Abstract
In recent years, many studies have shown that some types of tumors are characterized by the presence of cells with stem-like characteristics, called cancer stem cells (CSCs). These are considered cells that initiate the tumor and are probably responsible for tumor recurrence. CSCs have the capacity for self-renewal, the potential to give rise to one or more cell types within the tumor, and the ability to drive, in a continuous manner, the proliferation of malignant cells. The failure of current cancer therapies can be attributed to the relative ineffectiveness of drugs against CSCs, which remain viable while retaining their full ability to reproduce the tumor. The development of new strategies is currently hampered by the lack of reliable markers to identify CSCs. One promising surface marker of CSCs in head and neck cancer is the CD44 molecule, which has been shown in preliminary studies to have high specificity, although there are discrepant data because its prognostic value may depend on the specific tumor location. More rigorous studies are needed to investigate the usefulness of CD44 expression in head and neck tumors for possible clinical applicability.
Introduction
Emerging studies show that CD44 is an important biomarker of a cellular subpopulation – cancer stem cells (CSCs) – which are capable of self-renewal and have the capacity for initiation, progression, invasion, metastasis, tumor recurrence, and resistance to chemo- and radiotherapy.Citation1 This cell subpopulation was isolated for the first time by Bonnet and Dick from samples of acute myeloid leukemia.Citation2 CSCs have also been identified in solid tumors. Al-Hajj et al identified a subpopulation of CD44+/CD24− cells with tumorigenic capacity from breast cancer samples in 2003.Citation3 CSCs were also identified in brain tumors by Singh et al in 2003,Citation4 in prostate tumors by Collins et al in 2005,Citation5 in colorectal cancers by Dalerba et al in 2007,Citation6 in pancreatic tumors by Li et al,Citation7 and in lung tumors by Ho et al.Citation8
In 2007, Prince et al first identified a cellular subpopulation in head and neck tumors expressing the surface marker CD44 with stem-like characteristics; these cells were capable of reproducing when implanted into immunosuppressed mice.Citation9 In the same year, Harper et al studied the expression of CD44, CD29, and CD133 as presumed markers of CSCs in cell lines derived from head and neck tumors; they found that the greatest expression of CD44 correlated with increased clonogenicity.Citation10
CD44
CD44 is a type I transmembrane glycoprotein expressed in several cell types of mesenchymal and neuroectodermal origin.Citation11 CD44 functions as a major adhesion molecule and in the cellular internalization of hyaluronic acid.Citation12 The interaction between hyaluronic acid and CD44 influences adhesion to components of the extracellular matrix, and it is involved in the stimulation of aggregation, cell proliferation and migration, and angiogenesis.Citation13 All of these biological properties are essential to normal cell physiology, but in certain conditions they are associated with pathological activities, in particular those of cancer cells.Citation14
The bond between hyaluronic acid and the CD44 adhesion molecule may initiate a series of events that begin with modification of adhesion to the matrix and continue with activation of other molecules such as growth factors, degradation of the matrix, angiogenesis, permeation by blood vessels, and extravasation.Citation15 All of these steps are necessary in the initiation of metastasis.Citation16 In addition to hyaluronic acid, CD44 binds to fibronectin, the invariant part of the major histocompatibility complex class II,Citation17 and high-molecular-weight proteoglycans.Citation18 The heterogeneity of CD44 binding to these ligands reflects the fact that the gene encoding CD44 comprises 20 exons; the first and the last five are constant, and the central ten are subjected to alternative splicing, thus constituting the variable region of the receptor.Citation19 The most common isoform of the receptor is CD44 standard, which is highly expressed in hematopoietic cells. About 30 receptor variants (CD44v) have been identified, many of which appear to be expressed on tumor cells and arise from alternative splicing at the extracellular proximal portion of the receptor.Citation20 In pathological conditions such as cancer, the extracytoplasmic domain of the CD44 receptor detaches and is released into biological fluids as a soluble fraction of the receptor CD44sol.Citation21–Citation25
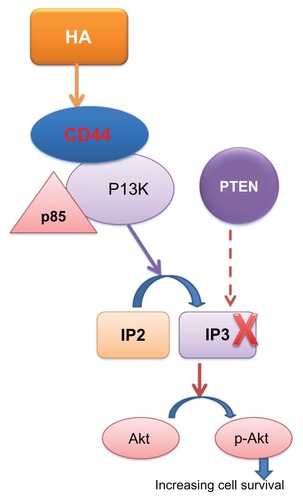
Numerous studies have highlighted the connection between CD44, hyaluronic acid, and the PI3K–Akt system, whose stimulation leads to phosphorylation of Akt (also known as protein kinase B). p-Akt is positively involved in the processes of cell survival and in the development of resistance to chemotherapy.Citation26 Activation of this enzyme triggers a series of reactions, all of which increase cell proliferation and survival through the transformation of phosphatidylinositol-4,5-bisphosphate, located in the cytoplasmic membrane, to phosphatidylinositol-3,4,5-triphosphate, which activates the effector molecule Akt. Akt is a Ser–Tyr kinase whose active form p-Akt phosphorylates a number of proteins involved in cell proliferation (). For example, Akt is involved in the maintenance of cell metabolism in growth-limiting conditions through adenosine triphosphate production via glycolysis; increased cellular uptake of glucose by glucose transporter type 4; mammalian target of rapamycin phosphorylation, which increases the synthesis of cyclin D; intracellular activation of transcription factors such as S6 kinase and apoptosis through BAD phosphorylation/inactivation; and reduction in proapoptotic gene transcription through phosphorylation of AFX, FKHR, and FKHRL1.Citation27,Citation28
Alteration in the T lymphocyte-mediated immune response can change the expression of CD44 and its role in lymphocyte homing. CD44 is also involved in the transport of circulating lymphocytes to lymph nodes and in lymphocytic–epithelial interactions, through which it modulates lymphocyte adhesion and activation.Citation29 These roles form the basis of the idea that CD44 plays an important role in lymph-node metastasis and in the potential carcinogenicity of certain forms of T-cell leukemia and lymphoma. The proposed pathway involves promoting the survival of T cells by increasing their resistance to apoptosis induced experimentally by corticosteroid treatment or ultraviolet rays through a p53-dependent mechanism involving the inhibition of DNA fragmentation.Citation30
Clinical studies
Currently, researchers continue to study the biological characteristics of the surface CD44 molecule as a marker of cancer stem cells (Allegra and Trapasso, unpublished data, 2012). However, there are conflicting findings about the clinical significance of CD44 expression.
Joshua et al have studied a lineage-CD44+ (Lin-CD44+) subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma, and they have observed a high frequency of Lin-CD44+ cells correlated with known poor prognostic factors such as advanced T classification and recurrence.Citation31 In some cases, the overexpression of CD44v (v3 and v6) seems to reflect the cellular invasiveness and leads to increased aggressiveness of tumors in the head/neck, such as in carcinoma of the oral cavity.Citation32 Understanding CD44 is important to the study of tumor progression and invasiveness because invasive tumors attack the extracellular matrix of surrounding tissues for expanding; the interaction between CD44 and hyaluronic acid plays a decisive role in various cellular pathways.Citation33,Citation34
There are clear discrepancies in the interpretation of the expression of CD44 in relation to tumors of various head and neck regions with different biological characteristics. In squamous cell carcinomas of the oral cavity, the evidence seems to indicate that a low expression of CD44 correlates with a greater capacity for metastasis and recurrence, with negative prognostic significance or no significant impact on prognosis.Citation35,Citation36 There are few studies of oropharyngeal cancer, and the results are inconsistent. Rajarajan et alCitation37 and Carinci et alCitation38 found no evidence of expression of CD44 or prognostic significance, whereas Lindquist et alCitation39 and Kokko et alCitation36 reported a correlation between high expression of CD44 and poor prognosis. In squamous cell carcinoma of the tongue, the few available clinical trials reported by Fonseca et al showed a relationship between lack of expression of CD44 and lateral cervical lymph-node metastases.Citation40 This finding is similar to those of Mostaan et al,Citation41 Rodrigo et al,Citation42 and Masuda et al,Citation43 who reported a correlation between the expression of CD44 and low propensity for metastasis and poor prognosis.
Instead, the high expression of CD44 in laryngeal tumors seems to correlate more strongly with a poor prognosis. This contrasts with other locations of head and neck cancer, in which high expression of CD44 correlates with a greater capacity for locoregional or distant metastasis and resistance to radiochemotherapy.Citation44–Citation46 It is becoming increasingly clear that differences in the ability for locoregional or distant metastasis and radioresistance seem to depend on the overexpression of specific CD44v: Sun et alCitation47 and Lu et alCitation48 have shown that high expression of CD44 correlates with a greater tendency to develop metastatic lymph nodes, recurrence, and radioresistance. The different isoforms CD44v3 and CD44v6 seem to correlate with lymph-node metastasis, systemic diffusion, and failure of radiation therapy.Citation49 The metastatic potential identified by markers of CSCs in tumors of the head and neck was recognized in a study that considered other candidate biomarkers of CSCs such as BMI1 with significant implications for clinical outcomes.Citation50 For example, in laryngeal carcinoma, high expression of BMI1 combined with the absence of p16 expression implies the presence of lymph-node metastases.Citation51
Considering the role of CD44 in the activation of cell replication, its antiapoptotic activity, and its potential as a marker of CSCs in epithelial tumors, we decided to study the role of CD44 standard in head and neck tumors. We studied the levels of CD44 sol in the saliva of patients with tumors of the larynx,Citation52 starting from the assumption that in the normal upper aerodigestive tract, CD44 is expressed on the basal surface, whereas in the histologically dysplastic epithelium, CD44 is expressed in all layers of the mucosa in more than 90% of cases. This overexpression is also present in 90% of invasive head and neck tumors.Citation52,Citation53 Our results were encouraging because we found high levels of CD44sol in most patients with laryngeal carcinoma with high specificity compared with controls, and the highest levels of CD44sol were observed in patients with advanced stages of disease. Our and Franzmann et al’s results are promising because of their high diagnostic power, and suggest that CD44sol could be a specific diagnostic marker of head and neck cancer.Citation52,Citation55–Citation57
These data are superior to those obtained by other studies using various markers with different methods of investigation such as loss of heterozygosity, the methylation-specific markers, telomerase activity, mitochondrial DNA mutations, and recently the multiplexed immunobeaded-based technology.Citation58–Citation63
Conclusion
CD44 appears to be a fairly reliable marker of head and neck tumors and to have potential diagnostic value, because its detection is easy and there are clinical benefits in terms of final outcomes. Cruz et al wrote, “The identification of a fraction of cancer stem cells (CSCs) associated with resistance to chemotherapy in most solid tumors leads to the dogma that eliminating this fraction will cure cancer.”Citation64
Further studies are needed to validate this theory and to consolidate the role of CD44 as a biomarker of CSCs in head and neck cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
- KoukourakisMIGiatromanolakiATsakmakiVDanielidisVSivridisECancer stem cell phenotype relates to radio-chemotherapy outcome in locally advanced squamous cell head-neck cancerBr J Cancer201210684685322333601
- BonnetDDickJEHuman acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cellNat Med199737307379212098
- Al-HajjMWichaMSBenito-HernandezAMorrisonSJClarkeMFProspective identification of tumorigenic breast cancer cellsProc Natl Acad Sci U S A20031003983398812629218
- SinghSKClarkeIDTerasakiMIdentification of a cancer stem cell in human brain tumorsCancer Res2003635821582814522905
- CollinsATBerryPAHydeCStowerMJMaitlandNJProspective identification of tumorigenic prostate cancer stem cellsCancer Res200565109461095116322242
- DalerbaPDyllaSJParkIKPhenotypic characterization of human colorectal cancer stem cellsProc Natl Acad Sci U S A2007104101581016317548814
- LiCHeidtDGDalerbaPIdentification of pancreatic cancer stem cellsCancer Res2007671030103717283135
- HoMMNgAVLamSHungJYSide population in human lung cancer cell lines and tumors is enriched with stem-like cancer cellsCancer Res2007674827483317510412
- PrinceMESivanandanRKaczorowskiAIdentification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinomaProc Natl Acad Sci U S A200710497397817210912
- HarperLJPiperKCommonJFortuneFMackenzieICStem cell patterns in cell lines derived from head and neck squamous cell carcinomaJ Oral Pathol Med20073659460317944752
- van der WindtGJSchoutenMZeerlederSFlorquinSvan der PollTCD44 is protective during hyperoxia-induced lung injuryAm J Respir Cell Mol Biol20114437738320463290
- PetersonLFWangYLoMCYanMKanbeEZhangDEThe multi-functional cellular adhesion molecule CD44 is regulated by the 8;21 chromosomal translocationLeukemia2007212010201917657222
- HanagiriTShinoharaSTakenakaMEffects of hyaluronic acid and CD44 interaction on the proliferation and invasiveness of malignant pleural mesotheliomaTumour Biol Epub August 11, 2012
- NaorDNedvetzkiSGolanIMelnikLFaitelsonYCD44 in cancerCrit Rev Clin Lab Sci20023952757912484499
- FangXJJiangHZhaoXPJiangWMThe role of a new CD44st in increasing the invasion capability of the human breast cancer cell line MCF-7BMC Cancer20111129021749678
- MarhabaRZöllerMCD44 in cancer progression: adhesion, migration and growth regulationJ Mol Histol20043521123115339042
- HuetSGrouxHCaillouBValentinHPrieurAMBernardACD44 contributes to T cell activationJ Immunol19891437988012568380
- Toyama-SorimachiNMiyasakaMA sulfated proteoglycan as a novel ligand for CD44J Dermatol1994217958017531724
- OlssonEHonethGBendahlPOCD44 isoforms are heterogeneously expressed in breast cancer and correlate with tumor subtypes and cancer stem cell markersBMC Cancer20111141821957977
- BrownRLReinkeLMDamerowMSCD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progressionJ Clin Invest20111211064107421393860
- PetersonRMYuQStamenkovicITooleBPPerturbation of hyaluronan interactions by soluble CD44 inhibits growth of murine mammary carcinoma cells in ascitesAm J Pathol20001562159216710854236
- LucasMGGreenAMTelenMJCharacterization of the serum In(Lu)-related antigen: identification of a serum protein related to erythrocyte p80Blood1989735966002917192
- HaynesBFHaleLPPattonKLMartinMEMcCallumRMMeasurement of an adhesion molecule as in indicator of inflammatory disease activity. Up-regulation of the receptor for hyaluronate (CD44) in rheumatoid arthritisArthritis Rheum199134143414431719988
- GuoYJLiuGWangXPotential use of soluble CD44 in serum as indicator of tumor burden and metastasis in patients with gastric or colon cancerCancer Res1994544224267506122
- Van HalNLVan DongenGATen BrinkCBEvaluation of soluble CD44v6 as a potential serum marker for head and neck squamous cell carcinomaClin Cancer Res199953534354110589769
- MisraSGhatakSZoltan-JonesATooleBPRegulation of multidrug resistance in cancer cells by hyaluronanJ Biol Chem2003278252852528812738783
- OsakiMKaseSAdachiKTakedaAHashimotoKItoHInhibition of the PI3K-Akt signaling pathway enhances the sensitivity of Fas-mediated apoptosis in human gastric carcinoma cell line, MKN-45J Cancer Res Clin Oncol200413081414605879
- CantleyLCThe phosphoinositide 3-kinase pathwayScience20022961655165712040186
- GrahamVAMarzoALToughDFA role for CD44 in T cell development and function during direct competition between CD44+ and CD44− cellsEur J Immunol20073792593417330818
- AyroldiECannarileLMiglioratiGBartoliANicolettiIRiccardiCCD44 (Pgp-1) inhibits CD3 and dexamethasone-induced apoptosisBlood199586267226787545465
- JoshuaBKaplanMJDoweckIFrequency of cells expressing CD44, a head and neck cancer stem cell marker: correlation with tumor aggressivenessHead Neck201234424921322081
- KunishiMKayadaYYoshigaKDown-regulated expression of CD44 variant 6 in oral squamous cell carcinomas and its relationship to regional lymph node metastasisInt J Oral Maxillofac Surg1997262802839258720
- NegiLMTalegaonkarSJaggiMAhmadFJIqbalZKharRKRole of CD44 in tumour progression and strategies for targetingJ Drug Target20122056157322758394
- NaganoOSayaHMechanism and biological significance of CD44 cleavageCancer Sci20049593093515596040
- WangSJWongGde HeerAMXiaWBourguignonLYCD44 variant isoforms in head and neck squamous cell carcinoma progressionLaryngoscope20091191518153019507218
- KokkoLLHurmeSMaulaSMSignificance of site-specific prognosis of cancer stem cell marker CD44 in head and neck squamous-cell carcinomaOral Oncol20114751051621514878
- RajarajanAStokesABloorBKCD44 expression in oro-pharyngeal carcinoma tissues and cell linesPLoS One20127e2877622242150
- CarinciFStabelliniGCalvittiMCD44 as prognostic factor in oral and oropharyngeal squamous cell carcinomaJ Craniofac Surg200213858911887001
- LindquistDAhrlund-RichterATarjánMTotTDalianisTIntense CD44 expression is a negative prognostic factor in tonsillar and base of tongue cancerAnticancer Res20123215316122213301
- FonsecaIPereiraTRosa-SantosJSoaresJExpression of CD44 isoforms in squamous cell carcinoma of the border of the tongue: a correlation with histological grade, pattern of stromal invasion, and cell differentiationJ Surg Oncol20117611512011223837
- MostaanLVKhorsandiMTSharifianSMCorrelation between E-cadherin and CD44 adhesion molecules expression and cervical lymph node metastasis in oral tongue SCC: predictive significance or notPathol Res Pract201120744845121632186
- RodrigoJPDomínguezFAlvarezCGonzálezMVHerreroASuárezCClinicopathologic significance of expression of CD44s and CD44v6 isoforms in squamous cell carcinoma of the supraglottic larynxAm J Clin Pathol2002118677212109858
- MasudaMKuratomiYShiratsuchiHNakashimaTNaonobuKKomiyamaSDecreased CD44H expression in early-stage tongue carcinoma associates with late nodal metastases following interstitial brachytherapyHead Neck20002266266511002320
- YüceIBayramACağlıSCanözOBayramSGüneyEThe role of CD44 and matrix metalloproteinase-9 expression in predicting neck metastasis of supraglottic laryngeal carcinomaAm J Otolaryngol20113214114620434807
- UwaNKataokaTRToriiICD44 expression is related to poor prognosis of hypopharyngeal squamous cell carcinomaActa Otolaryngol201113132332921142741
- de JongMCPramanaJvan der WalJECD44 expression predicts local recurrence after radiotherapy in larynx cancerClin Cancer Res2010165329533820837694
- SunBZhaoSZhouCYanQWangHDetection of PD4, CD44, PCNA protein and its clinical significance in human laryngeal carcinomaLin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi201024817819 Chinese21254647
- LuSTianJLvZThe probable role of tumor stem cells for lymph node metastasis in supraglottic carcinomaPathol Oncol Res201117333820407935
- BourguignonLYWongGEarleCHyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinomaJ Biol Chem201228739328003282422847005
- AllegraEPuzzoLZuccalàVNuclear BMI-1 expression in laryngeal carcinoma correlates with lymph node pathological statusWorld J Surg Oncol201210120623031716
- AllegraECaltabianoRAmorosiAVasquezEGarozzoAPuzzoLExpression of BMI1 and p16 in laryngeal squamous cell carcinomaHead Neck Epub June 22, 2012
- AllegraETrapassoSSaccoACD44 sol salivary levels detected by ELISA as a diagnostic test for laryngeal carcinomasJCST2012410333334
- IoachimEAssimakopoulosDGoussiaACPeschosDSkevasAAgnantisNJGlycoprotein CD44 expression in benign, premalignant and malignant epithelial lesions of the larynx: an immunohistochemical study including correlation with Rb, p53, Ki-67 and PCNAHistol Histopathol1999141113111810506927
- PereiraLHAdebisiINPerezASalivary markers and risk factor data: a multivariate modeling approach for head and neck squamous cell carcinoma detectionCancer Biomark20111024124922699785
- FranzmannEJReateguiEPPereiraLHSalivary protein and solCD44 levels as a potential screening tool for early detection of head and neck squamous cell carcinomaHead Neck20123468769522294418
- FranzmannEJReateguiEPPedrosoFSoluble CD44 is a potential marker for the early detection of head and neck cancerCancer Epidemiol Biomarkers Prev2007161348135517627000
- FranzmannEJReateguiEPCarrawayKLHamiltonKLWeedDTGoodwinWJSalivary soluble CD44: a potential molecular marker for head and neck cancerCancer Epidemiol Biomarkers Prev20051473573915767360
- SpaffordMFKochWMReedALDetection of head and neck squamous cell carcinoma among exfoliated oral mucosal cells by microsatellite analysisClin Cancer Res2001760761211297256
- CarvalhoALJeronimoCKimMMEvaluation of promoter hypermethylation detection in body fluids as a screening/diagnosis tool for head and neck squamous cell carcinomaClin Cancer Res2008149710718172258
- CalifanoJAhrendtSAMeiningerGWestraWHKochWMSidranskyDDetection of telomerase activity in oral rinses from head and neck squamous cell carcinoma patientsCancer Res199656572057228971181
- LinkovFLisovichAYurkovetskyZEarly detection of head and neck cancer: development of a novel screening tool using multiplexed immunobead-based biomarker profilingCancer Epidemiol Biomarkers Prev20071610210717220337
- HoffmanRMPeriodic PSA-based screening in men 55 to 69 years of age reduced prostate cancermortalityAnn Intern Med2012157JC1JC4
- AllegraEGarozzoALombardoNDe ClementeMCareyTEMutations and polymorphisms in mitochondrial DNA in head and neck cancer cell linesActa Otorhinolaryngol Ital200626418519018236634
- CruzMHSidénACalafGMDelwarZMYakisichJSThe stemness phenotype modelISRN Oncol2012201239264722928120