufficient to sustain erythropoiesis at a rate that will maintain normal RBC levels. Increased Epo concentrations result in an increased rate of erythropoiesis,Citation17–Citation19 thereby resulting in an increase in circulating RBCs with a maximal rate of erythropoiesis achieved at Epo concentrations of approximately 0.5–1 U/mL.Citation18,Citation20 Low Epo concentrations, on the other hand, result in apoptosis of precursor cells.Citation21 Epo concentrations below the normal circulating concentration therefore result in a decline in RBC numbers in peripheral blood because the rate of loss (~0.8%–1% per day) exceeds the rate of production.
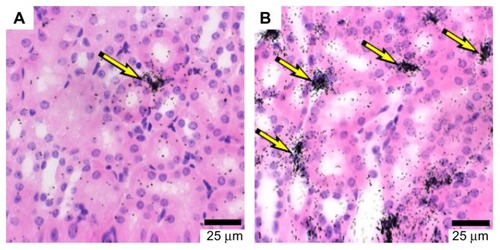
Epo expression increases with decreasing oxygen tension (hypoxia), and this mechanism appears to be the primary driver of erythropoiesis. Hypoxia by itself has little effect on erythropoiesis in vitro.Citation22 Hypoxia inducible factor (HIF), a heterodimer comprised of α- and β-subunits, is one of several transcription factors that regulate EPO gene expression,Citation23,Citation24 though HIF-2α has been shown to be the primary regulator of EPO transcription.Citation25–Citation28 HIFα (subunits HIF-1α or HIF-2α) protein levels are controlled by enzymes (HIF-prolyl hydroxylases [HIF-PH]) that hydroxylate the α-subunit of HIF, targeting it for ubiquitination by the Von Hippel–Lindau (VHL) protein and subsequent degradation by the proteosome.Citation29–Citation34 HIF-PH activity increases with increased levels of oxygen, iron, and 2-oxoglutarate, and thus HIF-PH can act as a “sensor” of oxygen tension, iron levels, and metabolic activity. As HIF protein levels increase due to decreased HIF-PH activity, the rate of Epo production in the kidney and liver as well as mobilization of iron to support increased erythropoiesis also increases. The renal Epo-producing cells appear to be either “on” or “off ” (), and thus increased Epo production is due to recruitment of increased numbers of producing cells and not due to an increase in rate per cell.Citation35,Citation36 Under conditions of severe anemia and therefore low O2 concentration, Epo levels can increase up to 1000-fold.Citation37
Figure 2 (A and B) Erythropoietin (Epo) mRNA is expressed in kidney interstitial cells. Mice were made anemic by withdrawing 0.5 mL blood and replacing with 0.5 mL saline 8, 16, and 24 hours prior to sacrifice. Standard in situ hybridization (ISH) on kidney sections was performed with an antisense 33P-labeled Epo probe. (A) ISH for mouse Epo mRNA in a control mouse; (B) ISH for mouse Epo mRNA in an anemic mouse.
The administration of Epo increases erythropoiesis, but has limited effects on other aspects of hematopoiesis. This conclusion is supported by a number of studies. Epo and EpoR knockout mice had an absence of post-CFU-E erythroid cells but numbers of earlier progenitor cell types – CFU-E, BFU-E, CFU-granulocyte macrophage, and CFU-megakaryocyte – in fetal liver were normal.Citation38 These observations indicated that Epo was not essential for the generation of these progenitor cells. Though administration of Epo to animals and humans resulted in a rapid stimulation of erythropoiesis, the total bone marrow (BM) cellularity and numbers of myeloid, lymphoid, and megakaryocytes remained unchanged.Citation17,Citation39–Citation43 Epo was also unable to stimulate early murine multipotential hematopoietic progenitor cells (Lin−, Sca+).Citation44 Finally, in humans, constitutive overexpression of Epo affected erythropoiesis but not other hematopoietic lineages,Citation45 and subjects with polycythemia due to a hypersensitive EpoR had normal white blood cell and platelet counts.Citation46
Epo is expressed primarily in the kidney and liver,Citation47,Citation48 with minimal levels of transcripts detected in most other tissues, including brain, heart, and lung.Citation36,Citation49–Citation57 In a normal adult animal, the kidney produces 70%–90% of the total Epo, with much of the remainder produced in the liver.Citation57–Citation60 The Epo-producing liver cell is a hepatocyte,Citation36 while in the kidney, it is a neuronal fibroblast cell type found in the interstitial region near the proximal tubular cells ().Citation36,Citation51,Citation55,Citation61,Citation62 Consistent with the detection of Epo transcripts primarily in kidney and liver, transgenic mice expressing LacZ or green fluorescent protein (GFP) under control of an Epo promoter showed B-gal activity/GFP in liver and kidney but not other tissues, including brain and lung.Citation36,Citation63 Although there are some reports that Epo expression may extend to other tissues and cell types (including cells in the brain), these data were based on Western immunoblot and immunohistochemical (IHC) methodologies that used nonspecific or insensitive antibodies or reverse transcription-polymerase chain reaction (RT-PCR).Citation64–Citation71 Therefore, the results of antibody studies are inconclusive. Furthermore, the significance of mRNA detection by nonquantitative RT-PCR is unclear, because there was no evidence provided that the transcripts were translated into significant amounts of Epo protein.
Erythropoietin receptors
The EPORCitation72–Citation74 is encoded by a single gene found on human chromosome 19p and mouse chromosome 9.Citation72,Citation75 It expresses a 2.0–2.2-kb mRNA that is translated into 508 aa (human) and 507 aa (mouse) proteins.Citation20,Citation74 After the removal of the 24 aa signal peptide, 484 aa (human) and 483 aa (mouse) proteins with a calculated molecular weight of approximately 53 kDa are generated.Citation76 Addition of an N-linked carbohydrate chain results in a protein with an estimated size of 56–57 kDa, which is comparable to the size of mature human and murine EpoR as determined by Western immunoblot analysis (~59–61 kDa).Citation76–Citation78 The mature form is then transported to the cell surface, making it accessible for binding to Epo. However, transport of EpoR to the cell surface is inefficient, and the majority of EpoR is detected in the endoplasmic reticulum, Golgi, and endosome-like structures.Citation79 Less than 10% of the total EpoR protein synthesized appears on the cell surface.Citation80–Citation83 The remainder is degraded, but EpoR “fragments” can be detected by Western blotting with specific anti-EpoR antibody A82.Citation78
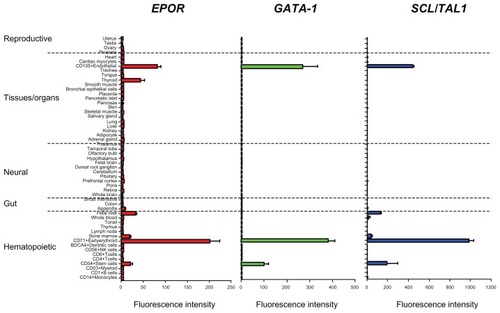
Cloning of the mouse and human EPOR genesCitation73,Citation74 allowed for the further identification of potential EpoR-expressing and Epo-responding cells. According to in situ hybridization studies using EPOR probes, EPOR transcripts were detected in erythroid progenitor cells, with no EpoR transcripts detected in other hematopoietic cell types or in nonhematopoietic tissues, including adult liver, heart, skeletal muscle, and kidney.Citation20,Citation74,Citation84–Citation86 High-level EPOR mRNA expression was detected by Northern blot analysis in megakaryocyte/erythroid cell lines, but levels were low to undetectable in other types, including pluripotent embryonic stem/carcinoma cells, multipotent hematopoietic cells, myeloid progenitors, and committed lymphoid and macrophage precursors.Citation87 With the advent of more sensitive PCR and microarray methodologies, EPOR transcripts were detected in multiple nonerythroid cell types from the BM compartment as well as in various normal and tumorous tissues.Citation56,Citation64,Citation84,Citation85,Citation88–Citation94 However, compared to erythroid progenitor cells and tissues containing them, levels are relatively low, as shown in .
Figure 3 Erythropoietin receptor (EPOR), GATA-1, and SCL/Tal1 have similar transcript profiles in normal human tissue.
The observation that EpoR transcripts could be detected at low levels outside the erythroid compartment suggested that EpoR protein could be generated and that therefore Epo could potentially have effects in nonerythroid tissues. Indeed, initial Western immunoblot and IHC experiments with anti-EpoR antibodies suggested that EpoR protein was widely expressed in nonerythroid cells at relatively high levels.Citation95 However, these results were confounded, as nonspecific antibodies with poor sensitivity and specificity were used.Citation76,Citation91,Citation96–Citation98 Concerns regarding anti-EpoR antibody specificity and sensitivity first became apparent when the reported size of putative EpoR proteins detected by Western blot differed from the calculated molecular size of EpoR in positive controls.Citation76 Furthermore, putative EpoR proteins were also detected in EpoR negative control cells with these anti-EpoR antibodies.Citation76 The use of nonvalidated anti-EpoR antibodies has caused significant confusion and conflicting data in the literature.Citation99,Citation100 This issue is not unique to EpoR, as nonspecificity of antibodies has caused issues in the reliable detection of many proteins.Citation101,Citation102 This has resulted in misdirected research and unnecessary or inappropriate clinical decisions.
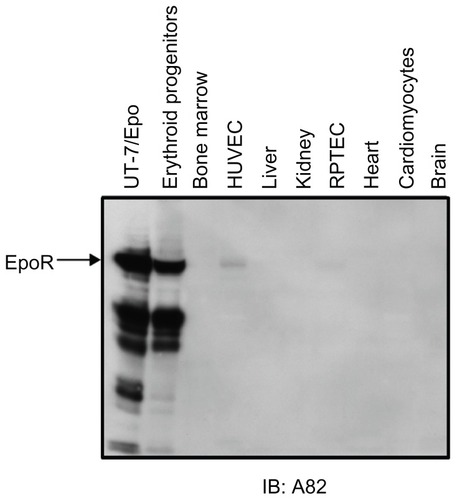
Another reason why the detection of EpoR protein has been problematic is that in nonerythroid cells, the levels of EpoR expression are generally very low, and therefore sensitive and specific detection methods are needed. For example, according to radiolabeled [125I]rHuEpo-binding assays, which are very sensitive, in erythroid progenitors EpoR was found to be expressed at <2 × 103 surface receptors/ cell.Citation103,Citation104 This contrasts with other receptors such as EGFR, which is expressed in epithelial cells at 1 × 105 to 1 × 106 receptors/cell.Citation105,Citation106 Using live freshly derived cells, Epo binding was detected on the surface of erythroid progenitor cells,Citation107,Citation108 but not on unfractionated bone marrow, macrophage, thymocytes, monocyte, granulocyte, or late myeloid precursor cells;Citation104,Citation108–Citation113 or on cells from normal tissues, including heart, kidney, brain, and lung.Citation8 Recently, a sensitive and more-specific anti-EpoR monoclonal antibody (A82) suitable to detect low levels of EpoR by Western immunoblot was described.Citation78 Results with A82 indicated that only erythroid cells had high levels of EpoR protein, with low to undetectable levels in other nonhematopoietic tissues and hematopoietic cell types ().Citation80,Citation94
Figure 4 High-level erythropoietin receptor (EpoR) protein expression is found in erythroid cells but not in other tissues. EpoR expression was analyzed by Western immunoblot analysis with anti-EpoR antibody A82 that was shown to specifically detect human EpoR in erythroid cells.Citation78 The arrow shows the location of full-length EpoR. Smaller proteins have been shown elsewhere to be EpoR fragments.Citation78 UT-7/ Epo cells (EpoR positive control) are derived from a megakaryoblastic leukemia and are Epo-dependent.Citation462
Abbreviations: HUVEC, human umbilical vein endothelial cells; RPTEC, renal proximal tubule epithelial cells.
Regulation of EpoR
During normal erythroid differentiation, EpoR mRNA and surface protein increase as cells progress through the BFU-E to CFU-E stage,Citation11,Citation20 with a decline thereafter and an absence of detectable expression on reticulocytes and RBCsCitation104,Citation110,Citation111,Citation114,Citation115 (, ). In knockout mice, neither Epo nor EpoR were required for the formation of BFU-E cells or the transition to the CFU-E stage.Citation116 EpoR is required for the Epo-dependent expansion and survival of erythroid progenitors as they differentiate from CFU-E into mature hemoglobinized RBCs, and Epo responsiveness correlates with EpoR expression level.Citation20,Citation104,Citation113,Citation115,Citation117 The observation that BFU-E grew with GM-CSF or interleukin (IL)-3 plus Epo but not with Epo alone, but did grow with Epo alone if EpoR expression was increased by forced overexpression using retrovirus-mediated gene transfer,Citation108 suggests that increased EpoR mRNA and protein expression is an important step preceding Epo responsiveness. However, increased EpoR mRNA is necessary but not sufficient for surface EpoR expression,Citation118 and other factors are required, such as JAK2, which acts as a key signaling intermediate as well as a chaperone.Citation119
Figure 5 Erythropoietin receptor (EpoR) expression in differentiating CD34+ hematopoietic progenitor cells grown with or without Epo.
Abbreviation: MWM, molecular weight marker in kilo Daltons (kDa).
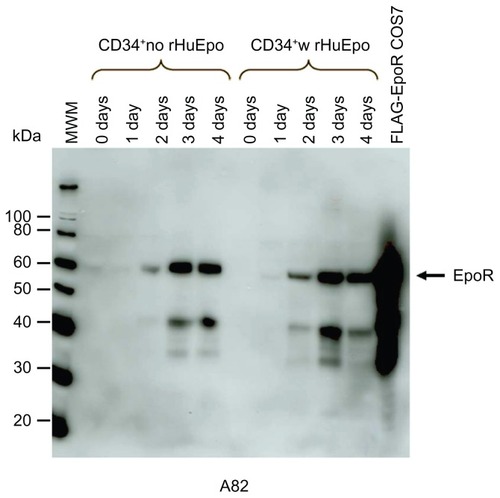
EpoR mRNA has a relatively long half-life, approximately 90 minutes in human cells and 75 minutes in murine cells,Citation20,Citation120 and the half-life is not affected by Epo or by cellular differentiation. The EpoR promoter was found to be active in erythroleukemia cell lines MEL and HEL, but not in nonerythroid cell types, including NIH3T3, HeLa, EL4, S194, WEHI-3, or COS.Citation121–Citation125 These findings suggested that EpoR gene transcription is controlled by essential erythroid-specific transcription factors that are limiting or absent in some cell types. In one study, the sequence of the EpoR in Epo-responsive and -unresponsive mouse erythroleukemia cells was the same,Citation126 suggesting that lack of response was not due to defects in EpoR itself.
Reporter experiments have been performed in transgenic mice to track the in vivo expression of endogenous EpoR in different cell populations. Using the Cre-Lox system, EpoR Cre mice were crossed to Lox Rosa26 enhanced yellow fluorescent protein (eYFP) reporter mice, and expression of eYFP was found to correlate with activity of the EpoR promoter.Citation127 In hematopoietic cells, eYFP was detected in erythroid cells up to the erythroblast stage. However, no eYFP was detected in megakaryocytes, platelets, macrophages, granulocytes, monocytes, or leukocytes. Further, eYFP was not detected in highly purified hematopoietic stem cells, mesenchymal, or osteoblastic enriched populations from the bone microenvironment. In a similar experiment, GFP-Cre was introduced into the EpoR locus by homologous recombination.Citation88 With this construct, EpoR-driven Cre activity was observed in Ter119+ erythroid cells but not in other hematopoietic lineages, including granulocytes, macrophages, monocytes, leukocytes, lymphoid cells, megakaryocytes, or platelets, nor in early Sca-1+ hematopoietic “stem cells.” Cre activity was observed in fetal liver and bone marrow, but not in any other tissue, including brain, heart, lung, and kidney. These observations are consistent with in situ EpoR hybridization experiments with tissues and purified hematopoietic cell types (see above) where high-level EpoR mRNA expression was detected only in erythroid cells or tissues containing erythroid cells.
EpoR expression does not appear to be controlled by Epo. In support of this, EpoR protein is increased in the absence of Epo in differentiating erythroid cells (), and in nonhematopoietic tissues, EpoR mRNA levels were not altered in Epo-deficient skeletal muscles,Citation128 nor were EpoR levels changed when endothelial cells were cultured with Epo.Citation129
EpoR also does not appear to be regulated by hypoxia. Neither EpoR transcriptsCitation22,Citation80,Citation91,Citation130–Citation135 nor protein levelsCitation80 were increased under hypoxic conditions. The lack of elevated EpoR transcription with hypoxia is consistent with the absence of a consensus hypoxia response element in the EpoR transcriptional regulatory regions. However, some reports have suggested EpoR expression is regulated by hypoxia.Citation132,Citation134,Citation136–Citation140 These latter data are confounded, because the studies were not appropriately controlled and conclusions were based on the use of nonspecific anti-EpoR antibodies to detect EpoR by IHC.
Several different transcription factors have been reported to play a role in regulating EpoR transcription, including GATA–1.Citation43,Citation123 GATA-1 knockout mice do not develop erythroid cells, but are able to develop other hematopoietic cell types.Citation141–Citation143 GATA-1 expression is primarily restricted to the erythroid lineage and is essential for high-level EpoR promoter activity.Citation123 Indeed, this relationship can be seen when Epo R and GATA-1 mRNA levels in various tissues are compared (). EpoR transcript levels correlate with GATA-1 transcript levels across tissue and cell types, levels of both change concomitantly during cell division,Citation144 both are expressed in the same cell types during erythropoiesis,Citation145 and GATA-1 levels correlate with Epo responsiveness in cell lines.Citation146,Citation147 However, GATA-1 alone is insufficient to drive EpoR expression, and other factors appear to be essential, including Friend of GATA (Fog1),Citation148 a factor that forms a complex with GATA-1;Citation149 the erythroid specific factor SCL/ Tal1,Citation150–Citation153 which demonstrates a similar expression profile as EpoR and GATA-1 (); and ETV6/RUNX1, which when overexpressed can also increase EPOR gene transcription.Citation154 Consistent with a similar tissue expression profile, SCL/Tal1 is coexpressed with GATA-1 in the same hematopoietic cells.Citation155 Another possible regulator is SP1, a transcription factor found in lysates from erythroid but not in nonerythroid cell lysates.Citation124
The EpoR promoter appears to be leaky because transcript levels are detected in numerous cell types, albeit at lower levels compared to erythroid cells. This is consistent with the finding that the EpoR gene promoter has characteristics of a ubiquitously expressed gene (ie, lacks a TATA box) and thus should have low basal transcription in nonerythroid cells.Citation156,Citation157
Activation of EpoR
Activation of EpoR is initiated by the direct binding of a single Epo molecule with two membrane-spanning EpoR proteinsCitation158–Citation160 that form a homodimer (). The binding of Epo induces a conformational change in EpoR that brings the transmembrane and intracellular regions of the receptor in close proximity. Following binding, the Epo–EpoR complex is activated, internalized, and some is degraded in lysosomes, with the remainder recycled to the cell surface.Citation8,Citation161 However, EpoR can also be internalized and degraded in lysosomes without Epo binding and activation.Citation162
Figure 6 Erythropoietin receptor (EpoR) activation and signaling with Epo in erythroid progenitor cells.
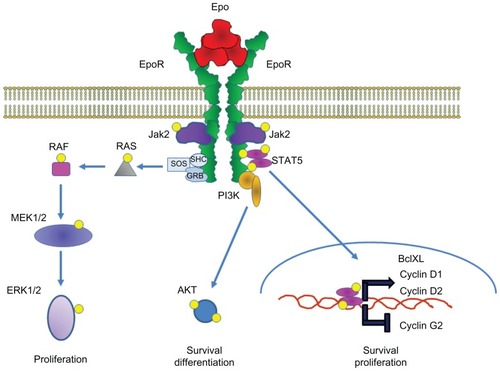
EpoR does not contain intrinsic tyrosine kinase activity but instead requires an accessory tyrosine kinase (JAK2) to induce the signaling cascade.Citation119 JAK2 interacts with EpoR at the juxtamembrane region,Citation119 and the conformational change induced by Epo binding to EpoRCitation163,Citation164 brings the JAK2 molecules into close proximity, resulting in their transphosphorylation.Citation165 The activation of JAK2 results in the phosphorylation of tyrosine residues in EpoR, which serve as docking sites for mediators of the STAT5, MAP kinase, and PI3 kinase/Akt signaling pathwaysCitation166 (). Following activation, negative regulators of EpoR, including Src homology region 2 domain-containing phosphatase 1 and suppressor of cytokine signaling proteins SOCS-1 and SOCS-2, down-modulate signaling responses.Citation167,Citation168 Further control of Epo-induced signaling in cells is mediated through inhibition of EpoR cell surface expression through ubiquitination and subsequent proteosomal degradation.Citation169
The rate of assembly of a functional EpoR homodimer is EpoR concentration–dependent.Citation158,Citation170 In HEL cells, the magnitude of increase in phosphorylated JAK2 after Epo treatment, minimal in the parental cells, is increased with overexpression of EpoR.Citation171 However, levels of surface EpoR are not always correlated with EpoR mRNA level.Citation172 Thus, low-level protein production and/or inefficient EpoR processing and surface translocation may be limiting factors for Epo–EpoR responses. In support of this possibility, increasing levels of EpoR in growth factor–dependent cell lines caused them to become demonstrably Epo-responsive. Citation20,Citation104,Citation108,Citation147,Citation171,Citation173,Citation174 EpoR levels also appear to affect magnitude of response to Epo in vivo. For example, mice that were haplo-insufficient (EpoR+/− mice) had reduced hematocrit and reduced responsiveness of CFU-E to Epo compared to normal mice.Citation175 While these studies indicate that a minimal level of EpoR expression is required for a functional response, the absolute level of EpoR required is unclear. SH-SY5Y cells (a neuroblastoma cell line) were reported to respond to rHuEpo despite very low levels of surface EpoR, less than 50 surface EpoR/cell.Citation176,Citation177 However, others could not detect responses in SH-SY5Y cells.Citation91,Citation94,Citation178
Another possible explanation for the lack of functional EpoR in some cells even though the receptor protein is expressed is that other accessory factors for functional responses are missing. Consistent with this proposal, the leukemia cell lines K562 and OCIM-1 do not respond to Epo (signaling or proliferation/survival) despite detectable EpoR expression on the cell surface using Epo-binding assays.Citation103,Citation112,Citation115 In addition, EpoR was detected at ~1000 receptors/cell in other cell lines derived from patients with acute myelogenous leukemia (AML), chronic myelogenous leukemia (CML), and erythroleukemias, but only some were responsive to Epo.Citation73,Citation103,Citation179–Citation182 This may be at least partly explained by constitutive activation of pathways making them nonresponsive to cytokine stimulation.Citation183 For example, K562 cells have the Bcr/Abl fusion,Citation184 while OCIM-1 cells have constitutive phosphorylation of STAT5, though the pathways contributing to this constitute activation are unknown.Citation185 However, other processes could also be defective in those cells, explaining the lack of Epo–EpoR response.
EpoR overexpression can confer Epo dependence in some cell types but not others, indicating EpoR expression is necessary but not sufficient for a response. For example, forced overexpression of EpoR resulted in Epo dependence for growth in factor-dependent murine progenitor cell lines (FDCP-1, 32D, BaF3) but not in others, such as mouse IL-2-dependent T-cell lines HT-2 and CTLL2Citation186–Citation195 or in NIH-3T3 cells,Citation121 which are dependent on platelet-derived or fibroblast growth factor for growth.Citation196 Infection of BM cells with virus expressing EpoR or a constitutive-active EpoR variant (R129C) resulted in an increase in erythroid, macrophage, and megakaryocyte cells but not other lineages, including lymphocytes, granulocytes, mast cells, and eosinophils.Citation108,Citation197–Citation199 This suggests that macrophage and megakaryocyte progenitors cells are programmed for a response but lack sufficient EpoR expression, while other cell types lack programming. For example, HT-2 cells expressing EpoR failed to grow with Epo despite Epo-induced phosphorylation of EpoR and JAK2. However, these cells had a deficit in Epo-induced STAT5 phosphorylation,Citation186 suggesting a deficiency in downstream signaling pathways. A somatic fusion of EpoR-expressing HT-2 cells with BaF3 cells resulted in Epo dependent growth and signaling, suggesting addition of an essential factor by BaF3 cells. Taken together, these observations suggest that in addition to the accumulation of a certain level of EpoR, the cells must contain the required intracellular signaling networks for a “programmed” response.
Is functional EpoR expressed in tumor cells?
The potential for ESAs to stimulate tumor growth has been of significant controversy since 2003, when it was reported that patients with head and neck cancer receiving rHuEpo had reduced locoregional control of their tumors compared to control subjects.Citation2,Citation200 This was followed by an analysis of patient samples for expression of EpoR,Citation201 in which an association between staining with the anti-EpoR antibody C-20 and negative clinical outcomes was reported. This raised the hypothesis that EpoR was expressed on tumors and that ESAs directly stimulated tumor growth. This hypothesis appeared to be supported by preclinical data that suggested that most tumors and cell lines expressed high levels of EpoR, and further that ESAs directly promoted tumor cell growth and survival.Citation100,Citation202,Citation203 However, these data contrasted with data from other groups that reported EpoR was not present on tumor cells and that ESAs did not have a direct tumorstimulating effect.Citation99,Citation204–Citation206 Further, with clinical data from other trials and meta-analyses, there was not a significant association between ESAs and tumor progression end points.Citation2 These conflicting data have caused considerable confusion and have led to calls for additional research. Here, we provide a critical evaluation of the research that pertains to the expression and function of EpoR in tumor cells.
Tumor growth is commonly driven by oncogenes, which are marked by shared characteristics, including overexpression due to genomic amplification, mutations that induce constitutive activation, and increased transcriptional/translational activity. Although EpoR genomic amplification and gene rearrangements have been described in some erythroleukemia and megakaryoblastic leukemias and derived cell lines (eg, UT-7 F36E and TF-1),Citation172,Citation207–Citation209 EpoR amplification is thought to be a rare event. Several studies failed to show amplification of EpoR or alterations to chromosome 19, the location of the EPOR gene,Citation209,Citation210 even in erythroleukemia, the disease above all others in which involvement of Epo/EpoR might have been predicted. Furthermore, in contrast to oncogenic receptors such as HER2 and EGFR, in a screen of >1000 different solid tumors, EpoR gene amplification was rarely found, and when observed was similar to the frequency and magnitude of amplification of other nononcogenes.Citation92
Constitutive activation of EpoR could theoretically also provide a growth advantage to tumors. This has been observed with Friend virus infection, which results in constitutive activation of EpoR through the binding of Env protein gp55 to EpoR, and has been shown to induce erythroleukemia in mice.Citation211,Citation212 An activating mutation in murine EpoR was identified (R129C) in a mutagenesis screening study that induced constitutive activation and conferred growth factor independence in IL-3-dependent BaF3 cells.Citation213 However, activating EpoR mutations do not appear to play a role in tumorigenesis, and naturally occurring activating EpoR mutations have not been found in human erythroleukemias.Citation209,Citation210 For example, EpoR sequence analysis was performed on six tumor cell lines (UT-7/Epo, MCF-7, 769-P, CAKI-2, SH-SY5Y, and HeLa), and no activating EpoR mutations were found (Amgen data on file). Moreover, while EpoR hyperactivating mutationsCitation214,Citation215 have been reported in patients with congenital erythrocytosis, these subjects had normal platelet and white blood cell counts and no increased incidence of tumors or leukemic transformation,Citation192,Citation209,Citation211,Citation216–Citation218 and were otherwise normal.
A prerequisite for a direct effect of ESAs on tumor cells is that they must express EpoR. Epo R mRNA was detected in multiple tumor cells and cell lines using RT-PCR. Citation20,Citation90,Citation96,Citation134,Citation219–Citation228 However, EpoR transcript levels were 10–1000-fold lower in tumor tissues and cell lines compared to Epo-responsive positive control cells.Citation64,Citation80,Citation91,Citation229–Citation234 These results were consistent with Northern analysis of solid tumor and leukemic cell lines, in which EpoR mRNA was expressed at low to undetectable levels.Citation87,Citation235 One group reported a direct correlation between EpoR transcript levels and poor clinical outcome in a subset of patients treated with ESAs, but definitive prognostic conclusions could not be made.Citation230 Moreover, levels of EpoR mRNA in tumors were similar to that of their normal counterpart.Citation92,Citation134 These data demonstrate that though the EpoR gene is expressed in normal tissues and tumor cells, EpoR mRNA transcripts are not overexpressed in tumors, with levels detected representing the low basal transcription seen in normal tissues.
As EpoR mRNA was detected in tumors, it seemed likely that EpoR protein was also present on tumor cells. Indeed, Henke et al reported that high levels of EpoR protein was expressed in tumors from head and neck cancer patients who had poor outcomes when treated with ESAs using IHC studies.Citation201 EpoR expression was also reported by multiple groups in various tumors and tumor cell lines by Western immunoblot and IHC using the same antibody (C-20).Citation236–Citation242 Over 30 different studies have been published with putative detection of EpoR in tumors and tumor cell lines that all used the C-20, M-20 and H194 antibodies (produced by the same manufacturer – Santa Cruz Biotechnology). These studies were thought to indicate that ESAs may stimulate EpoR expressed in tumors and thereby promote tumor growth and survival. However, analysis of the Henke et al clinical samples indicated that the level of EpoR protein expression suggested by the C-20 staining did not correlate with the level of Epo R mRNA.Citation230 In addition, not all groups reported correlations between C-20 antibody staining of other clinical tumor specimens and adverse clinical events.Citation243–Citation246 Further, in cells deemed to be EpoR-positive through staining with C-20 antibody, no cellular responses, such as changes in proliferation or viability, were observed.Citation247 These discordant results were highlighted in a study in which tumor cells from patients with B-CLL were reported to express EpoR using a nonspecific anti-EpoR antibody, but no EpoR protein was detected on the cell surface using a more specific digoxigenin-labeled rHuEpo binding method.Citation96
Several issues have recently come to light in the analysis of anti-EpoR antibodies, including C-20: the putative EpoR proteins detected with the antibodies varied in size by Western immunoblot analysis, were detected in negative control cell lines, differed in size from the EpoR detected in positive control samples, and in control studies many were shown to be nonspecific.Citation76,Citation91,Citation97,Citation98,Citation230,Citation248,Citation249 Therefore, it is likely that the putative EpoR detected with these antibodies were non-EpoR cross-reacting proteins, thereby giving false-positive results. One of the proteins detected by C-20 was 66 KDa in size and thought to be EpoR, but was subsequently shown to be heat shock protein (HSP)70.Citation76 Since HSP70 is ubiquitously expressed and expression is increased when cells and tumors undergo stress responses, the IHC results reported with C-20 may have reflected HSP70 biology and not EpoR. The use of nonspecific antibodies in general,Citation101 and anti-EpoR antibodies in particular,Citation76 is a well-recognized problem in research that has resulted in recommended guidelines for antibody validation.Citation250–Citation254
Recently, a specific and sensitive anti-EpoR antibody (A82) suitable for detecting EpoR by Western immunoblot analysis was described.Citation78 Using A82 in Western analyses of total protein lysates (intracellular and cell surface protein), EpoR was undetectable in normal nonhematopoietic human and mouse tissuesCitation94,Citation185 and in tumor specimens from breast, lung, ovary, colon, and skin.Citation255 In another analysis of 66 tumor cell lines with A82, 80% of the lines had over 100-fold lower or undetectable levels of EpoR compared to a positive control hematopoietic cell line.Citation80 The remaining cell lines had relatively low levels (5–100-fold lower) compared to that observed with a positive-control hematopoietic cell line. Only one tumor cell line (the NSCLC line NCI-H661), which had the highest level of total EpoR, had detectable EpoR on the cell surface according to [125I]rHuEpo-binding experiments. However, neither NCI-H661 nor any of the other solid tumor lines examined responded to ESAs in signaling studies.Citation80 Mouse monoclonal antibody MAB307 has also been used to detect cell surface EpoR by flow cytometry. While EpoR was detected on positive controls, including primary erythroid progenitors with MAB307, no EpoR was detected on the surface of viable tumor cells from over 180 different biopsies from patients with tumors including breast, colon, ovary, lung, head and neck, and kidney.Citation256 These findings are consistent with Western immunoblot data generated with A82.
Another method used to examine surface EpoR in tumor cells and cell lines is competitive binding experiments with labeled rHuEpo. Specific rHuEpo binding to some hematopoietic cells and certain myeloid and erythroleukemia cells and cell lines was reported.Citation103,Citation107,Citation112,Citation257 However, surface EpoR was not detected in primary hematopoietic leukemias, such as B-CLL or multiple myeloma,Citation258 or in most hematopoietic cell lines and nonhematopoietic cancer cell lines.Citation78,Citation80,Citation92,Citation103,Citation113,Citation115,Citation180,Citation259,Citation260 In a controlled flow cytometry study using biotinylated rHuEpo, 81/136 samples from AML patients were reported to bind rHuEpo, of which only 13 of 81 had an increase in growth with rHuEpo treatment.Citation257 However, there was no correlation between the amount of EpoR and the in vitro proliferative response to rHuEpo. In the same study, 4/14 acute lymphoblastic leukemia patient samples were reported to bind rHuEpo, but none proliferated with rHuEpo. In other studies, one group reported that rHuEpo increased colony number and plating efficiency with cells from CML patients.Citation261 In contrast, in other studies, no proliferative effect of ESAs in AML and B-cell leukemic cell types were found,Citation258,Citation262 and rHuEpo did not have an effect on STAT5 phosphorylation on those cells.Citation263
A few studies have evaluated [125I]rHuEpo binding in epithelial tumor cell lines. While some studies have reported specific binding to solid tumor cell lines,Citation235,Citation264,Citation265 other studies reported none.Citation80,Citation99 In Epo-responsive hematopoietic cell lines and primary erythroid cells, rHuEpo has a high binding affinity (Kd ~50–400 pM).Citation103,Citation104,Citation109,Citation172,Citation266,Citation267 In contrast, in the studies with solid tumor cells that reported binding, the rHuEpo binding affinity was unusually low (Kd ~1400–16,000 pM). The low affinities reported in these studies may be due to nonspecific interactions of rHuEpoCitation268 related to the hydrophobic nature of rHuEpo.
To independently determine if functional EpoR was present on the cell surface, investigators have also examined EpoR downstream signaling events after treatment of cells with ESAs in vitro. Signaling through EpoR is dependent on JAK2, which transduces downstream signaling though the STAT5, PI3K, and MAPK pathwaysCitation269 (). Thus, positive results showing phosphorylation of JAK2 or STAT5 with ESAs in tumor cells would be important evidence for activation of EpoR with Epo. However, there are a number of reports indicating no increased phosphorylation of JAK2 or STAT5 with rHuEpo in tumor cell lines,Citation80,Citation193,Citation270–Citation272 with only rare positive reports: SH-SY5Y (neuroblastoma), H838 (lung cancer), and several head and neck cell lines.Citation132,Citation224,Citation273,Citation274 However the results in the SH-SY5Y and H838 cell lines were not reproducible by others.Citation91,Citation94,Citation255
In other attempts to demonstrate specificity of potential responses to EpoR, a putative JAK2 inhibitor (AG490) has been used and effects on rHuEpo signaling and other functional effects in cell lines reported.Citation132,Citation246,Citation275–Citation278 However, AG490 shows minimal JAK2 inhibitory activity in vitro.Citation279 Further, AG490 has been reported to also inhibit JAK3, EGFR, HER2, guanylyl cyclase C, and BCR-ABL.Citation279–Citation283 These data raise significant questions as to the validity of results from studies that have used AG490 to ascribe effects mediated through EpoR and JAK2.
In the studies reporting positive signaling effects of ESAs on tumors or tumor cell lines, increases in phosphorylation of ERK or AKT were reported.Citation205,Citation229,Citation272,Citation275,Citation276,Citation284 However, those results are in conflict with results from other groups who reported no effect on the same pathways using the same or similar cell types.Citation80,Citation91,Citation223,Citation232,Citation233,Citation259,Citation285 Interestingly, there are several reports where rHuEpo had no effects on phosphorylation of JAK2 or STAT5, but did have effects on ERK phosphorylation.Citation271,Citation272,Citation276,Citation284,Citation286–Citation288 In those experiments, cells were serum-starved to increase the signal-to-noise ratio, making them sensitive to minor manipulation/stimulatory effects. Because the MAPK, PI3K/AKT, and JAK2-STAT5 pathways are stimulated by multiple receptor ligand complexes beyond Epo,Citation289–Citation291 contaminating factors could produce similar effects. Indeed, signaling that had been suggested to be mediated through EpoR was mimicked in cell lines using a media change alone.Citation292 ESA-induced signaling can also be mimicked with endotoxin, which can accumulate in contaminated preparations and can enhance AKT and ERK phosphorylation.Citation293,Citation294 Bovine serum albumin (frequently used to stabilize ESA preparations), can also support cell growth as well as stimulate ERK phosphorylation of cell lines, particularly when serum-starved cells are used,Citation292,Citation295 due to contaminants such as IGF1Citation296 and insulin.Citation297
ESAs have also been evaluated for potential chemotaxis activity. In some studies, ESAs were reported to increase movement of cells in Matrigel in vitro.Citation271,Citation276,Citation278,Citation288 These data supported the hypothesis that ESAs could promote metastases of tumor cells. However, others reported no effect of ESAs on migration with the same or similar cell types.Citation232,Citation233,Citation298–Citation300 In some of the cell lines reported to migrate in Matrigel with ESAs (eg, MCF-7, HeLa), EpoR protein was undetectable,Citation78,Citation80 raising questions about the significance of data generated with those cell lines. Furthermore, the effects reported to be mediated by ESAs were generally small compared to molecules known to induce migration, such as EGF or FGF,Citation298,Citation300 and could be a result of endotoxin, a contaminant that can similarly stimulate migration.Citation301–Citation304
Effects of ESAs on tumor cell line proliferation have also been evaluated. However, in most studies, ESAs had no effect.Citation99,Citation205 For example, in a controlled study, though estradiol increased the proliferation of 29 tumor cell lines derived from multiple tissue sources, rHuEpo treatment did not.Citation305 These findings were supported by studies in other groups that evaluated multiple different cell lines.Citation80,Citation91,Citation300 However, in one study, rHuEpo was reported to enhance proliferation in a head and neck cell line LU-HNSCC-7 in serum-free medium (<1.4-fold increase). Notably, the authors commented that the effects observed could have been due to the medium change, although no control for that was presented.Citation233
In primary tumors from renal and colorectal tumors, rHuEpo was also unable to stimulate proliferation.Citation306 More recently, in a study with biopsies from a large cohort of patient samples with epithelial tumors (>180) from breast, colorectal, lung, ovary, head and neck, and kidney, rHuEpo was unable to increase the phosphorylation of AKT, ERK, or STAT5 ex vivo.Citation256 The lack of response may be explained by the lack of EpoR expression on those cellsCitation256 or the high incidence of constitutive activation of pathways rendering them insensitive to growth factor stimulation.Citation263
In vivo xenograft studies have been used to examine the effect of exogenously administered ESAs on cell growth or the ability to prevent cell ablation with chemotherapeutic agents or radiotherapy in rodents. In 31 different studies, there was no tumor growth or survival-promoting effects observed, even when high doses of ESAs were usedCitation99,Citation205 (). This may be explained, in part, because most of the cell lines examined expressed little to no EpoR, and therefore would not be expected to directly respond to ESAs. However, the lack of a tumor-promoting effect was not solely explained by insufficient EpoR, because even with cells (eg, in ovarian carcinoma line A2780) having tenfold-higher levels of EpoR due to forced overexpression, no growth-promoting effects with rHuEpo were observed.Citation232 Further, one group performed studies using mice that produced spontaneous tumors, but again no increase in tumor incidence or growth with rHuEpo treatment was observed.Citation307
Table 1 Effect of erythropoiesis-stimulating agents in xenograft or syngenic tumor models
In contrast to xenograft studies with ESAs, in vivo Epo antagonism studies have been described where the blockade of Epo–EpoR inhibited tumor growth.Citation64,Citation227,Citation272,Citation308 However, these reports are inconsistent with in vitro experiments demonstrating that the cell lines used expressed little/no EpoR and had no detectable response when treated with ESAs. Antagonism studies can be impacted by other inhibitors and factors, such as endotoxin in the preparations, that can inhibit tumor cells.Citation309 The possibility that the tumor growth inhibition reported was due to the experimental design also cannot be excluded, as negative controls were not included in those studies. Taken together, these data suggest that functional EpoR is not expressed on tumor cells.
Epo–EpoR autocrine/paracrine loops
Paracrine stimulation of EpoR in cells has been reported to support growth of Epo-responsive cell lines.Citation310,Citation311 Accordingly, some groups have also suggested that both Epo and EpoR are coexpressed in tumor cells and this may be a mechanism that drives autocrine tumor growth.Citation312–Citation314 Consistent with this possibility, some erythroleukemia cells were reported to express EpoCitation315,Citation316 and Epo was reported to support their growth.Citation317 Erythrocytosis is observed in some patients with renal carcinomas, liver carcinomas, in Wilms’ tumors and cerebellar hemangioblastomas.Citation47,Citation48,Citation318–Citation320 In VHL syndrome patients that contain pVHL mutations, paraneoplastic Epo production and erythrocytosis is associated with renal carcinoma, cysts, cerebellar hemangioblastoma, and pheochromocytoma.Citation321 However, in many of these cases, it is likely that Epo production is secondary to activation of the HIF pathway, or alternatively, secondary to tumor formation in cell types that normally produce Epo (eg, hepatocytes). Alternatively, tumors may produce other substances that can synergise with Epo and promote erythropoiesis, such as thyroid hormone, glucocorticoids, SCF, IL-3, or GM-CSF.
The possibility that tumors express both Epo and EpoR and that this is a driver of their growth is not supported by other data. Indeed, anemia and not erythrocytosis is a general characteristic of patients with solid tumors, suggesting that most tumor cells do not express significant amounts of Epo. Several groups reported that an Epo–EpoR cytokine loop is not a general property of tumors.Citation80,Citation322 Forced expression of Epo in mouse erythroid cells, using a human EPO gene under the control of a human β-globin locus control regulatory element, resulted in autocrine stimulation of erythropoiesis and erythrocytosis in transgenic mice. However, those mice did not develop erythroleukemia.Citation45 Similarly, constitutive Epo expression in the bone marrow of mice using retroviral vectors with EpoR expression cassettes resulted in erythrocytosis but not erythroleukemia,Citation45 and Epo gene therapy in mice did not result in tumors when Epo was overproduced.Citation323,Citation324
The suggestion that tumor cells may express Epo at levels sufficient to activate resident EpoR is based almost exclusively on IHC experiments on tumor sections or Western immunoblot analysis on tumor cells using nonvalidated anti-Epo polyclonal antibodies. In the kidney, where Epo is expressed at relatively high levels, Epo is secreted efficiently, resulting in very low intracellular stores. Consequently, attempts to identify the Epo-producing cell type by IHC with anti-Epo antibodies would be difficult and have been unsuccessful.Citation16,Citation51 This indicates that it would be even more difficult to detect Epo in tissue sections that have even lower Epo expression levels than in the kidney.Citation50 In addition, similar to anti-EpoR antibodies, many available anti-Epo antibodies used by investigators are also nonspecific (Amgen, unpublished data) raising further questions about the significance of positive IHC or Western data with anti-Epo antibodies.
Epo and angiogenesis
Blood vessel development consists of two distinct phases – vasculogenesis and angiogenesis. Vasculogenesis is the assembly of vessels de novo and angiogenesis arises through the proliferation, movement, and incorporation of endothelial cells into existing vessels.Citation325 Given the important role that Epo and EpoR play in regulating oxygen delivery, hypothetically Epo may also play a role in regulating blood flow through effects on the endothelium or through stimulation of blood vessel formation. Supporting this possibility, in EpoR and Epo knockout mouse embryos, though de novo vasculogenesis remained intact,Citation326,Citation327 a defect in angiogenesis was reported. Positive effects of Epo on vasculogenesis or angiogenesis using bone marrow-derived endothelial progenitor cells (EPCs) in vitro and in vivo have also been reported by some groups,Citation328–Citation332 but positive effects were not observed by others.Citation333–Citation336 ESAs have been reported to increase circulating levels of EPCs,Citation337–Citation341 and in the case of a subject with erythrocytosis caused by a mutation in EpoR resulting in hypersensitivity to Epo, there were increased levels of circulating EPCs.Citation342 However, interpretation of some of this positive data can be confusing, because a surface marker found on endothelial cells (endoglin: CD105)Citation343 is also expressed on erythroid cells,Citation343,Citation344 resulting in possible false-positive identification of EPCs with that marker.
In contrast to the data described above, there are other reports that ESAs did not affect the vasculature. For example, rHuEpo did not affect endothelial progenitor levelsCitation345,Citation346 or endothelial markers in patients receiving hemodialysis in clinical studies,Citation347 and Epo did not recruit BM-derived endothelial progenitor cells in BM-transplanted mice to neointima in arteries with wire-induced injury despite accelerating reendothelialization. Citation348 Further confounding the data are other studies suggesting BM-derived endothelial progenitor cells do not contribute to the vasculature.Citation349,Citation350 These included a study where EpoR−/− mice had normal vascular endothelium,Citation38 as did EpoR−/− mice crossed with transgenic mice where EpoR expression was restricted to the erythroid compartment.Citation351 Therefore, if EPCs do not even contribute to the vasculature, the role of Epo itself in possibly mobilizing the EPC becomes irrelevant. These conflicting studies raise questions about the significance of reports that ESAs affect endothelial progenitors.
In several independent studies, endothelial cells were reported neither to express significant levels of EpoR nor to respond to ESAs. In one study using a specific anti-EpoR antibody, A82, endothelial cell preparations expressed very low levels of total EpoR protein, with no detectable protein on the cell surface and no response to ESAs in vitro.Citation94 In other studies, rHuEpo had no effect on endothelial cell preparations in controlled in vitro and in vivo experiments.Citation94,Citation352–Citation354 In tumor xenograft studies, no effect on angiogenesis was observed when animals were administered ESAs.Citation355–Citation357
While several groups have reported that EpoR was present in endothelial cell preparations, the studies were based on the detection of EpoR using anti-EpoR antibodies that suffered from the same antibody nonspecificity issues described above. In ESA response studies, effects were only observed at supraphysiologic and suprapharmacologic levels of rHuEpo (>10 U/mL), a concentration which may be more prone to provide false-positive results. Some groups reported that [125I]rHuEpo bound to endothelial cell preparations,Citation129,Citation358,Citation359 but the binding properties included unusually high EpoR density and low affinity, characteristics more consistent with nonspecific or off-target binding.Citation268 Further, the high EpoR density reported did not correlate with the relatively low EpoR transcript levels or EpoR protein levels detected by Western analysis with a specific anti-EpoR antibody.Citation94 Increased thymidine incorporation into brain capillary endothelial cells following addition of rHuEpo was reported in one study, but only if the addition was accompanied by a change in growth medium,Citation358 raising concerns about potential artifacts. Artifact was most likely the reason that rHuEpo reportedly induced increased vascularization in chicken eggs (chick chorioallantoic membrane assay),Citation337,Citation360,Citation361 because there is no evidence of cross-species activity between human Epo and chicken EpoR.Citation362–Citation364
Cytoprotective effect of Epo on normal nonhematopoietic cells and tissues
In addition to erythropoietic defects in Epo or EpoR knockout mice, nonhematopoietic developmental defects in the heart and vasculature were also reported, suggesting a functional role for Epo–EpoR in those organs.Citation326,Citation365 This possibility was further evaluated in transgenic mice with EpoR expression limited to the hematopoietic compartment using a GATA-1 promoter linked to the EpoR gene.Citation351 Though the GATA-1-EpoR transgenic mice had no detectable EpoR mRNA expression outside the erythroid compartment using RT-PCR analysis, the mice developed normally and had normal organ function and vasculature. These data suggested that EpoR was not required for normal nonhematopoietic organ development, and that reported nonhematopoietic effects may have been mediated though indirect mechanisms, such as insufficient oxygen delivery due to the defect in erythropoiesis.
Cytoprotection studies in animals have been performed to evaluate the possibility that ESAs have nonhematopoietic effects. Overall, in a number of different animal studies (rodents, pigs, rabbits), ESAs were reported to enhance angiogenesis after injury in models of hypoxia-induced hypertensionCitation366 and peripheral hind limb ischemia,Citation367 and reduce tissue injury in heart,Citation368–Citation374 brain,Citation375–Citation377 kidney,Citation378,Citation379 and other organsCitation204,Citation367,Citation380–Citation383 using different injury model systems. Though these data suggest that ESAs have direct effects on nonhematopoietic tissues, the positive findings from these studies may be related to RBC increases, such as enhanced oxygen delivery or changes in ferrokinetics.Citation384 In the particular case of neuroprotection by ESAs, cerebrospinal fluid (CSF) Epo levels did not correlate with plasma Epo levels,Citation385 ESAs were not transported into the brain at significant levels,Citation386 and even though there was some increase in CSF levels of Epo where there was blood–brain barrier dysfunction, Epo concentrations were still very lowCitation385,Citation387 (1–3 mU/mL vs 10–30 in serum), raising questions about possible direct effects of ESA addition on brain function in animal or human studies.
In a conditional EpoR knockout study in mice with brain-specific inactivation of the EpoR gene, endogenous Epo–EpoR was found nonessential for protecting neurons from ischemic injury, though a role was suggested in poststroke neurogenesis.Citation388 In this study, mice with no EpoR expression in the brain had a slight reduction in proliferation and migration of neuroblasts to the peri-infarct cortex. A similar role of endogenous Epo–EpoR was suggested using another conditional EpoR knockout system.Citation389 In the absence of neural EpoR, a twofold increase in neural cell apoptosis and a two- to threefold decrease in neural progenitor cell proliferation compared to wild type was reported. However, the functional neurological impact of the findings in these two studies was not reported.
Although ESAs were reported to have cytoprotective activities by directly interacting with EpoR present on cells, the data supporting this hypothesis are confounded by a number of issues similar to those associated with the hypothesis that ESAs directly stimulate tumor cells. Some investigators reported EpoR mRNA was expressed in nonerythroid tissues and suggested functional EpoR protein may also be present.Citation176,Citation286,Citation358,Citation365,Citation370,Citation389–Citation392 However, EpoR mRNA levels in nonhematopoietic tissues were 5–1000 times lower than in bone marrow (see also ), and detection of EpoR mRNA in cell lines and endothelial cells did not predict surface expression.Citation94 Many of the investigators that reported EpoR protein expression in normal nonhematopoietic tissuesCitation390,Citation391,Citation393 used antibodies known to be nonspecific, most likely resulting in false-positive results.Citation76,Citation91,Citation97,Citation98,Citation248,Citation249,Citation394 Alternative approaches to determine surface protein, such as radiolabeled [125I]rHuEpo binding studies, found EpoR characteristics (high receptor number, low affinity) that are substantially different from EpoR characteristics on erythroid progenitor cells (low receptor number, high affinity).Citation11,Citation129,Citation235,Citation358,Citation359,Citation391 Recently, results using a specific anti-EpoR antibody (A82) indicated that EpoR was undetectable in most nonhematopoietic tissues from humans and mice (see ), raising further questions about the potential for ESAs to have a direct effect on nonhematopoietic tissues.Citation94,Citation255
ESAs were reported to activate downstream antiapoptotic signaling pathways in nonhematopoietic tissues, a mechanism that could inhibit cell death associated with tissue insult (eg, ischemia, reperfusion injury, and exposure to cytotoxins) in vitro.Citation369,Citation372,Citation375,Citation376,Citation389 For example, rHuEpo was reported to activate AKT and ERK signaling in cardiac myocytes in vitro, reducing apoptosis by ~30% upon exposure to hydrogen peroxide.Citation395 In studies evaluating the effects of ESAs on nonhematopoietic cell proliferation, signaling, or inhibition of apoptosis, modest effects (two- to threefold increases that are within the experimental noise of the system) were reported.Citation368,Citation375,Citation378,Citation395,Citation396 Many of these studies used cells starved of serum and did not describe the use of an appropriate vehicle control, both of which raise the possibility of nonspecific effects.Citation286,Citation375,Citation395,Citation397,Citation398 Furthermore, rHuEpo doses used for the in vitro studies were approximately tenfold higher (>10 U/mL) than levels achievable in patients with modest responses reported, raising the possibility of artifacts as well as questions about the physiological and clinical relevance of these findings.Citation286,Citation368,Citation370,Citation378,Citation396,Citation399
While the possibility that ESAs may be cytoprotective is supported by some studies, many of the in vivo studies with ESAs are conflicting. For example, though in two studies rHuEpo reduced ischemia reperfusion-induced renal injury and preserved renal function,Citation400,Citation401 in another study rHuEpo did not preserve renal function.Citation402 In studies using the same transgenic mouse model of amyotrophic lateral sclerosis, mixed findings have been reported. In one, rHuEpo delayed symptom onset and prolonged survival times.Citation403 In a second, rHuEpo delayed disease onset in females but not males,Citation404 and in the third, rHuEpo had minimal improvement in motor neuron function, with no effect on motor neuron loss or overall survival.Citation405 In another central nervous system (CNS) model, though high doses of rHuEpo (500–5000 U/kg daily) were reported to inhibit CNS inflammatory effects rats with experimental autoimmune encephalomyelitis,Citation406 no protective effect was found in animals with adjuvant arthritis, even when the same high-dosing regimen was used.Citation406
In other in vivo animal studies, ESAs did not provide nonhematopoietic protective effects. Pretreatment of rats with darbepoetin alfa did not alter endotoxin-evoked myocardial depression or the expression of proapoptotic or antiapoptotic genes in the heart.Citation407 rHuEpo was unable to provide neuroprotective effects in a rabbit bacterial meningitis model, even though the systemically administered rHuEpo was reported to penetrate the CNS in infected rabbits.Citation408 rHuEpo was also unable to prevent endotoxinemia-induced liver and kidney damage in rats.Citation408 Human clinical studies with tissue-protective end points have also been performed. To date, the cytoprotective effects reported in animal models have generally not translated into a clinical benefit in humans (reviewed in SøllingCitation409) who had injury to brain,Citation410–Citation412 heart,Citation413–Citation419 or kidney.Citation420–Citation426 Further, in a recent study, rHuEpo had no effect on intracellular signalling with human skeletal muscle.Citation427 Taken together, these data suggest that ESAs may not have the broad, reproducible, robust, nonhematopoietic protective abilities described by some investigators.
Alternative receptor complexes for Epo and Epo derivatives
An alternative receptor complex that can bind ESAs and mediate cytoprotective activity has been proposed based on the unusual binding affinities of ESA reported on nonhematopoietic cells. The proposed alternative receptor was reported to consist of a heteromeric complex of EpoR and the GM-CSF/ IL-3/IL-5 β-common chain (βc).Citation393 It was further proposed that a chemically modified Epo molecule (carbamoylated Epo [cEpo]) bound the alternative receptor complex and provided tissue-protective effects in the absence of stimulation of erythropoiesis. Citation428 Similar to rHuEpo, a number of model systems with various cytotoxic insults have been used to describe this cytoprotective activity of cEpo, such as inhibition of cardiac-myocyte apoptosis,Citation393,Citation429 improvement in cardiac function after permanent ischemia,Citation429 inhibition of renal tubule apoptosis, improvement in renal function after ischemia-reperfusion or obstructive injury,Citation430–Citation432 and reduction in neural lesions and apoptosis in the CNS with various rodent model systems.Citation433–Citation435 Data used to support the hypothetical cytoprotective role of the βc–EpoR heteromer were generated using mice in which the GM-CSF βc had been knocked out. Based on these data, cEpo and ESAs were reported to bind to the heteromer, activate signaling pathways, and prevent apoptosis in several normal nonhematopoietic tissues.Citation393,Citation397,Citation428 However, this hypothesis is controversial, as other investigators have found βc does not play a role in preventing apoptosis with ESAs.Citation176 It is particularly noteworthy that the investigators who initially generated the GM-CSF βc knockout miceCitation436 examined the receptor status and responsiveness of those animals thoroughly and concluded that there was no evidence of an interaction between the GM-CSF βc and EpoR.Citation437–Citation439
Summary and conclusions
Epo is an essential cytokine that binds and activates EpoR resident on the surface of erythroid progenitor cells, thereby promoting erythropoiesis. To this end, ESAs are currently indicated for treatment of anemia in patients with chronic kidney disease and chemotherapy-induced anemia. Epo has also been reported to have effects beyond erythropoiesis, such as tissue-protective effects and promotion of tumor cell growth or survival. This Epo–EpoR tumor stimulation hypothesis has been used to explain the safety signals seen in some clinical trails in anemic cancer patients treated with ESAs. However, putative positive results for this hypothesis are generally confounded by the absence of controls to detect false-positive effects and the use of nonspecific reagents in many studies. EpoR levels outside the erythroid compartment are very low, and the data that such low-level EpoR can bind significant amounts of Epo and promote a functional response are unconvincing. Further, in controlled clinical trials, the cytoprotective benefits observed in animal studies have not as yet translated into benefit in the clinic. The totality of evidence suggests that ESAs do not directly stimulate tumor cells and that similarly the cytoprotective and other nonhematopoietic effects of ESA treatment reported are not a direct effect of ESAs acting through EpoR on nonerythroid cells.
Acknowledgments
The authors thank Chip Stark for guidance on the manuscript.
Disclosure
Both authors are employees and/or hold stock in Amgen, Inc, a manufacturer of ESAs.
References
- KrantzSBErythropoietinBlood19917734194341991159
- GlaspyJCrawfordJVansteenkisteJErythropoiesis-stimulating agents in oncology: a study-level meta-analysis of survival and other safety outcomesBr J Cancer2010102230131520051958
- BohliusJToniaTSchwarzerGTwist and shout: one decade of metaanalyses of erythropoiesis-stimulating agents in cancer patientsActa Haematol20101251–2556721150188
- PapayannopoulouTAbkowitzJD’AndreaABiology of erythropoiesis, erythroid maturation and differentiationHoffmanRBenzEJShattilSJHematology Basic Principals and Practice3rd edPhiladelphiaChurchill Livingston2000202219
- HuntJAHalf-life and rate of synthesis of globin messenger ribonucleic acid. Determination of half-life of messenger ribonucleic acid and its relative synthetic rate in erythroid cellsBiochem J197413834874984429545
- DevAFangJSathyanarayanaPPradeepAEmersonCWojchowskiDMDuring EPO or anemia challenge, erythroid progenitor cells transit through a selectively expandable proerythroblast poolBlood2010116245334534620810925
- GlaserKLimarziLRPoncherHGCellular composition of the bone marrow in normal infants and childrenPediatrics19506578982414797342
- SawyerSTKrantzSBGoldwasserEBinding and receptor-mediated endocytosis of erythropoietin in Friend virus-infected erythroid cellsJ Biol Chem198726212555455623032937
- MigliaccioGMigliaccioARVisserJWSynergism between erythropoietin and interleukin-3 in the induction of hematopoietic stem cell proliferation and erythroid burst colony formationBlood19887239449513262001
- BackxBBroedersLHoefslootLHWognumBLowenbergBErythropoiesis in myelodysplastic syndrome: expression of receptors for erythropoietin and kit ligandLeukemia19961034664728642863
- SawadaKKrantzSBDaiCHPurification of human blood burst-forming units-erythroid and demonstration of the evolution of erythropoietin receptorsJ Cell Physiol199014222192302154501
- SmithJAExercise, training and red blood cell turnoverSports Med19951919317740249
- EschbachJWThe anemia of chronic renal failure: pathophysiology and the effects of recombinant erythropoietinKidney Int19893511341482651751
- LawMLCaiGYLinFKChromosomal assignment of the human erythropoietin gene and its DNA polymorphismProc Natl Acad Sci U S A19868318692069243462737
- JacobsKShoemakerCRudersdorfRIsolation and characterization of genomic and cDNA clones of human erythropoietinNature198531360058068103838366
- LeHirMEckardtKUKaisslingBKourySTKurtzAStructure-function correlations in erythropoietin formation and oxygen sensing in the kidneyKlin Wochenschr199169135675751753679
- EschbachJWEgrieJCDowningMRBrowneJKAdamsonJWCorrection of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trialNew Engl J Med1987316273783537801
- ElliottSEgrieJBrowneJControl of rHuEPO biological activity: the role of carbohydrateExp Hematol200432121146115515588939
- EgrieJCStricklandTWLaneJCharacterization and biological effects of recombinant human erythropoietinImmunobiology19861723–52132243542810
- WickremaAKrantzSBWinkelmannJCBondurantMCDifferentiation and erythropoietin receptor gene expression in human erythroid progenitor cellsBlood1992808194019491391953
- KouryMJBondurantMCErythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cellsScience199024849533783812326648
- RogersHMYuXWenJSmithRFibachENoguchiCTHypoxia alters progression of the erythroid programExp Hematol2008361172717936496
- WangGLJiangBHRueEASemenzaGLHypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tensionProc Natl Acad Sci U S A19959212551055147539918
- JiangBHRueEWangGLRoeRSemenzaGLDimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1J Biol Chem19962713017771177788663540
- RankinEBBijuMPLiuQHypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivoJ Clin Invest200711741068107717404621
- YamashitaTOhnedaOSakiyamaAIwataFOhnedaKFujii-KuriyamaYThe microenvironment for erythropoiesis is regulated by HIF-2alpha through VCAM-1 in endothelial cellsBlood200811241482149218451309
- KapitsinouPPLiuQUngerTLHepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemiaBlood2010116163039304820628150
- PaliegeARosenbergerCBondkeAHypoxia-inducible factor- 2alpha-expressing interstitial fibroblasts are the only renal cells that express erythropoietin under hypoxia-inducible factor stabilizationKidney Int201077431231820016470
- EpsteinACGleadleJMMcNeillLAC. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylationCell20011071435411595184
- MassonNWillamCMaxwellPHPughCWRatcliffePJIndependent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylationEMBO J200120185197520611566883
- BruickRKMcKnightSLA conserved family of prolyl-4-hydroxylases that modify HIFScience200129455451337134011598268
- IvanMKondoKYangHHIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensingScience2001292551646446811292862
- HonWCWilsonMIHarlosKStructural basis for the recognition of hydroxyproline in HIF-1 alpha by pVHLNature2002417689297597812050673
- MaxwellPHPughCWRatcliffePJInsights into the role of the von Hippel-Lindau gene product. A key player in hypoxic regulationExp Nephrol20019423524011423722
- KourySTKouryMJBondurantMCCaroJGraberSEQuantitation of erythropoietin-producing cells in kidneys of mice by in situ hybridization: correlation with hematocrit, renal erythropoietin mRNA, and serum erythropoietin concentrationBlood19897426456512752138
- ObaraNSuzukiNKimKNagasawaTImagawaSYamamotoMRepression via the GATA box is essential for tissue-specific erythropoietin gene expressionBlood2008111105223523218202227
- ErslevAJWilsonJCaroJErythropoietin titers in anemic, nonuremic patientsJ Lab Clin Med198710944294333102659
- LinCSLimSKD’AgatiVCostantiniFDifferential effects of an erythropoietin receptor gene disruption on primitive and definitive erythropoiesisGenes Dev19961021541648566749
- MizunoSSasakiJSuzukiCKonoHKojimaSEffect of recombinant human erythropoietin administration on peripheral blood neutrophil counts of premature infantsJ Pediatr199412434674708120723
- ShannonKMMentzerWCAbelsRIRecombinant human erythropoietin in the anemia of prematurity: results of a placebo-controlled pilot studyJ Pediatr199111869499552040933
- WardCSWestwoodNBEmmersonAJPearsonTCThe in vitro effect of high-dose recombinant human erythropoietin on granulocyte-macrophage colony production in premature infants using a defined serum deprived cell culture systemBr J Haematol19928133253301382542
- UlichTRdelCJYinSMEgrieJCThe erythropoietic effects of interleukin 6 and erythropoietin in vivoExp Hematol199119129341989892
- SingbrantSRussellMRJovicTErythropoietin couples erythropoiesis, B-lymphopoiesis, and bone homeostasis within the bone marrow microenvironmentBlood2011117215631564221421837
- RamsfjellVBorgeOJVeibyOPThrombopoietin, but not erythropoietin, directly stimulates multilineage growth of primitive murine bone marrow progenitor cells in synergy with early acting cytokines: distinct interactions with the ligands for c-kit and FLT3Blood19968812448144928977240
- MadanALinCWangZCurtinPTAutocrine stimulation by erythropoietin in transgenic mice results in erythroid proliferation without neoplastic transformationBlood Cells Mol Dis2003301828912667989
- FurukawaTNaritaMSakaueMPrimary familial polycythaemia associated with a novel point mutation in the erythropoietin receptorBr J Haematol19979912222279359528
- SherwoodJBThe chemistry and physiology of erythropoietinVitam Horm1984411612116397910
- JelkmannWErythropoietin: structure, control of production, and functionPhysiol Rev19927224494891557429
- BeruNMcDonaldJLacombeCGoldwasserEExpression of the erythropoietin geneMol Cell Biol198667257125753466025
- BachmannSLeHMEckardtKUCo-localization of erythropoietin mRNA and ecto-5′-nucleotidase immunoreactivity in peritubular cells of rat renal cortex indicates that fibroblasts produce erythropoietinJ Histochem Cytochem19934133353418429197
- MaxwellPHOsmondMKPughCWIdentification of the renal erythropoietin-producing cells using transgenic miceKidney Int1993445114911628264149
- TanCCEckardtKURatcliffePJOrgan distribution of erythropoietin messenger RNA in normal and uremic ratsKidney Int199140169761921157
- SemenzaGLKourySTNejfeltMKGearhartJDAntonarakisSECell-type-specific and hypoxia-inducible expression of the human erythropoietin gene in transgenic miceProc Natl Acad Sci U S A19918819872587291924331
- FandreyJBunnHFIn vivo and in vitro regulation of erythropoietin mRNA: measurement by competitive polymerase chain reactionBlood19938136176238381307
- SuzukiNObaraNYamamotoMUse of gene-manipulated mice in the study of erythropoietin gene expressionMethods Enzymol200743515717717998054
- DavidRBLimGBMoritzKMKoukoulasIWintourEMQuantitation of the mRNA levels of Epo and EpoR in various tissues in the ovine fetusMol Cell Endocrinol20021881–220721811911958
- BondurantMCKouryMJKourySTSemenzaGErythropoietin ontogeny and organ distribution in miceSemin Hematol1991283 Suppl 320251891721
- ErslevAJCaroJKansuESilverRRenal and extrarenal erythropoietin production in anaemic ratsBr J Haematol198045165727378330
- BondurantMCKouryMJAnemia induces accumulation of erythropoietin mRNA in the kidney and liverMol Cell Biol198667273127333785209
- EckardtKURatcliffePJTanCCBauerCKurtzAAge-dependent expression of the erythropoietin gene in rat liver and kidneysJ Clin Invest19928937537601541670
- KourySTBondurantMCKouryMJSemenzaGLLocalization of cells producing erythropoietin in murine liver by in situ hybridizationBlood19917711249725032039831
- FredeSFreitagPGeutingLKonietznyRFandreyJOxygen-regulated expression of the erythropoietin gene in the human renal cell line REPCBlood2011117184905491421406725
- LoyaFYangYLinHGoldwasserEAlbitarMTransgenic mice carrying the erythropoietin gene promoter linked to lacZ express the reporter in proximal convoluted tubule cells after hypoxiaBlood1994846183118368080988
- JeongJYFeldmanLSolarPSzenajchJSytkowskiAJCharacterization of erythropoietin receptor and erythropoietin expression and function in human ovarian cancer cellsInt J Cancer2008122227428017893874
- JeongJYHoxhajGSochaALSytkowskiAJFeldmanLAn erythropoietin autocrine/paracrine axis modulates the growth and survival of human prostate cancer cellsMol Cancer Res2009771150115719567780
- AcsGZhangPJRebbeckTRAcsPVermaAImmunohistochemical expression of erythropoietin and erythropoietin receptor in breast carcinomaCancer200295596998112209679
- GombosZDanihelLRepiskaVAcsGFurthEExpression of erythropoietin and its receptor increases in colonic neoplastic progression: the role of hypoxia in tumorigenesisIndian J Pathol Microbiol201154227327821623073
- SanchezPENavarroFPFaresRPErythropoietin receptor expression is concordant with erythropoietin but not with common beta chain expression in the rat brain throughout the life spanJ Comp Neurol2009514440341419330822
- Castillo-MelendezMYanEWalkerDWExpression of erythropoietin and its receptor in the brain of late-gestation fetal sheep, and responses to asphyxia caused by umbilical cord occlusionDev Neurosci2005272–422022716046857
- ChungYHKimSIJooKMAge-related changes in erythropoietin immunoreactivity in the cerebral cortex and hippocampus of ratsBrain Res20041018114114615262216
- KnabeWKnerlichFWashausenSExpression patterns of erythropoietin and its receptor in the developing midbrainAnat Embryol (Berl)2004207650351214770308
- WinkelmannJCPennyLADeavenLLForgetBGJenkinsRBThe gene for the human erythropoietin receptor: analysis of the coding sequence and assignment to chromosome 19pBlood199076124302163695
- JonesSSD’AndreaADHainesLLWongGGHuman erythropoietin receptor: cloning, expression, and biologic characterizationBlood199076131352163696
- D’AndreaADLodishHFWongGGExpression cloning of the murine erythropoietin receptorCell19895722772852539263
- BudarfMHuebnerKEmanuelBAssignment of the erythropoietin receptor (EPOR) gene to mouse chromosome 9 and human chromosome 19Genomics1990835755781962754
- ElliottSBusseLBassMBAnti-Epo receptor antibodies do not predict Epo receptor expressionBlood200610751892189516249375
- AtkinsHLBroudyVCPapayannopoulouTCharacterization of the structure of the erythropoietin receptor by ligand blottingBlood19917712257725821646043
- ElliottSBusseLMcCafferyIIdentification of a sensitive antierythropoietin receptor monoclonal antibody allows detection of low levels of EpoR in cellsJ Immunol Methods201035212613919887071
- KettelerRHeinrichACOffeJKA functional green fluorescent protein-erythropoietin receptor despite physical separation of JAK2 binding site and tyrosine residuesJ Biol Chem200227729265472655211997394
- SwiftSEllisonARKassnerPAbsence of functional EpoR expression in human tumor cell linesBlood2010115214254426320124514
- NeumannDWikstromLWatowichSSLodishHFIntermediates in degradation of the erythropoietin receptor accumulate and are degraded in lysosomesJ Biol Chem19932681813639136498514796
- HiltonDJWatowichSSMurrayPJLodishHFIncreased cell surface expression and enhanced folding in the endoplasmic reticulum of a mutant erythropoietin receptorProc Natl Acad Sci U S A19959211901947816815
- Supino-RosinLYoshimuraAAltaratzHNeumannDA cytosolic domain of the erythropoietin receptor contributes to endoplasmic reticulum-associated degradationEur J Biochem1999263241041910406949
- BilliaFBarbaraMMcEwenJTrevisanMIscoveNNResolution of pluripotential intermediates in murine hematopoietic differentiation by global complementary DNA amplification from single cells: confirmation of assignments by expression profiling of cytokine receptor transcriptsBlood20019782257226811290586
- LiuZYChinKNoguchiCTTissue specific expression of human erythropoietin receptor in transgenic miceDev Biol199416611591697958443
- ChibaTIkawaYTodokoroKGATA-1 transactivates erythropoietin receptor gene, and erythropoietin receptor-mediated signals enhance GATA-1 gene expressionNucleic Acids Res19911914384338481650452
- HeberleinCFischerKDStoffelMThe gene for erythropoietin receptor is expressed in multipotential hematopoietic and embryonal stem cells: evidence for differentiation stage-specific regulationMol Cell Biol1992124181518261312671
- HeinrichACPelandaRKlingmullerUA mouse model for visualization and conditional mutations in the erythroid lineageBlood2004104365966615090451
- AshiharaEVannucchiAMMigliaccioGMigliaccioARGrowth factor receptor expression during in vitro differentiation of partially purified populations containing murine stem cellsJ Cell Physiol199717133433569180904
- ChibaSTakahashiTTakeshitaKSelective expression of mRNA coding for the truncated form of erythropoietin receptor in hematopoietic cells and its decrease in patients with polycythemia veraBlood1997901971049207443
- LaugschMMetzenESvenssonTDeppingRJelkmannWLack of functional erythropoietin receptors of cancer cell linesInt J Cancer200812251005101117990315
- SinclairAMRogersNBusseLErythropoietin receptor transcription is neither elevated nor predictive of surface expression in human tumour cellsBr J Cancer20089861059106718349818
- KellerMAAddyaSVadigepalliRTranscriptional regulatory network analysis of developing human erythroid progenitors reveals patterns of coregulation and potential transcriptional regulatorsPhysiol Genomics200628111412816940433
- SinclairAMCoxonAMcCafferyIFunctional erythropoietin receptor is undetectable in endothelial, cardiac, neuronal, and renal cellsBlood2010115214264427220124513
- HardeeMEArcasoyMOBlackwellKLKirkpatrickJPDewhirstMWErythropoietin biology in cancerClin Cancer Res200612233233916428469
- KokhaeiPAbdallaAOHanssonLExpression of erythropoietin receptor and in vitro functional effects of epoetins in B-cell malignanciesClin Cancer Res200713123536354417575216
- BrownWMMaxwellPGrahamANErythropoietin receptor expression in non-small cell lung carcinoma: a question of antibody specificityStem Cells200725371872217110616
- KirkebyAvanBJNielsenJLeistMHelboeLFunctional and immunochemical characterisation of different antibodies against the erythropoietin receptorJ Neurosci Methods20071641505817524492
- SinclairAMToddMDForsytheKKnoxSJElliottSBegleyCGExpression and function of erythropoietin receptors in tumors: implications for the use of erythropoiesis-stimulating agents in cancer patientsCancer2007110347748817582631
- OsterborgAAaproMCornesPHaselbeckAHaywardCRJelkmannWPreclinical studies of erythropoietin receptor expression in tumour cells: impact on clinical use of erythropoietic proteins to correct cancer-related anaemiaEur J Cancer200743351051917150352
- KirkpatrickPSpecificity concerns with antibodies for receptor mappingNat Rev Drug Discov20098427819348032
- BordeauxJWelshAAgarwalSAntibody validationBiotechniques201048319720920359301
- BroudyVCLinNEgrieJIdentification of the receptor for erythropoietin on human and murine erythroleukemia cells and modulation by phorbol ester and dimethyl sulfoxideProc Natl Acad Sci U S A19888517651365172842774
- BroudyVCLinNBriceMNakamotoBPapayannopoulouTErythropoietin receptor characteristics on primary human erythroid cellsBlood19917712258325901646044
- RadinskyRRisinSFanDLevel and function of epidermal growth factor receptor predict the metastatic potential of human colon carcinoma cellsClin Cancer Res19951119319815883
- FoonKAYangXDWeinerLMPreclinical and clinical evaluations of ABX-EGF, a fully human anti-epidermal growth factor receptor antibodyInt J Radiat Oncol Biol Phys200458398499014967460
- SasakiRYanagawaSHitomiKChibaHCharacterization of erythropoietin receptor of murine erythroid cellsEur J Biochem1987168143482822405
- McArthurGALongmoreGDKlinglerKJohnsonGRLineage-restricted recruitment of immature hematopoietic progenitor cells in response to Epo after normal hematopoietic cell transfection with EpoRExp Hematol19952376456547601257
- SawadaKKrantzSBSawyerSTCivinCIQuantitation of specific binding of erythropoietin to human erythroid colony-forming cellsJ Cell Physiol198813723373453192618
- WognumAWLansdorpPMHumphriesRKKrystalGDetection and isolation of the erythropoietin receptor using biotinylated erythropoietinBlood19907646977052166605
- WognumAWKrystalGEavesCJEavesACLansdorpPMIncreased erythropoietin-receptor expression on CD34-positive bone marrow cells from patients with chronic myeloid leukemiaBlood19927936426491370638
- ShinjoKTakeshitaAHiguchiMOhnishiKOhnoRErythropoietin receptor expression on human bone marrow erythroid precursor cells by a newly-devised quantitative flow-cytometric assayBr J Haematol19979635515589054663
- MayeuxPBillatCJacquotRThe erythropoietin receptor of rat erythroid progenitor cells. Characterization and affinity cross-linkageJ Biol Chem19872622913985139902820989
- HoshinoSTeramuraMTakahashiMExpression and characterization of erythropoietin receptors on normal human bone marrow cellsInt J Cell Cloning1989731561672543713
- FraserJKLinFKBerridgeMVExpression of high affinity receptors for erythropoietin on human bone marrow cells and on the human erythroleukemic cell line, HELExp Hematol198816108368422844573
- WuHLiuXJaenischRLodishHFGeneration of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptorCell199583159677553874
- MitjavilaMTNatazawaMBrignaschiPDebiliNBreton-GoriusJVainchenkerWEffects of five recombinant hematopoietic growth factors on enriched human erythroid progenitors in serum-replaced culturesJ Cell Physiol198913836176232647773
- CullVTilbrookPAAdenanASDominant action of mutated erythropoietin receptors on differentiation in vitro and erythroleukemia development in vivoOncogene200019795396010702804
- HuangLJConstantinescuSNLodishHFThe N-terminal domain of Janus kinase 2 is required for Golgi processing and cell surface expression of erythropoietin receptorMol Cell2001861327133811779507
- WickremaABondurantMCKrantzSBAbundance and stability of erythropoietin receptor mRNA in mouse erythroid progenitor cellsBlood1991789226922751657247
- LodishHFHiltonDLongmoreGWatowichSSYoshimuraAThe erythropoietin receptor: dimerization, activation, and tumorigenesisCold Spring Harb Symp Quant Biol199257951061339709
- YoussoufianHZonLIOrkinSHD’AndreaADLodishHFStructure and transcription of the mouse erythropoietin receptor geneMol Cell Biol1990107367536822162479
- ZonLIYoussoufianHMatherCLodishHFOrkinSHActivation of the erythropoietin receptor promoter by transcription factor GATA-1Proc Natl Acad Sci U S A1991882310638106411660143
- MaoucheLCartronJPChretienSDifferent domains regulate the human erythropoietin receptor gene transcriptionNucleic Acids Res19942233383468127671
- YoussoufianHFurther characterization of cis-acting regulatory sequences in the genomic locus of the murine erythropoietin receptor: evidence for stage-specific regulationBlood1994835142814358118044
- KuramochiSIkawaYTodokoroKCharacterization of murine erythropoietin receptor genesJ Mol Biol199021635675752175360
- SingbrantSRussellMRJovicErythropoietin couples erythropoiesis, B lymphopoiesis, and bone homeostasis within the bone marrow microenvironmentBlood2011117215631564221421837
- HagstromLAgbulutOEl-Hasnaoui-SaadaniREpo is relevant neither for microvascular formation nor for the new formation and maintenance of mice skeletal muscle fibres in both normoxia and hypoxiaJ Biomed Biotechnol2010201013781720414335
- BanerjeeDRodriguezMNagMAdamsonJWExposure of endothelial cells to recombinant human erythropoietin induces nitric oxide synthase activityKidney Int20005751895190410792608
- OhigashiTYoshiokaKFisherJWAutocrine regulation of erythropoietin gene expression in human hepatocellular carcinoma cellsLife Sci19965854214278594307
- DigicayliogluMBichetSMartiHHLocalization of specific erythropoietin binding sites in defined areas of the mouse brainProc Natl Acad Sci U S A1995929371737207731971
- MohyeldinADalgardCLLuHSurvival and invasiveness of astrocytomas promoted by erythropoietinJ Neurosurg2007106233835017410721
- BrameyTFreitagPFandreyJNo evidence for protective erythropoietin alpha signalling in rat hepatocytesBMC Gastroenterol200992619383129
- WinterSCShahKACampoLRelation of erythropoietin and erythropoietin receptor expression to hypoxia and anemia in head and neck squamous cell carcinomaClin Cancer Res200511217614762016278379
- BernaudinMBellailAMartiHHNeurons and astrocytes express EPO mRNA: oxygen-sensing mechanisms that involve the redox-state of the brainGlia200030327127810756076
- WincewiczAKodaMSulkowskaMKanczuga-KodaLWincewiczDSulkowskiSSTAT3 and hypoxia induced proteins – HIF-1alpha, EPO and EPOR in relation with Bax and Bcl-xL in nodal metastases of ductal breast cancersFolia Histochem Cytobiol200947342543020164027
- BatraSPerelmanNLuckLRShimadaHMalikPPediatric tumor cells express erythropoietin and a functional erythropoietin receptor that promotes angiogenesis and tumor cell survivalLab Invest200383101477148714563949
- LamSYTipoeGLFungMLUpregulation of erythropoietin and its receptor expression in the rat carotid body during chronic and intermittent hypoxiaAdv Exp Med Biol200964820721419536483
- ChenZYWangLAsavaritkraiPNoguchiCTUp-regulation of erythropoietin receptor by nitric oxide mediates hypoxia preconditioningJ Neurosci Res201088143180318820806411
- AcsGAcsPBeckwithSMErythropoietin and erythropoietin receptor expression in human cancerCancer Res20016193561356511325818
- PevnyLLinCSD’AgatiVSimonMCOrkinSHCostantiniFDevelopment of hematopoietic cells lacking transcription factor GATA-1Development199512111631727867497
- PevnyLSimonMCRobertsonEErythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1Nature199134963062572601987478
- SimonMCPevnyLWilesMVKellerGCostantiniFOrkinSHRescue of erythroid development in gene targeted GATA-1- mouse embryonic stem cellsNat Genet19921292981302015
- KomatsuNKiritoKKashiiYCell-cycle-dependent regulation of erythropoietin receptor geneBlood1997894118211889028940
- SuzukiNSuwabeNOhnedaOIdentification and characterization of 2 types of erythroid progenitors that express GATA-1 at distinct levelsBlood2003102103575358312893747
- CrottaSNicolisSRonchiAProgressive inactivation of the expression of an erythroid transcriptional factor in GM- and G-CSF-dependent myeloid cell linesNucleic Acids Res19901823686368691702202
- MigliaccioARMigliaccioGD’AndreaAResponse to erythropoietin in erythroid subclones of the factor-dependent cell line 32D is determined by translocation of the erythropoietin receptor to the cell surfaceProc Natl Acad Sci U S A1991882411086110901722318
- TsangAPFujiwaraYHomDBOrkinSHFailure of megakaryopoiesis and arrested erythropoiesis in mice lacking the GATA-1 transcriptional cofactor FOGGenes Dev1998128117611889553047
- SnowJWOrkinSHTranslational isoforms of FOG1 regulate GATA1- interacting complexesJ Biol Chem200928443293102931919654328
- KassoufMTHughesJRTaylorSGenome-wide identification of TAL1’s functional targets: insights into its mechanisms of action in primary erythroid cellsGenome Res20102081064108320566737
- MikkolaHKKlintmanJYangHHaematopoietic stem cells retain long-term repopulating activity and multipotency in the absence of stem-cell leukaemia SCL/tal-1 geneNature2003421692254755112540851
- GreenARSalvarisEBegleyCGErythroid expression of the ‘helix-loop- helix’ gene, SCLOncogene1991634754792011404
- VisvaderJBegleyCGAdamsJMDifferential expression of the LYL, SCL and E2 A helix-loop-helix genes within the hemopoietic systemOncogene1991621871942000219
- InthalAKrapfGBeckDRole of the erythropoietin receptor in ETV6/RUNX1-positive acute lymphoblastic leukemiaClin Cancer Res200814227196720419010836
- GreenARLintsTVisvaderJHarveyRBegleyCGSCL is coexpressed with GATA-1 in hemopoietic cells but is also expressed in developing brainOncogene1992746536601565464
- MaoucheLCartronJPChretienSDifferent domains regulate the human erythropoietin receptor gene transcriptionNucleic Acids Res19942233383468127671
- NoguchiCTBaeKSChinKWadaYSchechterANHankinsWDCloning of the human erythropoietin receptor geneBlood19917810254825561668606
- PhiloJSAokiKHArakawaTNarhiLOWenJDimerization of the extracellular domain of the erythropoietin (EPO) receptor by EPO: one high-affinity and one low-affinity interactionBiochemistry1996355168116918634300
- SyedRSReidSWLiCEfficiency of signalling through cytokine receptors depends critically on receptor orientationNature199839567015115169774108
- WatowichSSActivation of erythropoietin signaling by receptor dimerizationInt J Biochem Cell Biol199931101075108810582340
- GrossAWLodishHFCellular trafficking and degradation of erythropoietin and novel erythropoiesis stimulating protein (NESP)J Biol Chem200628142024203216286456
- BeckmanDLLinLLQuinonesMELongmoreGDActivation of the erythropoietin receptor is not required for internalization of bound erythropoietinBlood19999482667267510515870
- NarhiLOAokiKHPhiloJSArakawaTChanges in conformation and stability upon formation of complexes of erythropoietin (EPO) and soluble EPO receptorJ Protein Chem19971632132259155092
- RemyIWilsonIAMichnickSWErythropoietin receptor activation by a ligand-induced conformation changeScience199928354049909939974393
- LuXGrossAWLodishHFActive conformation of the erythropoietin receptor: random and cysteine-scanning mutagenesis of the extracellular juxtamembrane and transmembrane domainsJ Biol Chem2006281117002701116414957
- WojchowskiDMGregoryRCMillerCPPanditAKPircherTJSignal transduction in the erythropoietin receptor systemExp Cell Res1999253114315610579919
- JegalianAGWuHDifferential roles of SOCS family members in EpoR signal transductionJ Interferon Cytokine Res200222885386012396724
- MinooPZadehMMRottapelRLebrunJJAliSA novel SHP-1/ Grb2-dependent mechanism of negative regulation of cytokine-receptor signaling: contribution of SHP-1 C-terminal tyrosines in cytokine signalingBlood200410341398140714551136
- VerdierFWalrafenPHubertNProteasomes regulate the duration of erythropoietin receptor activation by controlling down-regulation of cell surface receptorsJ Biol Chem200027524183751838110849444
- KubatzkyKFRuanWGurezkaRSelf assembly of the transmembrane domain promotes signal transduction through the erythropoietin receptorCurr Biol200111211011511231127
- BinderCLafayetteAArchibequeIOptimization and utilization of the SureFire phospho-STAT5 assay for a cell-based screening campaignAssay Drug Dev Technol200861273718336085
- TakahashiTChibaSHiranoNYazakiYHiraiHCharacterization of three erythropoietin (Epo)-binding proteins in various human Eporesponsive cell lines and in cells transfected with human Epo-receptor cDNABlood19958511061147803787
- SantucciMAPierceJHZanniniSErythropoietin increases the radioresistance of a clonal hematopoietic progenitor cell line expressing a transgene for the erythropoietin receptorStem Cells19941255065137804124
- YawataHYasukawaKNatsukaSStructure-function analysis of human IL-6 receptor: dissociation of amino acid residues required for IL-6-binding and for IL-6 signal transduction through gp130EMBO J1993124170517128467812
- JegalianAGAcurioADranoffGWuHErythropoietin receptor haploinsufficiency and in vivo interplay with granulocytemacrophage colony-stimulating factor and interleukin 3Blood20029972603260511895800
- UmMGrossAWLodishHFA “classical” homodimeric erythropoietin receptor is essential for the antiapoptotic effects of erythropoietin on differentiated neuroblastoma SH-SY5Y and pheochromocytoma PC-12 cellsCell Signal200719363464517045782
- PregiNVittoriDPerezGLeirosCPNesseAEffect of erythropoietin on staurosporine-induced apoptosis and differentiation of SH-SY5Y neuroblastoma cellsBiochim Biophys Acta20061763223824616500719
- RosslerJStolzeIFredeSHypoxia-induced erythropoietin expression in human neuroblastoma requires a methylation free HIF-1 binding siteJ Cell Biochem200493115316115352172
- DrexlerHGMatsuoYMacLeodRAMalignant hematopoietic cell lines: in vitro models for the study of erythroleukemiaLeuk Res200428121243125115475063
- TodokoroKKanazawaSAmanumaHIkawaYCharacterization of erythropoietin receptor on erythropoietin-unresponsive mouse erythroleukemia cellsBiochim Biophys Acta198894323263302840960
- MayeuxPBillatCJacquotRMurine erythroleukaemia cells (Friend cells) possess high-affinity binding sites for erythropoietinFEBS Lett198721122292333467981
- EhrenmanKStJTThe erythropoietin receptor gene: cloning and identification of multiple transcripts in an erythroid cell line OCIM1Exp Hematol19911999739771654273
- ZouXCalameKSignaling pathways activated by oncogenic forms of Abl tyrosine kinaseJ Biol Chem199927426181411814410373409
- LozzioCBLozzioBBHuman chronic myelogenous leukemia cell-line with positive Philadelphia chromosomeBlood1975453321334163658
- ElliottSBusseLSwiftSLack of expression and function of erythropoietin receptors in kidneyNephrol Dial Transplant2012 Epub December 13, 2011
- GaffenSLLaiSYLongmoreGDLiuKDGoldsmithMAGenetic evidence for an additional factor required for erythropoietin-induced signal transductionBlood1999941748610381500
- D’AndreaADJonesSSActivation of the erythropoietin receptor in stable lymphoid and myeloid transfectantsSemin Hematol19912821521571652158
- ShikamaYBarberDLD’AndreaADSieffCAA constitutively activated chimeric cytokine receptor confers factor-independent growth in hematopoietic cell linesBlood19968824554648695792
- WakaoHHaradaNKitamuraTMuiALMiyajimaAInterleukin 2 and erythropoietin activate STAT5/MGF via distinct pathwaysEMBO J19951411252725357781605
- YamamuraYKageyamaYMatuzakiTNodaMIkawaYDistinct downstream signaling mechanism between erythropoietin receptor and interleukin-2 receptorEMBO J19921113490949151464316
- MinamotoSTreismanJHankinsWDSugamuraKRosenbergSAAcquired erythropoietin responsiveness of interleukin-2- dependent T lymphocytes retrovirally transduced with genes encoding chimeric erythropoietin/interleukin-2 receptorsBlood1995866228122877662975
- LongmoreGDLodishHFAn activating mutation in the murine erythropoietin receptor induces erythroleukemia in mice: a cytokine receptor superfamily oncogeneCell1991676108911021662116
- KangJKChangCHNamHJDownregulation of erythropoietin receptor by overexpression of phospholipase C-gamma 1 is critical for decrease on focal adhesion in transformed cellsCell Oncol20113411121
- LayonMEAckleyCJWestRJLowreyCHExpression of GATA-1 in a non-hematopoietic cell line induces beta-globin locus control region chromatin structure remodeling and an erythroid pattern of gene expressionJ Mol Biol2007366373774417196618
- MiuraYMiuraOIhleJNAokiNActivation of the mitogen-activated protein kinase pathway by the erythropoietin receptorJ Biol Chem19942694729962299697961995
- ZhanXGoldfarbMGrowth factor requirements of oncogene-transformed NIH 3T3 and BALB/c 3T3 cells cultured in defined mediaMol Cell Biol1986610354135443796592
- PharrPNOgawaMHofbauerALongmoreGDExpression of an activated erythropoietin or a colony-stimulating factor 1 receptor by pluripotent progenitors enhances colony formation but does not induce differentiationProc Natl Acad Sci U S A19949116748274868052607
- LongmoreGDPharrPNeumannDLodishHFBoth megakaryocytopoiesis and erythropoiesis are induced in mice infected with a retrovirus expressing an oncogenic erythropoietin receptorBlood1993828238623958400289
- LongmoreGDPharrPNLodishHFA constitutively activated erythropoietin receptor stimulates proliferation and contributes to transformation of multipotent, committed nonerythroid and erythroid progenitor cellsMol Cell Biol1994144226622778139532
- HenkeMLaszigRRubeCErythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trialLancet200336293921255126014575968
- HenkeMMatternDPepeMDo erythropoietin receptors on cancer cells explain unexpected clinical findings?J Clin Oncol200624294708471317028293
- McKinneyMArcasoyMOErythropoietin for oncology supportive careExp Cell Res20113179 Special Issue SI1246125421396935
- SzenajchJWcisloGJeongJYSzczylikCFeldmanLThe role of erythropoietin and its receptor in growth, survival and therapeutic response of human tumor cells. From clinic to bench – a critical reviewBiochim Biophys Acta201018061829520406667
- JelkmannWBohliusJHallekMSytkowskiAJThe erythropoietin receptor in normal and cancer tissuesCrit Rev Oncol Hematol2008671396118434185
- NowrousianMRDunstJVaupelPErythropoiesis-stimulating agents: favorable safety profile when used as indicatedStrahlenther Onkol2008184312113618330508
- HadlandBKLongmoreGDErythroid-stimulating agents in cancer therapy: potential dangers and biologic mechanismsJ Clin Oncol2009254217422619636005
- WinkelmannJCWardJMayeuxPLacombeCSchimmentiLJenkinsRBA translocated erythropoietin receptor gene in a human erythroleukemia cell line (TF-1) expresses an abnormal transcript and a truncated proteinBlood19958511791857803793
- ChretienSMoreau-GachelinFApiouFPutative oncogenic role of the erythropoietin receptor in murine and human erythroleukemia cellsBlood1994837181318218142650
- GondaTJD’AndreaRJActivating mutations in cytokine receptors: implications for receptor function and role in diseaseBlood19978923553699002936
- Le CouedicJPMitjavilaMTVillevalJLMissense mutation of the erythropoietin receptor is a rare event in human erythroid malignanciesBlood1996874150215118608241
- D’AndreaADMoreauJFShowersMOMolecular mimicry of erythropoietin by the spleen focus-forming virus gp55 glycoprotein: the first stage of Friend virus-induced erythroleukemiaBiochim Biophys Acta19921114131411390869
- Moreau-GachelinFMulti-stage Friend murine erythroleukemia: molecular insights into oncogenic cooperationRetrovirology200859918983647
- YoshimuraALongmoreGLodishHFPoint mutation in the exoplasmic domain of the erythropoietin receptor resulting in hormone-independent activation and tumorigenicityNature199034863026476492174515
- RivesSPahlHLFlorensaLMolecular genetic analyses in familial and sporadic congenital primary erythrocytosisHaematologica200792567467717488692
- Al-SheikhMMazurierEGardieBA study of 36 unrelated cases with pure erythrocytosis revealed three new mutations in the erythropoietin receptor geneHaematologica20089371072107518492694
- WatowichSSXieXKlingmullerUErythropoietin receptor mutations associated with familial erythrocytosis cause hypersensitivity to erythropoietin in the heterozygous stateBlood19999472530253210498627
- de la ChapelleATraskelinALJuvonenETruncated erythropoietin receptor causes dominantly inherited benign human erythrocytosisProc Natl Acad Sci U S A19939010449544998506290
- GordeukVRSergueevaAIMiasnikovaGYCongenital disorder of oxygen sensing: association of the homozygous Chuvash polycythemia VHL mutation with thrombosis and vascular abnormalities but not tumorsBlood2004103103924393214726398
- AcsGZhangPJMcGrathCMHypoxia-inducible erythropoietin signaling in squamous dysplasia and squamous cell carcinoma of the uterine cervix and its potential role in cervical carcinogenesis and tumor progressionAm J Pathol200316261789180612759237
- ArcasoyMOJiangXHaroonZAExpression of erythropoietin receptor splice variants in human cancerBiochem Biophys Res Commun20033074999100712878211
- BatraSPerelmanNLuckLRShimadaHMalikPPediatric tumor cells express erythropoietin and a functional erythropoietin receptor that promotes angiogenesis and tumor cell survivalLab Invest200383101477148714563949
- FeldmanLWangYRhimJSBhattacharyaNLodaMSytkowskiAJErythropoietin stimulates growth and STAT5 phosphorylation in human prostate epithelial and prostate cancer cellsProstate200666213514516161153
- KataokaMMoriyaYMoriguchiYEffect of erythropoietin on human tumor growth in xenograft modelsMol Med Report2010319510121472206
- LaiSYChildsEEXiSErythropoietin-mediated activation of JAK-STAT signaling contributes to cellular invasion in head and neck squamous cell carcinomaOncogene200524274442444915856028
- LarssonAMJirstromKFredlundEErythropoietin receptor expression and correlation to tamoxifen response and prognosis in breast cancerClin Cancer Res200915175552555919706814
- WestphalGNiederbergerEBlumCErythropoietin and G-CSF receptors in human tumor cells: expression and aspects regarding functionalityTumori200288215015912088257
- YasudaYFujitaYMatsuoTErythropoietin regulates tumour growth of human malignanciesCarcinogenesis20032461021102912807756
- YasudaYFujitaYMasudaSErythropoietin is involved in growth and angiogenesis in malignant tumours of female reproductive organsCarcinogenesis200223111797180512419827
- ShiZHodgesVMDunlopEAErythropoietin-induced activation of the JAK2/STAT5, PI3K/Akt, and Ras/ERK pathways promotes malignant cell behavior in a modified breast cancer cell lineMol Cancer Res20108461562620353997
- MillerCPloweKAValliant-SaundersKEvaluating erythropoietin- associated tumor progression using archival tissues from a phase III clinical trialStem Cells20092792353236119544471
- NingSHartleyCMolineuxGKnoxSJDarbepoietin alfa potentiates the efficacy of radiation therapy in mice with corrected or uncorrected anemiaCancer Res200565128429015665305
- ParaghGKumarSMRakosyZChoiSCXuXAcsGRNA interference-mediated inhibition of erythropoietin receptor expression suppresses tumor growth and invasiveness in A2780 human ovarian carcinoma cellsAm J Pathol200917441504151419264915
- SasakiYKjellenEMinetaHWennerbergJEkbladLNo direct effects of erythropoietin beta on a head and neck squamous cell carcinoma cell line which is growth stimulated in vivoActa Oncol20094871062106919412811
- WilliamsDMZimmersTAPierceJHThe expression and role of human erythropoietin receptor in erythroid and nonerythroid cellsAnn N Y Acad Sci19947182322437514379
- MasudaSNagaoMTakahataKFunctional erythropoietin receptor of the cells with neural characteristics. Comparison with receptor properties of erythroid cellsJ Biol Chem19932681511208112167684373
- SaintignyPBesseBCallardPErythropoietin and erythropoietin receptor coexpression is associated with poor survival in stage I non-small cell lung cancerClin Cancer Res200713164825483117699861
- Belda-IniestaCPeronaRCarpenoJCHuman recombinant erythropoietin does not promote cancer growth in presence of functional receptors expressed in cancer cellsCancer Biol Ther20076101600160517938574
- McBroomJWAcsGRoseGSKrivakTCMohyeldinAVermaAErythropoietin receptor function and expression in epithelial ovarian carcinomaGynecol Oncol200599357157716051335
- RadesDSetterCDahlOSchildSENoackFPrognostic Impact of erythropoietin expression and erythropoietin receptor expression on locoregional control and survival of patients irradiated for stage ii/iii non-small-cell lung cancerInt J Radiat Oncol Biol Phys201180249950520646855
- RadesDGolkeHSchildSEKilicEThe impact of tumor expression of erythropoietin receptors and erythropoietin on clinical outcome of esophageal cancer patients treated with chemoradiationInt J Radiat Oncol Biol Phys200871115215917967510
- RibattiDMarzulloANicoBCrivellatoERiaRVaccaAErythropoietin as an angiogenic factor in gastric carcinomaHistopathology200342324625012605644
- RibattiDNicoBPerraMTErythropoietin is involved in angiogenesis in human primary melanomaInt J Exp Pathol201091649549920804540
- BrunotteJBockHCBruckWHemmerleinBStrikHHigh expression levels of erythropoietin and its receptor are not correlated with shorter survival in human glioblastomaExp Ther Med20112229529922977501
- LonnrothCSvenssonMWangWSurvival and erythropoietin receptor protein in tumours from patients randomly treated with rhEPO for palliative careMed Oncol2008251222918188711
- MittelbronnMCapperDBunzBDe novo erythropoietin receptor (EPO-R. expression in human neoplastic glial cells decreases with grade of malignancy but is favourably associated with patient survivalNeuropathol Appl Neurobiol200733329930717493011
- PelekanouVKampaMKafousiMErythropoietin and its receptor in breast cancer: correlation with steroid receptors and outcomeCancer Epidemiol Biomarkers Prev200716102016202317932349
- SarteletHFabreMCastaingMExpression of erythropoietin and its receptor in neuroblastomasCancer200711051096110617647284
- Della RagioneFCucciollaVBorrielloAOlivaAPerrottaSErythropoietin receptors on cancer cells: a still open questionJ Clin Oncol200725131812181317470877
- SturialeACampoSCrasciEErythropoietin and its lost receptorNephrol Dial Transplant20072251484148517267537
- LorinczANusserZSpecificity of immunoreactions: the importance of testing specificity in each methodJ Neurosci200828379083908618784286
- LeongASPitfalls in diagnostic immunohistologyAdv Anat Pathol2004112869315090844
- BordeauxJWelshAAgarwalSAntibody validationBiotechniques201048319720920359301
- SaperCBA guide to the perplexed on the specificity of antibodiesJ Histochem Cytochem20095711518854594
- FritschyJMIs my antibody-staining specific? How to deal with pitfalls of immunohistochemistryEur J Neurosci200828122365237019087167
- ElliottSSwiftSBusseLRossiJMcCafferyIAbsence of functional Epo receptors in normal and cancerous human tissues and cell linesBlood2011118213169
- RossiJMcCafferyIPaweletzKAnalysis of cell surface erythropoietin receptor (EpoR) expression and function in human epithelial tumor tissues [abstract]J Clin Oncol20092715SAbstr 11104
- TakeshitaAShinjoKHiguchiMQuantitative expression of erythropoietin receptor (EPO-R) on acute leukaemia cells: relationships between the amount of EPO-R and CD phenotypes, in vitro proliferative response, the amount of other cytokine receptors and clinical prognosis. Japan Adult Leukaemia Study GroupBr J Haematol20001081556310651724
- KokhaeiPAbdallaAOHanssonLExpression of erythropoietin receptor and in vitro functional effects of epoetins in B-cell malignanciesClin Cancer Res200713123536354417575216
- LaMontagneKRButlerJMarshallDJRecombinant epoetins do not stimulate tumor growth in erythropoietin receptor-positive breast carcinoma modelsMol Cancer Ther20065234735516505108
- LiuWMPowlesTShamashJPropperDOliverTJoelSEffect of haemopoietic growth factors on cancer cell lines and their role in chemosensitivityOncogene200423498199014647427
- MitjavilaMTVillevalJLCramerPEffects of granulocyte-macrophage colony-stimulating factor and erythropoietin on leukemic erythroid colony formation in human early erythroblastic leukemiasBlood19877049659733498522
- AsanoYOkamuraSShibuyaTHaradaMNihoYGrowth of clonogenic myeloblastic leukemic cells in the presence of human recombinant erythropoietin in addition to various human recombinant hematopoietic growth factorsBlood1988725168216862460160
- KolonicsAApatiANahajevszkySGatiRBrozikAMagocsiMUnregulated activation of STAT-5, ERK1/2 and c-Fos may contribute to the phenotypic transformation from myelodysplastic syndrome to acute leukaemiaHaematologia200131212513811583024
- OhigashiTYoshiokaKFisherJWAutocrine regulation of erythropoietin gene expression in human hepatocellular carcinoma cellsLife Sci19965854214278594307
- WestenfelderCBaranowskiRLErythropoietin stimulates proliferation of human renal carcinoma cellsKidney Int200058264765710916088
- KitamuraTTojoAKuwakiTIdentification and analysis of human erythropoietin receptors on a factor-dependent cell line, TF-1Blood19897323753802537111
- NakazawaMMitjavilaMTDebiliNKU 812: a pluripotent human cell line with spontaneous erythroid terminal maturationBlood1989737200320132540861
- van ZoelenEJReceptor-ligand interaction: a new method for determining binding parameters without a priori assumptions on non-specific bindingBiochem J198926225495562553000
- PelletierSGingrasSFunakoshi-TagoMHowellSIhleJNTwo domains of the erythropoietin receptor are suff icient for Jak2 binding/activation and functionMol Cell Biol200626228527853816982687
- ShannonAMBouchier-HayesDJCondronCMToomeyDCorrection of anaemia through the use of darbepoetin alfa improves chemotherapeutic outcome in a murine model of Lewis lung carcinomaBr J Cancer200593222423215999100
- FuPJiangXArcasoyMOConstitutively active erythropoietin receptor expression in breast cancer cells promotes cellular proliferation and migration through a MAP-kinase dependent pathwayBiochem Biophys Res Commun2009379369670119133231
- HardeeMECaoYFuPErythropoietin blockade inhibits the induction of tumor angiogenesis and progressionPloS One20076e54917579721
- DunlopEAPercyMJBolandMPMaxwellAPLappinTRInduction of signalling in non-erythroid cells by pharmacological levels of erythropoietinNeurodegener Dis200631–29410016909043
- UmMLodishHFAntiapoptotic effects of erythropoietin in differentiated neuroblastoma SH-SY5Y cells require activation of both the STAT5 and AKT signaling pathwaysJ Biol Chem200628195648565616407271
- LiangKEstevaFJAlbarracinCRecombinant human erythropoietin antagonizes trastuzumab treatment of breast cancer cells via Jak2-mediated Src activation and PTEN inactivationCancer Cell201018542343521075308
- MirmohammadsadeghAMariniAGustrauARole of erythropoietin receptor expression in malignant melanomaJ Invest Dermatol2010130120121019536148
- ArcasoyMOAminKKarayalAFFunctional significance of erythropoietin receptor expression in breast cancerLab Invest200282791191812118093
- HamadmadSNHohlRJErythropoietin stimulates cancer cell migration and activates RhoA protein through a mitogen-activated protein kinase/extracellular signal-regulated kinase-dependent mechanismJ Pharmacol Exp Ther200832431227123318079357
- SinclairAArchibequeIZhanJJanus kinase (JAK) 2 potency and selectivity assessment of small molecules: widely used AG490 inhibitor is neither potent nor selective for JAK2Blood201111821478021868576
- KirkenRAErwinRATaubDTyrphostin AG-490 inhibits cytokine-mediated JAK3/STAT5a/b signal transduction and cellular proliferation of antigen-activated human T cellsJ Leuke Biol1999656891899
- GazitAOsherovNPosnerITyrphostins. 2. Heterocyclic and alpha-substituted benzylidenemalononitrile tyrphostins as potent inhibitors of EGF receptor and ErbB2/neu tyrosine kinasesJ Med Chem1991346189619071676428
- JaleelMShenoyARVisweswariahSSTyrphostins are inhibitors of guanylyl and adenylyl cyclasesBiochem200443258247825515209521
- KaurGGazitALevitzkiAStoweECooneyDASausvilleEATyrphostin induced growth inhibition: correlation with effect on p210bcr-abl autokinase activity in K562 chronic myelogenous leukemiaAnticancer Drugs1994522132228049505
- GewirtzDADiXWalkerTDSawyerSTErythropoietin fails to interfere with the antiproliferative and cytotoxic effects of antitumor drugsClin Cancer Res2006127 Pt 12232223816609039
- FandreyJDicatoMExamining the involvement of erythropoiesis-stimulating agents in tumor proliferation (erythropoietin receptors, receptor binding, signal transduction), angiogenesis, and venous thromboembolic eventsOncologist200914Suppl 1344219762515
- AmmarguellatFLloveraMKellyPAGoffinVLow doses of EPO activate MAP kinases but not JAK2-STAT5 in rat vascular smooth muscle cellsBiochem Biophys Res Commun200128441031103811409898
- HardeeMECaoYFuPErythropoietin blockade inhibits the induction of tumor angiogenesis and progressionPloS One20076e54917579721
- LesterRDJoMCampanaWMGoniasSLErythropoietin promotes MCF-7 breast cancer cell migration by an ERK/mitogen-activated protein kinase-dependent pathway and is primarily responsible for the increase in migration observed in hypoxiaJ Biol Chem200528047392733927716207704
- SeidelHMLambPRosenJPharmaceutical intervention in the JAK/ STAT signaling pathwayOncogene200019212645265610851064
- JatianiSSBakerSJSilvermanLRPremkumarREJAK/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapiesGenes Cancer201011097999321442038
- ParganasEWangDStravopodisDJak2 is essential for signaling through a variety of cytokine receptorsCell19989333853959590173
- SwiftSElliottSSinclairABegleyCGErythropoietin receptor in ovarian cancer cells – LetterMol Cancer Ther2010941070107120371727
- HsuRYChanCHSpicerJDLPS-induced TLR4 signaling in human colorectal cancer cells increases beta1 integrin-mediated cell adhesion and liver metastasisCancer Res20117151989199821363926
- DoanHQBowenKAJacksonLAEversBMToll-like receptor 4 activation increases Akt phosphorylation in colon cancer cellsAnticancer Res20092972473247819596916
- KeenanJDooleyMPearsonDClynesMRecombinant human albumin in cell culture: evaluation of growth-promoting potential for NRK and SCC-9 cells in vitroCytotechnology199624243252
- SlaabyRAndersenASBrandtJIGF-I binding to the IGF-I receptor is affected by contaminants in commercial BSA: the contaminants are proteins with IGF-I binding propertiesGrowth Horm IGF Res200818426727417945524
- EthertonTDChungCSWigginsJPReceptor-dependent and independent degradation of insulin by isolated swine adipocytes at 37 CJ Anim Sci19845923663756384170
- BlairKJKiangAWang-RodriguezJYuMADohertyJKOngkekoWMEGF and bFGF promote invasion that is modulated by PI3/ Akt kinase and Erk in vestibular schwannomaOtol Neurotol201132230831421178801
- HassounaISperlingSKimEErythropoietin augments survival of glioma cells after radiation and temozolomideInt J Radiat Oncol Biol Phys200872392793419014782
- LaMontagneKRButlerJMarshallDJRecombinant epoetins do not stimulate tumor growth in erythropoietin receptor-positive breast carcinoma modelsMol Cancer Ther20065234735516505108
- QuesenberryPLevinJZuckermanKRencriccaNSullivanRTylerWStem cell migration induced by erythropoietin or haemolytic anaemia: the effects of actinomycin and endotoxin contamination of erythropoietin preparationsBr J Haematol197941225326985457
- HarmeyJHBucanaCDLuWLipopolysaccharide-induced metastatic growth is associated with increased angiogenesis, vascular permeability and tumor cell invasionInt J Cancer2002101541542212216068
- KilleenSDWangJHAndrewsEJRedmondHPBacterial endotoxin enhances colorectal cancer cell adhesion and invasion through TLR-4 and NF-kappaB-dependent activation of the urokinase plasminogen activator systemBr J Cancer2009100101589160219436306
- WangJHManningBJWuQDBlanksonSBouchier-HayesDRedmondHPEndotoxin/lipopolysaccharide activates NF-kappa B and enhances tumor cell adhesion and invasion through a beta 1 integrin-dependent mechanismJ Immunol2003170279580412517943
- SugawaMFukuiHEffects of recombinant human erythropoietin on the growth of various human tumor cellsBiotherapy2005192181196
- BauerEDanhauser-RiedlSDeRWEffects of recombinant human erythropoietin on clonogenic growth of primary human tumour specimens in vitroEur J Cancer199228A1017691389501
- MillerCPValliant-SaundersKBlauCALimitations of a murine transgenic breast cancer model for studies of erythropoietin-induced tumor progressionClin Transl Oncol201033176180
- YasudaYMushaTTanakaHInhibition of erythropoietin signal-ling destroys xenografts of ovarian and uterine cancers in nude miceBr J Cancer200184683684311259101
- YarkoniEGorenMBRappHJRegression of a transplanted guinea pig hepatoma after intralesional injection of an emulsified mixture of endotoxin and mycobacterial sulfolipidInfect Immun1979242357362378852
- VillevalJLMitjavilaMTDusanter-FourtIWendlingFMayeuxPVainchenkerWAutocrine stimulation by erythropoietin (Epo) requires Epo secretionBlood1994848264926627919379
- RaskoJEMetcalfDGoughNMBegleyCGThe cytokine receptor repertoire specifies autocrine growth factor production in factor-dependent cellsExp Hematol19952354534607720817
- PalumboCBattistiSCarboneDRecombinant erythropoietin differently affects proliferation of mesothelioma cells but not sensitivity to cisplatin and pemetrexedCancer Chemother Pharmacol200861589390117922127
- AcsGChenMXuXAcsPVermaAKochCJAutocrine erythropoietin signaling inhibits hypoxia-induced apoptosis in human breast carcinoma cellsCancer Lett2004214224325115363551
- KumarSMAcsGFangDHerlynMElderDEXuXFunctional erythropoietin autocrine loop in melanomaAm J Pathol2005166382383015743794
- HankinsWDSchooleyJEastmentCErythropoietin, an autocrine regulator? Serum-free production of erythropoietin by cloned erythroid cell linesBlood19866812632683459557
- QianRLChinKKimJKChinHMConeJHankinsWDPurification of murine erythropoietin produced in serum-free cultures of erythro-leukemia cellsBlood19866812582623459556
- MitjavilaMTLe CouedicJPCasadevallNAutocrine stimulation by erythropoietin and autonomous growth of human erythroid leukemic cells in vitroJ Clin lnvest1991883789797
- SherwoodJBShouvalDContinuous production of erythropoietin by an established human renal carcinoma cell line: development of the cell lineProc Natl Acad Sci U S A19868311651693455754
- ShouvalDSherwoodJBProduction of erythropoietin by an established human renal carcinoma cell line: in vitro and in vivo studiesAdv Exp Med Biol19882413193283223410
- HammondDWinnickSParaneoplastic erythrocytosis and ectopic erythropoietinsAnn N Y Acad Sci19742302192274595944
- KaelinWGJrThe von Hippel-Lindau tumour suppressor protein: O2 sensing and cancerNat Rev Cancer200881186587318923434
- BerdelWEDanhauser-RiedlSOberbergDZafferaniMEffects of hematopoietic growth factors on malignant nonhematopoietic cellsSemin Oncol1992192 Suppl 441451553574
- HamamoriYSamalBTianJKedesLPersistent erythropoiesis by myoblast transfer of erythropoietin cDNAHum Gene Ther1994511134913567893806
- MuramatsuTArakawaSFukazawaKIn vivo gene electroporation in skeletal muscle with special reference to the duration of gene expressionInt J Mol Med200171374211115606
- GeudensIGerhardtHCoordinating cell behaviour during blood vessel formationDevelopment2011138214569458321965610
- WuHLeeSHGaoJLiuXIruela-ArispeMLInactivation of erythropoietin leads to defects in cardiac morphogenesisDevelopment1999126163597360510409505
- KerteszNWuJChenTHSucovHMWuHThe role of erythropoietin in regulating angiogenesisDev Biol2004276110111015531367
- DavidoffAMNgCYBrownPBone marrow-derived cells contribute to tumor neovasculature and, when modified to express an angiogenesis inhibitor, can restrict tumor growth in miceClin Cancer Res2001792870287911555605
- DePalmaMNaldiniLRole of haematopoietic cells and endothelial progenitors in tumour angiogenesisBiochim Biophys Acta20061766115916616857321
- ZwezdarykKJCoffeltSBFigueroaYGErythropoietin, a hypoxia-regulated factor, elicits a pro-angiogenic program in human mesenchymal stem cellsExp Hematol2007354640652 [Erratum, Exp Hematol. 2007;35(7):1153–1161]17379074
- HaoQLiuJRPappuRContribution of bone marrow-derived cells associated with brain angiogenesis is primarily through leukocytes and macrophagesArterioscler Thromb Vasc Biol200828122151215718802012
- Muller-EhmsenJSchmidtAKrausgrillBSchwingerRHBlochWRole of erythropoietin for angiogenesis and vasculogenesis: from embryonic development through adulthoodAm J Physiol Heart Circ Physiol20062901H331H34016024562
- PerryTESongMDespresDJBone marrow-derived cells do not repair endothelium in a mouse model of chronic endothelial cell dysfunctionCardiovasc Res200984231732519578071
- ScherschelJASoonpaaMHSrourEFFieldLJRubartMAdult bone marrow-derived cells do not acquire functional attributes of cardiomyocytes when transplanted into peri-infarct myocardiumMol Ther20081661129113718431364
- GothertJRGustinSEvan EekelenJAGenetically tagging endothelial cells in vivo: bone marrow-derived cells do not contribute to tumor endotheliumBlood200410461769177715187022
- ZiegelhoefferTFernandezBKostinSBone marrow-derived cells do not incorporate into the adult growing vasculatureCirc Res200494223023814656934
- CrivellatoENicoBVaccaADjonovVPrestaMRibattiDRecombinant human erythropoietin induces intussusceptive microvascular growth in vivoLeukemia200418233133614671634
- JaquetKKrauseKTawakol-KhodaiMGeidelSKuckKHErythropoietin and VEGF exhibit equal angiogenic potentialMicrovasc Res200264232633312204656
- BahlmannFHDeGrootKDuckertTEndothelial progenitor cell proliferation and differentiation is regulated by erythropoietinKidney Int20036451648165214531796
- BahlmannFHSongRBoehmSMLow-dose therapy with the long-acting erythropoietin analogue darbepoetin alpha persistently activates endothelial Akt and attenuates progressive organ failureCirculation200411081006101215302785
- HeeschenCAicherALehmannRErythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilizationBlood200310241340134612702503
- PerrottaSCucciollaVFerraroMEPO receptor gain-of-function causes hereditary polycythemia, alters CD34 cell differentiation and increases circulating endothelial precursorsPloS One2010582010
- DuffSELiCGarlandJMKumarSCD105 is important for angiogenesis: evidence and potential applicationsFASEB J200317998499212773481
- BuhringHJMullerCALetarteMEndoglin is expressed on a subpopulation of immature erythroid cells of normal human bone marrowLeukemia19915108418471961019
- KimSNMoonJHKimJGMobilization effects of G-CSF, GM-CSF, and darbepoetin-alpha for allogeneic peripheral blood stem cell transplantationJ Clin Apher200924517317919753648
- TaniguchiNSawadaTMatsubaraKErythropoietin prevention trial of coronary restenosis and cardiac remodeling after ST-elevated acute myocardial infarction (EPOC-AMI): a pilot, randomized, placebo-controlled studyCirc J201074112365237120834185
- PawlakKPawlakDMysliwiecMLong-term erythropoietin therapy does not affect endothelial markers, coagulation activation and oxidative stress in haemodialyzed patientsThromb Res2007120679780317391740
- TsuzukiMBone marrow-derived cells are not involved in reendothelialized endothelium as endothelial cells after simple endothelial denudation in miceBasic Res Cardiol2009104560161119333644
- PetersBADiazLAPolyakKContribution of bone marrow-derived endothelial cells to human tumor vasculatureNat Med200511326126215723071
- MacheinMRRenningerSde Lima-HahnEPlateKHMinor contribution of bone marrow-derived endothelial progenitors to the vascularization of murine gliomasBrain Pathol200313458259714655762
- SuzukiNOhnedaOTakahashiSErythroid-specific expression of the erythropoietin receptor rescued its null mutant mice from lethalityBlood200210072279228812239135
- AmmarguellatFGogusevJDruekeTBDirect effect of erythropoietin on rat vascular smooth-muscle cell via a putative erythropoietin receptorNephrol Dial Transplant19961146876928671860
- Lopez OngilSLSauraMLamasSRodriguezPMRodriguezPDRecombinant human erythropoietin does not regulate the expression of endothelin-1 and constitutive nitric oxide synthase in vascular endothelial cellsExp Nephrol19964137428788598
- FusteBaz-RicartMCasesALopez-PedretJOrdinasAEscolarGErythropoietin does not modify the prothrombotic effect induced by uremic media on endothelial cellsHaematologica20028791006100812217816
- HardeeMEKirkpatrickJPShanSHuman recombinant erythropoietin (rEpo) has no effect on tumour growth or angiogenesisBr J Cancer200593121350135516288305
- SairahAKRasedeeASheikhORozitaRALHajNThe effects of recombinant human erythropoietin and tamoxifen on growth and angiogenesis of mammary tumor in Sprague-Dawley ratAm J Pharmacol Toxicol2009411216
- PinelSBarberi-HeyobMCohen-JonathanEErythropoietin-induced reduction of hypoxia before and during fractionated irradiation contributes to improvement of radioresponse in human glioma xenograftsInt J Radiat Oncol Biol Phys200459125025915093922
- YamajiROkadaTMoriyaMBrain capillary endothelial cells express two forms of erythropoietin receptor mRNAEur J Biochem199623924945008706759
- AnagnostouALeeESKessimianNLevinsonRSteinerMErythropoietin has a mitogenic and positive chemotactic effect on endothelial cellsProc Natl Acad Sci U S A19908715597859822165612
- RibattiDPrestaMVaccaAHuman erythropoietin induces a pro-angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivoBlood19999382627263610194442
- NicoBAnneseTGuidolinDFinatoNCrivellatoERibattiDEpo is involved in angiogenesis in human gliomaJ Neurooncol20111021515820614229
- SamarutJNigonVIn vitro development of chicken erythropoietin-sensitive cellsExp Cell Res19761002245248945769
- SteinleinPDeinerELeutzABeugHRecombinant murine erythropoietin receptor expressed in avian erythroid progenitors mediates terminal erythroid differentiation in vitroGrowth Factors19941011168179929
- KooBCKwonMSLeeHTetracycline-dependent expression of the human erythropoietin gene in transgenic chickensTransgenic Res201019343744719795218
- YuXShackaJJEellsJBErythropoietin receptor signalling is required for normal brain developmentDevelopment2002129250551611807041
- SatohKKagayaYNakanoMImportant role of endogenous erythropoietin system in recruitment of endothelial progenitor cells in hypoxia-induced pulmonary hypertension in miceCirculation2006113111442145016534010
- NakanoMSatohKFukumotoYImportant role of erythropoietin receptor to promote VEGF expression and angiogenesis in peripheral ischemia in miceCirc Res2007100566266917293480
- CalvilloLLatiniRKajsturaJRecombinant human erythropoietin protects the myocardium from ischemia-reperfusion injury and promotes beneficial remodelingProc Natl Acad Sci U S A200310084802480612663857
- MoonCKrawczykMAhnDErythropoietin reduces myocardial infarction and left ventricular functional decline after coronary artery ligation in ratsProc Natl Acad Sci U S A200310020116121161714500913
- TramontanoAFMuniyappaRBlackADErythropoietin protects cardiac myocytes from hypoxia-induced apoptosis through an Akt-dependent pathwayBiochem Biophys Res Commun2003308499099412927817
- BakerJEKozikDHsuAKFuXTweddellJSGrossGJDarbepoetin alfa protects the rat heart against infarction: dose-response, phase of action, and mechanismsJ Cardiovasc Pharmacol200749633734517577097
- ParsaCJKimJRielRUCardioprotective effects of erythropoietin in the reperfused ischemic heart: a potential role for cardiac fibroblastsJ Biol Chem200427920206552066215020586
- AsaumiYKagayaYTakedaMProtective role of endogenous erythropoietin system in nonhematopoietic cells against pressure overload-induced left ventricular dysfunction in miceCirculation2007115152022203217404160
- TadaHKagayaYTakedaMEndogenous erythropoietin system in non-hematopoietic lineage cells plays a protective role in myocardial ischemia/reperfusionCardiovasc Res200671346647716781691
- SirenALFratelliMBrinesMErythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stressProc Natl Acad Sci U S A20019874044404911259643
- SakanakaMWenTCMatsudaSIn vivo evidence that erythropoietin protects neurons from ischemic damageProc Natl Acad Sci U S A1998958463546409539790
- GorioAGokmenNErbayraktarSRecombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord traumaProc Natl Acad Sci U S A200299149450945512082184
- SalahudeenAKHaiderNJenkinsJAntiapoptotic properties of erythropoiesis-stimulating proteins in models of cisplatin- induced acute kidney injuryAm J Physiol Renal Physiol20082946F1354F136518385271
- BahlmannFHFliserDErythropoietin and renoprotectionCurr Opin Nephrol Hypertens2009181152019077684
- LombarderoMKovacsKScheithauerBWErythropoietin: a hormone with multiple functions [review]Pathobiology2011781415321474975
- ArcasoyMOThe non-haematopoietic biological effects of erythropoietinBr J Haematol20081411143118324962
- MinhKLKlemmKAbshagenKEipelCMengerMDVollmarBAttenuation of inflammation and apoptosis by pre- and posttreatment of darbepoetin-alpha in acute liver failure of miceAm J Pathol200717061954196317525263
- SepodesBMaioRPintoRRecombinant human erythropoietin protects the liver from hepatic ischemia-reperfusion injury in the ratTranspl Int2006191191992617018128
- KatavetinPTungsangaKEiam-OngSNangakuMAntioxidative effects of erythropoietinKidney Int200772Suppl 107S10S15
- MartiHHGassmannMWengerRHDetection of erythropoietin in human liquor: intrinsic erythropoietin production in the brainKidney Int19975124164189027715
- BanksWAJumbeNLFarrellCLNiehoffMLHeatheringtonACPassage of erythropoietic agents across the blood-brain barrier: a comparison of human and murine erythropoietin and the analog darbepoetin alfaEur J Pharmacol20045051–39310115556141
- JuulSEHarcumJLiYChristensenRDErythropoietin is present in the cerebrospinal fluid of neonatesJ Pediatr199713034284309063419
- TsaiPTOhabJJKerteszNA critical role of erythropoietin receptor in neurogenesis and post-stroke recoveryJ Neurosci20062641269127416436614
- ChenXChenYBiYPreventive cardioprotection of erythropoietin against doxorubicin-induced cardiomyopathyCardiovasc Drugs Ther200721536737417924179
- JuulSEYachnisATChristensenRDTissue distribution of erythropoietin and erythropoietin receptor in the developing human fetusEarly Hum Dev19985232352499808074
- WestenfelderCBiddleDLBaranowskiRLHuman, rat, and mouse kidney cells express functional erythropoietin receptorsKidney Int199955380882010027918
- CaiZManaloDJWeiGHearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injuryCirculation20031081798512796124
- BrinesMGrassoGFiordalisoFErythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptorProc Natl Acad Sci U S A200410141149071491215456912
- AgarwalNGordeukVRPrchalJTAre erythropoietin receptors expressed in tumors? Facts and fiction – more careful studies are neededJ Clin Oncol200725131813181417470878
- ParsaCJMatsumotoAKimJA novel protective effect of erythropoietin in the infarcted heartJ Clin Invest20031127999100714523037
- AkimotoTKusanoEInabaTErythropoietin regulates vascular smooth muscle cell apoptosis by a phosphatidylinositol 3 kinase-dependent pathwayKidney Int200058126928210886572
- FiordalisoFChimentiSStaszewskyLA nonerythropoietic derivative of erythropoietin protects the myocardium from ischemia-reperfusion injuryProc Natl Acad Sci U S A200510262046205115671158
- ZhangDZhangFZhangYErythropoietin enhances the angiogenic potency of autologous bone marrow stromal cells in a rat model of myocardial infarctionCardiology2007108422823617106196
- RuscherKFreyerDKarschMErythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro modelJ Neurosci20022223102911030112451129
- YangCWLiCJungJYPreconditioning with erythropoietin protects against subsequent ischemia-reperfusion injury in rat kidneyFASEB J200317121754175512958199
- VeseyDACheungCPatBEndreZGobeGJohnsonDWErythropoietin protects against ischaemic acute renal injuryNephrol Dial Transplant200419234835514736958
- NemotoTYokotaNKeaneWFRabbHRecombinant erythropoietin rapidly treats anemia in ischemic acute renal failureKidney Int200159124625111135077
- KohSHKimYKimHYChoGWKimKSKimSHRecombinant human erythropoietin suppresses symptom onset and progression of G93A-SOD1 mouse model of ALS by preventing motor neuron death and inflammationEur J Neurosci20072571923193017439481
- GrunfeldJFBarhumYBlondheimNRabeyJMMelamedEOffenDErythropoietin delays disease onset in an amyotrophic lateral sclerosis modelExp Neurol2007204126026317174305
- GrignaschiGZennaroETortaroloMCalvaresiNBendottiCErythropoietin does not preserve motor neurons in a mouse model of familial ALSAmyotroph Lateral Scler200781313517364433
- AgnelloDBiginiPVillaPErythropoietin exerts an anti- inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitisBrain Res2002952112813412363412
- BrendtPFreyUAdamzikMSchaferSTPetersJDarbepoetin alpha, a long-acting erythropoeitin derivate, does not alter LPS evoked myocardial depression and gene expression of Bax, Bcl-Xs, Bcl-XL, Bcl-2, and TNF-alphaShock2009311505418497705
- SpreerAGerberJHanssenMNauRNo neuroprotective effect of erythropoietin under clinical treatment conditions in a rabbit model of Escherichia coli meningitisPediatr Res200762668068317957150
- SøllingCOrgan-protective and immunomodulatory effects of erythropoietin – an update on recent clinical trialsBasic Clin Pharmacol Toxicol20121102113121
- EhrenreichHWeissenbornKPrangeHRecombinant human erythropoietin in the treatment of acute ischemic strokeStroke20094012e647e65619834012
- SpringborgJBMollerCGideonPJorgensenOSJuhlerMOlsenNVErythropoietin in patients with aneurysmal subarachnoid haemorrhage: a double blind randomised clinical trialActa Neurochir (Wien)2007149111089110017876497
- TalvingPLustenbergerTKobayashiLErythropoiesis stimulating agent administration improves survival after severe traumatic brain injury: a matched case control studyAnn Surg201025111419779323
- BinbrekASRaoNSAlKNAssaqqafJSobelBEErythropoietin to augment myocardial salvage induced by coronary thrombolysis in patients with ST segment elevation acute myocardial infarctionAm J Cardiol200981035104019801020
- ClelandJGColettaAPClarkALCullingtonDClinical trials update from the American College of Cardiology 2009: ADMIRE-HF, PRIMA, STICH, REVERSE, IRIS, partial ventricular support, FIX-HF- 5, vagal stimulation, REVIVAL-3, pre-RELAX-AHF, ACTIVE-A, HF-ACTION, JUPITER, AURORA, and OMEGAEur J Heart Fail200911662263019468023
- FerrarioMArbustiniEMassaMHigh-dose erythropoietin in patients with acute myocardial infarction: a pilot, randomised, placebo-controlled studyInt J Cardiol2011147112413119906454
- NajjarSSRaoSVMelloniCIntravenous erythropoietin in patients with ST-segment elevation myocardial infarction: REVEAL: a randomized controlled trialJAMA2011305181863187221558517
- OttISchulzSMehilliJErythropoietin in patients with acute st-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention a randomized, double-blind trialCirc Cardiovasc Interv20103540841320736448
- RaoSVKilaruRPovsicTJA randomized placebo controlled trial of intravenous erythropoietin to reduce infarct size after st-segment elevation myocardial infarction: primary results of the REVEAL trialCirculation2010122212222
- VoorsAABelonjeAMZijlstraFA single dose of erythropoietin in ST-elevation myocardial infarctionEur Heart J201031212593260020802250
- BeckerBNBeckerYTLeversonGEHeiseyDMErythropoietin therapy may retard progression in chronic renal transplant dysfunctionNephrol Dial Transplant20021791667167312198221
- HaferCBeckerTKielsteinJTHigh-dose erythropoietin has no effect on short-or long-term graft function following deceased donor kidney transplantationKidney Int201281331432022012130
- KamarNRebouxA-HCointaultOImpact of very early high doses of recombinant erythropoietin on anemia and allograft function in de novo kidney-transplant patientsTranspl Int201023327728419821956
- MartinezFKamarNPalletNHigh dose epoetin beta in the first weeks following renal transplantation and delayed graft function: results of the Neo-PDGF study [brief communication]Am J Transplant201010717041709
- EndreZHWalkerRJPickeringJWEarly intervention with erythropoietin does not affect the outcome of acute kidney injury (the EARLYARF trial)Kidney Int201077111020103020164823
- ParkJGageBFVijayanAUse of EPO in critically ill patients with acute renal failure requiring renal replacement therapyAmerican Journal of Kidney Diseases200546579179816253718
- SongYRLeeTYouSJPrevention of acute kidney injury by erythropoietin in patients undergoing coronary artery bypass grafting: a pilot studyAm J Nephrol200930325326019494484
- ChristensenBLundbyCJessenNEvaluation of functional erythropoietin receptor status in skeletal muscle in vivo: acute and prolonged studies in healthy human subjectsPloS One201272e3185722384088
- LeistMGhezziPGrassoGDerivatives of erythropoietin that are tissue protective but not erythropoieticScience2004305568123924215247477
- MoonCKrawczykMPaikDErythropoietin, modified to not stimulate red blood cell production, retains its cardioprotective propertiesJ Pharmacol Exp Ther20063163999100516306273
- ImamuraRIsakaYIchimaruNTakaharaSOkuyamaACarbamylated erythropoietin protects the kidneys from ischemia-reperfusion injury without stimulating erythropoiesisBiochem Biophys Res Commun2007353378679217196938
- ImamuraROkumiMIsakaYCarbamylated erythropoietin improves angiogenesis and protects the kidneys from ischemia-reperfusion injuryCell Transplant2008171–213514118468243
- KitamuraHIsakaYTakabatakeYNonerythropoietic derivative of erythropoietin protects against tubulointerstitial injury in a unilateral ureteral obstruction modelNephrol Dial Transplant20082351521152818194980
- KingVRAverillSAHewazyDPriestleyJVTorupLMichael-TitusATErythropoietin and carbamylated erythropoietin are neuroprotective following spinal cord hemisection in the ratEur J Neurosci20072619010017614942
- MonteroMPoulsenFRNorabergJComparison of neuroprotective effects of erythropoietin (EPO) and carbamylerythropoietin (CEPO) against ischemia-like oxygen-glucose deprivation (OGD) and NMDA excitotoxicity in mouse hippocampal slice culturesExp Neurol2007204110611717157835
- SchmidtREGreenKGFengDErythropoietin and its carbamylated derivative prevent the development of experimental diabetic autonomic neuropathy in STZ-induced diabetic NOD-SCID miceExp Neurol2008209116117017967455
- RobbLDrinkwaterCCMetcalfDHematopoietic and lung abnormalities in mice with a null mutation of the common beta subunit of the receptors for granulocyte-macrophage colony-stimulating factor and interleukins 3 and 5Proc Natl Acad Sci U S A19959221956595697568173
- NicolaNARobbLMetcalfDCaryDDrinkwaterCCBegleyCGFunctional inactivation in mice of the gene for the interleukin-3 (IL-3)- specific receptor beta-chain: implications for IL-3 function and the mechanism of receptor transmodulation in hematopoietic cellsBlood1996877266526748639882
- ScottCLHughesDACaryDNicolaNABegleyCGRobbLFunctional analysis of mature hematopoietic cells from mice lacking the betac chain of the granulocyte-macrophage colony-stimulating factor receptorBlood19989211411941279834217
- ScottCLRobbLPapaevangeliouBMansfieldRNicolaNABegleyCGReassessment of interactions between hematopoietic receptors using common beta-chain and interleukin-3-specific receptor beta-chain-null cells: no evidence of functional interactions with receptors for erythropoietin, granulocyte colony-stimulating factor, or stem cell factorBlood20009641588159010942411
- MittelmanMNeumannDPeledAKanterPHaran-GheraNErythropoietin induces tumor regression and antitumor immune responses in murine myeloma modelsProc Natl Acad Sci U S A20019895181518611309490
- MittelmanMThe implications of anemia in multiple myelomaClin Lymphoma20034Suppl 1S23S2914556675
- StubenGThewsOPottgenCImpact of anemia prevention by recombinant human erythropoietin on the sensitivity of xenografted glioblastomas to fractionated irradiationStrahlenther Onkol2003179962062514628128
- SigounasGSallahSSigounasVYErythropoietin modulates the anticancer activity of chemotherapeutic drugs in a murine lung cancer modelCancer Lett2004214217117915363543
- ThewsOKoenigRKelleherDKKutznerJVaupelPEnhanced radiosensitivity in experimental tumours following erythropoietin treatment of chemotherapy-induced anaemiaBr J Cancer19987867527569743294
- SilverDFPiverMSEffects of recombinant human erythropoietin on the antitumor effect of cisplatin in SCID mice bearing human ovarian cancer: a possible oxygen effectGynecol Oncol199973228028410329047
- StubenGThewsOPottgenCKnuhmannKVaupelPStuschkeMRecombinant human erythropoietin increases the radiosensitivity of xenografted human tumours in anaemic nude miceJ Cancer Res Clin Oncol2001127634635011414194
- ThewsOKelleherDKVaupelPErythropoietin restores the anemia-induced reduction in cyclophosphamide cytotoxicity in rat tumorsCancer Res20016141358136111245434
- GolabJOlszewskaDMrozPErythropoietin restores the antitumor effectiveness of photodynamic therapy in mice with chemotherapy- induced anemiaClin Cancer Res2002851265127012006547
- TovariJGillyRRasoERecombinant human erythropoietin alpha targets intratumoral blood vessels, improving chemotherapy in human xenograft modelsCancer Res200565167186719316103069
- LoveyJBereczkyBGillyRRecombinant human erythropoietin alpha improves the efficacy of radiotherapy of a human tumor xenograft, affecting tumor cells and microvesselsStrahlenther Onkol200818411718188516
- GolabJZagozdzonRStoklosaTJakobisiakMPojdaZMachajEErythropoietin prevents the development of interleukin-12-induced anemia and thrombocytopenia but does not decrease its antitumor activity in miceBlood19989111438743889596689
- BlackwellKLKirkpatrickJPSnyderSAHuman recombinant erythropoietin significantly improves tumor oxygenation independent of its effects on hemoglobinCancer Res200363196162616514559797
- BianchiRGilardiniARodriguez-MenendezVCisplatin-induced peripheral neuropathy: neuroprotection by erythropoietin without affecting tumour growthEur J Cancer200743471071717251006
- KelleherDKMattheinsenUThewsOVaupelPBlood flow, oxygenation, and bioenergetic status of tumors after erythropoietin treatment in normal and anemic ratsCancer Res19965620472847348840991
- KirkpatrickJPHardeeMESnyderSAThe effect of darbepoetin alfa on growth, oxygenation and radioresponsiveness of a breast adenocarcinomaRadiat Res2006165219220116518899
- van HalterenHKBongaertsGPVerhagenCARecombinant human erythropoietin attenuates weight loss in a murine cancer cachexia modelJ Cancer Res Clin Oncol2004130421121614745550
- HardeeMERabbaniZNArcasoyMOErythropoietin inhibits apoptosis in breast cancer cells via an Akt-dependent pathway without modulating in vivo chemosensitivityMol Cancer Ther20065235636116505109
- KjellenESasakiYKjellstromJZackrissonBWennerbergJRecombinant erythropoietin beta enhances growth of xenografted human squamous cell carcinoma of the head and neck after surgical traumaActa Otolaryngol2006126554554716698707
- OkazakiTEbiharaSAsadaMYamandaSNiuKAraiHErythropoietin promotes the growth of tumors lacking its receptor and decreases survival of tumor-bearing mice by enhancing angiogenesisNeoplasia200810993293918714393
- RupertusKSperlingJCorstenMDarbepoetin-alpha enhances hepatectomy-associated stimulation of colorectal liver metastatic growthAnn Surg2010252113114120531003
- MirabelliPDiNRLoPCExtended flow cytometry characterization of normal bone marrow progenitor cells by simultaneous detection of aldehyde dehydrogenase and early hematopoietic antigens: implication for erythroid differentiation studiesBMC Physiol200881318510759
- KomatsuNYamamotoMFujitaHEstablishment and characterization of an erythropoietin-dependent subline, UT-7/Epo, derived from human leukemia cell line, UT-7Blood19938224564648329702