Abstract
Increased cardiovascular mortality has been associated with rheumatoid arthritis (RA). There are reports indicating that tumor necrosis factor (TNF) blockers may exert favorable but transient effects on the lipid profile, flow-mediated vasodilatation (FMD) of the brachial artery, and the common carotid intima–media thickness (ccIMT) in RA. We evaluated 38 RA patients (33 females and five males with a mean age of 66.7 ± 10.2 years) who were unresponsive to TNF blockers. The patients received one or more courses of two rituximab (RTX) 1000 mg infusions. Disease activity was evaluated at each visit. Investigations included erythrocyte sedimentation rate, C-reactive protein (CRP) levels, the 28-joint disease activity score (DAS28), DAS28CRP, the Health Assessment Questionnaire, the FMD percent change from baseline (FMD%), and the postnitroglycerine endothelium-independent vasodilatation. In comparison with the baseline, there was a significant improvement in clinical variables and acute-phase reactants 24 months after the start of RTX therapy. There was also a major improvement in FMD% (from baseline 5.24 ± 1.12 to 5.43 ± 1.16; P = −0.03) and a smaller change in the ccIMT (from baseline 0.69 ± 0.16 to 0.67 ± 0.12 mm P = 0.25). Univariate analysis showed that global health (P < 0.034) was associated with the improvement in FMD%. Multivariate models showed that GH (odds ratio [OR] 0.91; 95% CI: 0.99–0.83; P = 0.032), CD19+ cells (OR 1.024; 95% CI: 1.045–1.003; P = 0.025), IgM (OR 1.025; 95% CI: 1.045–1.004; P = 0.016), and interleukin (IL)-8 (OR 0.487; 95% CI: 0.899–0.264; P = 0.021) were statistically associated with the improvement of FMD%, and that IL-8 (OR 0.717; 95% CI: 0.926–0.555; P = 0.018) was also statistically associated with improvement of ccIMT. The findings of the study confirm that RTX reduces the progression of accelerated atherosclerosis in patients with RA. They also show that improvement in CD19+ cells, IgM and GH after treatment are statistically associated with the improvement of FMD%, and that improvement in IL-8 levels after treatment is statistically associated with improved FMD% and with decrease in the ccIMT.
Introduction
A number of studies have demonstrated that patients with rheumatoid arthritis (RA) are at increased risk of cardiovascular (CV) morbidity and mortality, and that this risk appears early during the course of the disease.Citation1,Citation2 Epidemiological studies have also shown that RA is associated with an increased risk of premature CV diseases (CVD), including acute myocardial infarction and CV-related mortality.Citation3–Citation7 These findings are not only due to the traditional CV risk factors but also to various mediators of inflammation,Citation8,Citation9 although recent studies have shown that inflammation also plays a key role in atherosclerosis in patients without inflammatory disease.Citation10
Endothelial dysfunction is involved in the development of atherosclerosis and contributes to the late stages of vascular disease.Citation11 Moreover, as there is a correlation between the function of the endothelium of the brachial and coronary arteries, vascular ultrasonography of the brachial artery is now used as a noninvasive means of examining endothelium-dependent flow-mediated vasodilatation (FMD).Citation11 Patients with long-standing RA treated with methotrexate are affected by endothelial dysfunction,Citation12 but a rapid and transient improvement in FMD has been found in patients who were refractory to conventional disease-modifying antirheumatic drugs, after the administration of tumor necrosis factor (TNF) blockers.Citation13
Rituximab (RTX) is a monoclonal antibody that selectively targets CD20-positive B cells and has recently been approved for the treatment of patients with active RA who inadequately respond to or cannot tolerate TNF blockers. It has been found that 24 weeks of treatment with RTX significantly improves disease activity in patients with long-standing active RA who have inadequately responded to one or more anti-TNF drugs,Citation14 and inhibits the progression of structural joint damage.Citation15
A number of studies have investigated the factors that predict a clinical response to RTX treatment and the role of B cells in the pathogenesis of RA.Citation16 On the basis of their findings, the primary aim of this study was to assess whether RTX improves endothelial function in patients with RA who are refractory to anti-TNF therapy, by measuring brachial FMD as an indicator of endothelial dysfunction and common carotid intima–media thickness (ccIMT) as a marker of atherosclerosis before and after RTX therapy. The secondary aim was to assess factors associated with improved endothelial function during RTX therapy.
Material and methods
The study involved 38 RA patients (33 females [81.6%] and five males [18.4%]; mean age 66.4 ± 10.6 years [range 41–83]) diagnosed on the basis of the American College of Rheumatology criteria.Citation17 Written consent was obtained from all patients enrolled in the study, according to the Declaration of Helsinki. The mean disease duration was 5.8 years (range 1–9 years). All of the patients were treated with prednisone (mean dose 6.4 ± 1.2 mg/day) and methotrexate (MTX) (mean dose 12.4 ± 1.3 mg/week), and all had failed to respond to treatment with one or two anti-TNF agents (mean 1.8 ± 0.4). before beginning treatment with RTX.
Treatments
The patients received at least one course of RTX (ie, two infusions of 1000 mg, each separated by a 2-week interval). Subsequent courses were administered on the basis of the repopulation of CD19+ lymphocytes: the mean retreatment time was 38.1 ± 7.11 weeks, and 30 patients received two courses, 20 patients received three courses, eleven patients received four courses, and four patients received five courses.
Clinical evaluation
The clinical evaluation was performed at baseline, T6, T12 and T24. At the time RTX was started, the patients underwent swollen- and tender-joint counts (28 joints) and were asked to provide a global health (GH) assessment. Ritchie’s Index was used to measure joint tenderness.Citation18 In addition to their use in evaluation of the patient, these measures were also used to compute the 28-joint disease activity score (DAS28) and the DAS28/C-reactive protein (CRP) score, in order to establish whether the patients were treatment responders on the basis of the European League Against Rheumatism criteria.Citation19 Details of their past and present antirheumatic therapies and current comorbidities were also recorded, and they were asked to complete a separate questionnaire that included the Italian adaptation of the Health Assessment Questionnaire (HAQ).Citation20
Laboratory data
The following parameters were evaluated at baseline and every 6 months up to 24 months: TNFα (Human TNF-alpha Quantikine Immunoassay; R&D Systems Inc, Minneapolis, MN, USA); interleukin (IL)-6 (Human IL6 Instant ELISA; Bender MedSystems GmbH, Vienna, Austria), IL-10 (Human IL10 Instant ELISA; Bender MedSystems), and IL-8 (Human IL-8 Instant ELISA, Bender MedSystems); the erythrocyte sedimentation rate (ESR) and/or CRP levels (Unicel Coulter DxC 800 Synchron Central System; Beckman Coulter Inc, Brea, CA, USA); CD3+, CD3+/CD4+, CD3+/CD8+, CD19+(B), CD20+(B), CD19+/CD38+(B), NK, CD3-CD56+CD16+, and CD45+Ro/Ra peripheral mononuclear cells (Cytomics FC 500, Beckman Coulter Inc); rheumatoid factor (RF) IgM (N Latex RF, Siemens AG, Munich, Germany); RF IgA and RF IgG (Enzyme Immuno Assay Orgentec Diagnostika GmbH, Mainz, Germany); serum free light chain κ and λ levels were measured with Freelite® assays (The Binding Site Group Ltd, Birmingham, UK) on a BNII nephelometric analyzer (Siemens AG); anticyclic citrullinated peptide (anti-CCP) (Anti-CCP EDIA™; Euro-Diagnostica, Malmö, Sweden); immunoglobulins IgA, IgG, and IgM were measured with a BNII nephelometric analyzer (Siemens); antinuclear antibody (ANA) titers (IIF assay [indirect immunofluorescence test] on Hep-2 cells ANA Hep2 IgG assay; Scimedx Corporation, Denville, NJ, USA and BioPlex 2200 ANA screen; Bio-Rad Laboratories Inc, Hercules, CA, USA).
Assessment of brachial artery FMD
The brachial artery FMD was assessed as previously described.Citation21 Briefly, a B-mode longitudinal section of the right arm brachial artery above the antecubital fossa was obtained by a single trained sonographer, using a 10 MHz linear array transducer (HP Sonos 5500; Hewlett-Packard Development Co, Palo Alto, CA, USA), after the patients had rested for 30 minutes in a temperature-controlled room. In order to assess the FMD, reactive hyperemia was induced by releasing a pneumatic cuff that had been positioned on the forearm and inflated to suprasystolic pressure for 4.5 minutes. After deflation, the maximal flow velocity and arterial diameter were recorded for 90 minutes, ECG-gated, and detected offline. The FMD was assessed at baseline (before the first infusion) and after 24 months and was expressed as the percent change from baseline (resting) (FMD%). Although, there is no generally accepted normal range, an FMD of <5% was considered impaired.
Determination of ccIMT
The measurements were made as previously described.Citation21 Briefly, common carotid artery ultrasonography was performed by a single observer using a duplex ultrasound system (10 MHz linear array transducer, HP Sonos 5500). The patients were supine with the neck extended and the chin turned contralaterally to the examined side, and the carotid arteries were scanned in the transverse and longitudinal planes. The measurement was made 1 cm distal to the carotid bifurcation, in the posterior wall of both the right and left carotid arteries. The IMT was defined as the distance between the leading edges of the lumen interfaces and the media-adventitia interface of the far wall, and the average of three measurements was recorded. In order to assess stiffness parameters, the right and the left common carotid arteries were similarly studied about 1 cm proximal to the bulb region. The ccIMT was defined as the distance between the first and second echogenic lines from the lumen, taking the average of measurements on both sides. The ccIMT values were assessed at baseline (before the first infusion) and after 24 months, and were expressed as millimeters. Although there is no accepted normal range, an increase of ≥1 mm was considered abnormal.
Statistical analysis
The data were analyzed using SAS statistical software, version 8.2 (SAS Institute Inc, Cary, NC, USA). All of the tests were two-tailed, and probability (P) values of <0.05 were considered statistically significant.
Student’s t-test was used to evaluate the changes in disease activity. The correlations between disease activity, the hematological and serological parameters, and the brachial FMD and ccIMT were evaluated by means of regression analysis. Kendall’s tau-b parameter was calculated after stratifying for the line of therapy. The potential predictors of endothelial improvement were identified using multivariate binary logistic regression models
Results
shows the clinical characteristics of the patients at baseline and after treatment with RTX. After 24 months of RTX therapy, there was a significant improvement in the tender-joint count (from 6.88 ± 1.99 to 3.1 ± 1), the swollen-joint count (from 5.15 ± 1.64 to 2.1 ± 1), global health (GH) (from 49.6 ± 14.2 to 10 ± 1.2), Ritchie’s Index (from 11.8 ± 3.2 to 5.3 ± 2.5), ESR (from 63.6 ± 28.0 to 21 ± 0.5 mm/h), CRP (from 2.5 ± 1.8 to 1.3 ± 0.4 mg/dL), DAS28 (from 5.84 ± 0.8 to 3.6 ± 0.1), and the HAQ (from 2.24 ± 0.44 to 0.8 ± 0.12).
Table 1 Clinical parameters of the patients at baseline and after treatment with RTX
shows the laboratory parameters at different time points. At 24 months after the start of RTX therapy, there were statistically significant changes in various cell types, a major improvement in FMD% (from baseline 5.24 ± 1.12 to 5.43 ± 1.16; P = −0.03), and a smaller change in ccIMT (from baseline 0.69 ± 0.16 to 0.67 ± 0.12 mm; P = 0.25).
Table 2 Laboratory parameters at baseline and at different times after RTX therapy
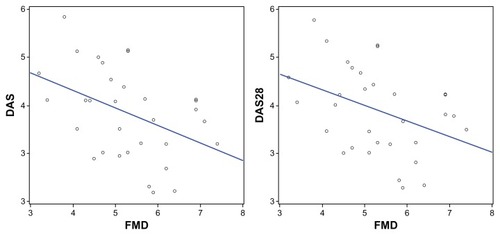
The dramatic improvement in FMD% observed after 12 months was associated with a significant decrease in DAS and DAS28 (), whereas the only correlation after 24 months was with CD19+ cells. There was also a correlation between the improvement in IMT and kappa and lambda chain levels after 12 and 24 months of RTX therapy.
Figure 1 Correlations between FMD% and disease activity scores DAS-DAS28.
Univariate analysis showed that GH (P < 0.034) was associated with the improved FMD%, but none of the other clinical and laboratory parameters seemed to be correlated. IL-8 was the only parameter associated with improved ccIMT (P = 0.0161). Multivariate models showed that after the treatment, GH (odds ratio [OR] 0.91; 95% CI: 0.99–0.83; P = 0.032), levels of CD19+ cells (OR 1.024; 95% CI: 1.045–1.003; P = 0.025), IgM (OR 1.025; 95% CI: 1.045–1.004; P = 0.016), and IL-8 (OR 0.487; 95% CI: 0.899–0.264; P = 0.021) were statistically associated with improved FMD%, and that IL-8 (OR 0.717; 95% CI: 0.926–0.555; P = 0.018) was also a statistically associated with improved ccIMT.
Discussion
The findings of this study confirm that RTX reduces the progression of accelerated atherosclerosis in RA patients and shows that there is a correlation between FMD% and the cells involved in the atherosclerotic process, such as macrophages and lymphocytes, and between ccIMT and the kappa and lambda chains expressed by B cells (the targets of RTX treatment).Citation22
The change in FMD% appeared to be related to changes in disease activity, and the decrease in DAS28 suggests that inflammatory and immune-mediated mechanisms play a central role in both atherosclerosis and RA and that the two disorders have a number of common pathogenic mechanisms.Citation23 Various disease-related mechanisms may be involved in the development of premature vascular damage in RA patients, including an increased synthesis of proinflammatory mediators (cytokines, chemokines, and adhesion molecules), the production of autoantibodies against endothelial cell components, perturbations in T-cell subsets, genetic polymorphisms, hyperhomocysteinemia, oxidative stress, abnormal vascular repair, and iatrogenic factors. It is recognized that organic arterial wall damage is usually preceded by endothelial dysfunction, which is considered the earliest but reversible stage of atheroma development.Citation23 Altered arterial endothelium function has been detected in patients with early RA and is thought to be the result of a chronic inflammatory process, as in the case of other systemic rheumatic diseases.Citation24 As it is now clear that altered cytokine production predates the onset of RACitation25 and that endothelial dysfunction may be reverted by antirheumatic drugs, the pharmacological strategies currently used in the early stages of RA may also benefit RA-related CVD complications. The results of our study suggest that this may also be true of RTX.Citation26
Two other published studies have investigated the effects of RTX in RA patients with atherosclerosis. The firstCitation27 studied five patients treated with two intravenous infusions of RTX 1000 mg and found a prompt and sustained improvement in FMD; the mean improvement was 30% after 2 weeks, 22% after 6 weeks, and 81% after 16 weeks. The authors supposed that RTX would have only a slight effect on atherosclerosis over this short period but actually observed that ccIMT decreased by 10% after 2 weeks, by 9% after 6 weeks, and by 2% after 16 weeks. Moreover, RTX induced a 3%–11% decrease in triglyceride levels and a 14%–35% increase in high density lipoprotein (HDL)-cholesterol levels. The second studyCitation28 involved RTX-treated patients with active disease that was refractory to TNF blockers, and evaluated FMD% and postnitroglycerine endothelium-independent vasodilatation. After 2 weeks, all of the patients showed a dramatic increase in FMD% values in comparison with baseline, and these remained higher after 6 months. Moreover, the significant improvement in FMD% was associated with a significant decrease in CRP levels and DAS28.
Our study not only confirms these findings but also revealed correlations between FMD%, disease activity, and the cells involved in the atherosclerotic process, thus suggesting that RTX acts on various mechanisms of RA and accelerated atherosclerosis. Moreover, the correlation between the changes in ccIMT and the kappa and lambda chains of immunoglobulin suggests that B cells play an important role in atherosclerosis. Recent data have shown that B-cell depletion induced by a CD20-specific monoclonal antibody significantly reduces atherosclerosis in different mouse models, maintains the production of natural and protective anti-oxidized low-density lipoprotein (oxLDL) IgM autoantibodies over anti-oxLDL IgG antibodies, significantly reduces pathogenic T cell activation and T cell–derived interferon-γ secretion, and enhances the production of IL-17.Citation29
Furthermore, selective B-cell depletion in apolipoprotein E-deficient mice using a well-characterized anti-mouse CD20 monoclonal antibody reduced the development and progression of atherosclerosis without having any effect on hyperlipidemia induced by a high-fat diet. These findings suggest that B2 cells can promote atherosclerosis alone, in the absence of other lymphocytes.Citation30 B-cell depletion affects proatherogenic IgG anti-oxLDL antibodies, as the link between oxLDL and anti-oxLDL IgG leads to the formation of immune complexes that bind the surface Fcγ receptors on macrophages, thus causing foam cell formation and the release of inflammatory cytokines.Citation31
Our findings also show that GH, the number of CD19+ cells, and IgM and IL-8 levels after the treatment were associated with an improvement of FMD%. GH reflects the health status and the quality of life, and it is known that a poor quality of life and a sedentary lifestyle are risk factors for CVD in the general population and in RA patients.Citation32–Citation34
In line with the effect of RTX therapy on B cells, we found that CD19+ cells and IgM values after the treatment were correlated with improved FMD%. The risk associated with RTX therapy is dose independent, but it is not clear whether there is a threshold below which it is efficacious, as improved FMD% has also been observed in patients receiving only one cycle of RTX.Citation27
Also IL-8 levels after the treatment were associated with an improvement in both FMD% and ccIMT. This suggests that IL-8 (a chemokine produced by the macrophages and endothelial cells involved in the inflammatory process) may be involved in the pathogenesis of atherosclerosis and CV events.Citation33 The effect of RTX on cytokines is supported by the fact that we observed a decrease in all of the inflammatory parameters considered in our study.
Finally, RTX is safe in patients with CVD. This was shown by a large-scale randomized controlled trial, which found that the patients treated with RTX and those treated with placebo experienced a similar number of CV events (acute myocardial infarction, angina, and stroke),Citation34 but it is worth pointing out that RTX also halts the progression of atherosclerosis via a different pathway. On the basis of our findings, we believe that the potent anti-inflammatory effects of RTX may explain the improvement in endothelial function.
In conclusion, our data suggest that RTX therapy in RA is associated with a small but significant improvement of FMD%, which is also associated with GH, CD19+ cells, and IgM and IL-8 levels. The decrease of ccIMT was also associated with IL-8 values after RTX therapy. These findings confirm the results obtained in animal models indicating that RTX plays a role in preventing accelerated atherosclerosis in RA patients. However, further studies of a large number of patients and national registers are required to validate these preliminary results.
Acknowledgments
This project was financially supported by our Department. The authors wish to thank MD Paola Baiardi for her statistical support.
Disclosure
The authors report no conflicts of interest in this work.
References
- KremersHMCrowsonCSTherneauTMRogerVLGabrielSEHigh ten-year risk of cardiovascular disease in newly diagnosed rheumatoid arthritis patients: a population-based cohort studyArthritis Rheum20085882268227418668561
- Aviña-ZubietaJAChoiHKSadatsafaviMElminanJMEsdaileJMLacailleDRisk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studiesArthritis Rheum200859121690169719035419
- SolomonDHGoodsonNJKatzJNPatterns of cardiovascular risk in rheumatoid arthritisAnn Rheum Dis200665121608161216793844
- Maradit-KremersHNicolaPJCrowsonCSBallmanKVGabrielSECardiovascular death in rheumatoid arthritis: a population-based studyArthritis Rheum200552372273215751097
- SolomonDHKarlsonEWRimmEBCardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritisCirculation200310791303130712628952
- del RincónIDWilliamsKSternMPFreemanGLEscalanteAHigh incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factorsArthritis Rheum200144122737274511762933
- WolfeFMichaudKThe risk of myocardial infarction and pharmacologic and nonpharmacologic myocardial infarction predictors in rheumatoid arthritis: a cohort and nested case-control analysisArthritis Rheum20085892612262118759273
- Del RincónIWilliamsKSternMPFreemanGLO’LearyDHEscalanteAAssociation between carotid atherosclerosis and markers of inflammation in rheumatoid arthritis patients and healthy subjectsArthritis Rheum20034871833184012847676
- GoodsonNJSymmonsDPScottDGBunnDLuntMSilmanAJBaseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: a ten-year followup study of a primary care-based inception cohortArthritis Rheum20055282293229916052597
- Van DoornumSMcCollGWicksIPAccelerated atherosclerosis: an extraarticular feature of rheumatoid arthritis?Arthritis Rheum200246486287311953961
- VitaJAKeaneyJFJrEndothelial function: a barometer for cardiovascular risk?Circulation2002106664064212163419
- Gonzalez-JuanateyCTestaAGarcia-CasteloAHLA-DRB1 status affects endothelial function in treated patients with rheumatoid arthritisAm J Med2003114864765212798452
- Gonzalez-JuanateyCTestaAGarcia-CasteloAGarcia-PorruaCLlorcaJGonzalez-GayMAActive but transient improvement of endothelial function in rheumatoid arthritis patients undergoing long-term treatment with anti-tumor necrosis factor alpha antibodyArthritis Rheum200451344745015188332
- CohenSBEmeryPGreenwaldMWthe REFLEX Trial GroupRituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeksArthritis Rheum20065492793280616947627
- KeystoneEEmeryPPeterfyCGRituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapiesAnn Rheum Dis200968221622118388156
- BenucciMManfrediMPuttiniPCAtzeniFPredictive factors of response to rituximab therapy in rheumatoid arthritis: What do we know today?Autoimmun Rev201091280180320656069
- ArnettFCEdworthySMBlochDAThe American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritisArthritis Rheum19883133153243358796
- RitchieDMBoyleJAMcInnesJMClinical studies with an articular index for the assessment of joint tenderness in patients with rheumatoid arthritisQJMed196837147393406
- FransenJvan RielPLThe disease activity score and EULAR response criteriaClin Exp Rheumatol2005235 suppl 39939515789894
- RanzaRMarchesoniACaloriGThe Italian version of the Functional Disability Index of the Health Assessment Questionnaire. A reliable instrument for multicenter studies in rheumatoid arthritisClin Exp Rheumatol19931121231288508554
- KerekesGSzekaneczZDérHEndothelial dysfunction and atherosclerosis in rheumatoid arthritis: a multiparametric analysis using imaging techniques and laboratory markers of inflammation and autoimmunityJ Rheumatol200835339840618203326
- SellamJHendel-ChavezHRouanetSB cell activation biomarkers as predictive factors for the response to rituximab in rheumatoid arthritis: a six-month, national, multicenter, open-label studyArthritis Rheum201163493393821225699
- Sarzi-PuttiniPAtzeniFGerliRCardiac involvement in systemic rheumatic diseases: An updateAutoimmun Rev201091284985220692379
- GerliRVaudoGBocciEBFunctional impairment of arterial wall in primary Sjögren’s syndrome: combined action of immunological and inflammatory factorsArthritis Care Res (Hoboken)201062571271820191479
- KokkonenHSöderströmIRocklövJHallmansGLejonKRantapää DahlqvistSUp-regulation of cytokines and chemokines predates the onset of rheumatoid arthritisArthritis Rheum201062238339120112361
- AtzeniFTurielMCaporaliRThe effect of pharmacological therapy on the cardiovascular system of patients with systemic rheumatic diseasesAutoimmun Rev201091283583920678592
- KerekesGSoltészPDérHEffects of rituximab treatment on endothelial dysfunction, carotid atherosclerosis, and lipid profile in rheumatoid arthritisClin Rheumatol200928670571019319624
- Gonzalez-JuanateyCLlorcaJVazquez-RodriguezTRDiaz-VarelaNGarcia-QuirogaHGonzalez-GayMAShort-term improvement of endothelial function in rituximab-treated rheumatoid arthritis patients refractory to tumor necrosis factor alpha blocker therapyArthritis Rheum200859121821182419035415
- Ait-OufellaHHerbinOBouazizJDB cell depletion reduces the development of atherosclerosis in miceExp Med2010207815791587
- KyawTTayCKhanAConventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosisJ Immunol201018574410441920817865
- Van LeeuwenMDamoiseauxJDuijvestijnATervaertJWThe therapeutic potential of targeting B cells and anti-oxLDL antibodies in atherosclerosisAutoimmun Rev200991535719285155
- TremblayMSColleyRCSaundersTJHealyGNOwenNPhysiological and health implications of a sedentary lifestyleAppl Physiol Nutr Metab201035672574021164543
- Ait-OufellaHTalebSMallatZTedguiARecent advances on the role of cytokines in atherosclerosisArterioscler Thromb Vasc Biol201131596997921508343
- van VollenhovenRFEmeryPBinghamCO3rdLongterm safety of patients receiving rituximab in rheumatoid arthritis clinical trialsJ Rheumatol201037355856720110520