Abstract
Purpose
The main purpose of this paper is to explore the interaction between GAS5 and miR-135b-5p to understand their function in the metastasis, invasion, and proliferation of glioma. This may provide new ideas for the pathogenesis and treatment of glioma.
Patients and Methods
Western blotting assays and RT‑qPCR were employed to investigate the expression of related genes in glioma tissues or cell lines. CCK-8 was used to examine the impact of GAS5 on cell viability. Motile activities were adopted by the transwell and wound healing experiments. A double luciferase experiment was performed to elucidate transcriptional regulation.
Results
GAS5 showed low expression in glioma cells and tissues, and up-regulation of GAS5 could depress the invasion, proliferation, and metastasis of glioma. GAS5 negatively regulates miR-135b-5p, which can counteract the cellular effects caused by GAS5. APC was the target of miR-135b-5p, and GAS5 can regulate the expression of APC by sponging miR-135b-5p. APC overexpression reversed the effects of miR-135b-5p promotion on glioma cells, while miR-135b-5p has the opposite function. As a downstream target gene of GAS5, miR-135b-5p was negatively regulated by GAS5. The restoration of miR-135b-5p can remarkably reverse the impact of GAS5 on glioma cells. In addition, GAS5 increased the expression of APC in glioma cells by inhibiting miR-135b-5p.
Conclusion
GAS5 increased APC expression by restraining miR-135b-5p and partially blocked the progression of glioma, suggesting that it could be an advantageous therapeutic target for glioma intervention.
Keywords:
Introduction
Gliomas are responsible for the majority of deaths from primary brain tumours.Citation1 Gliomas originate from glial cells or stem cells and are the most common primary tumors of the brain and spinal cord,Citation2 accounting for 80% of primary malignant tumors of the brain.Citation3 The main glial tumor groups included oligodendroglial tumors, astrocytic tumors, oligoastrocytic tumors, neuronal tumors, ependymal tumors, and mixed neuronal-glial tumors. Among adults with primary intracranial tumors, gliomas account for the majority.Citation4 For children, the most common glioma types are pilocytic astrocytomas and diffuse midline gliomas including diffuse intrinsic pontine gliomas of various grades.Citation1 The incidence of gliomas in general increases with age, with the most pronounced increase in glioblastoma. Many environmental factors have been studied in relation to glioma, but ionizing radiation (exposure to therapeutic doses) is the only established factor that has been identified as a causative agentitive factor.Citation5–7 Genome-wide association studies have shown that glioma risk is associated with single-nucleotide polymorphisms (SNPs).Citation8,Citation9 In addition, telomerase RNA component (TERC) and telomerase reverse transcriptase (TERT), which are involved in telomere length regulation, were identified as candidate genes for increased glioma risk.Citation10,Citation11
The World Health Organization (WHO) classifies gliomas into four different grades based on tumor mitotic rate, necrosis, histology, angiogenesis, and nuclear atypia.Citation12 The malignant degree of glioma is strongly linked to prognosis.Citation13 To date, the conventional therapy for glioma consists of surgical resection, temozolomide (TMZ), and radiation, which is far from sufficient in combating cancer development.Citation14 The recurrence rate of patients is high due to the depth of glioma malignancy, and the 5-year survival rate is no more than 5%. Early identification and treatment of these tumors will greatly improve the treatment effect. There is an urgent need for new diagnostic and prognostic markers to identify the disease at an early stage and to differentiate subtypes of these tumors, thereby improving the current treatment modalities.Citation15
LncRNAs are more than 200 bases in length and are noncoding RNAs. LncRNAs can widely participate in the regulation and expression of genes in eukaryotic cells. Additionally, abnormal expression of lncRNAs is strongly linked to the appearance and progression of tumors and is regarded as an emerging biomarker and potential therapeutic target in cancer epigenetics.Citation16 Long noncoding RNA growth arrest-specific 5 (GAS5) is known to act as a tumor suppressor and apoptosis promoter, and its downregulation is involved in a variety of tumors, such as lymphoma, cervical carcinoma, and osteosarcoma.Citation17–19 GAS5 exerts biological functions mainly through its intron encoding multiple snoRNAs.Citation20 In MA’s study,Citation17 GAS5 inhibited the expression of miR-221-3p and upregulated the expression of IRF2 in non-small cell lung cancer. GAS5 also acted as a microRNA-23a sponge to promote autophagy and enhance autophagosome formation after GAS5 overexpression in breast cancer.Citation21 In addition, GAS5 slowed glioma progression by eradicating Sirtuin 1 and microRNA-10b in A172 and U251 cells.Citation22 As a competitive endogenous RNA, GAS5 restrained the proliferation and invasion of osteosarcoma cells through a combination of miR-23a-3p and promoted PTEN expression by mediating the PI3K/AKT pathway.Citation22 Moreover, GAS5 targets miR-106b to regulate the expression of PTEN, thus affecting the progression of EMT, as well as the invasion, migration, and proliferation of glioma cells.
MicroRNAs are noncoding small RNAs containing 20 ~ 40 nucleotides. Most of them have important functions.Citation23,Citation24 In particular, diverse functions of miRNA have been reported in many diseases, including apoptosis, migration, differentiation, proliferation, and invasion.Citation25,Citation26 Due to their essential role in eukaryotic cells, dysregulation of miRNAs can lead to the activation of oncogenes or suppressors in tumor diseases.Citation27–29 MiR-135b-5p is a conserved transcript among mammalians and is located in the gene locus of 1q32.1 in humans.Citation30 In gastric cancer, miR-135b-5p upregulates and maintains the expression of Kruppel-like factor 4 (KLF4), thereby promoting the proliferation, viability, migration, and invasion of cancer cells.Citation31 The expression of miR-135b-5p was significantly up-regulated in feces of CRC patients, and miR-135-5p can directly target the mRNA of ZNRF3, thereby activating the Wnt pathway. MiR-135b-5p is expected to be a non-invasive biomarker for the diagnosis of colorectal cancer patients at stage TNM III/IV and a potential candidate for colorectal cancer intervention strategies.Citation32
The adenomatous polyposis coli (APC) gene is a tumor suppressor gene located in the human chromosome region 5q21–22,Citation33,Citation34 which plays a vital role in cellular proliferation, migration, DNA repair, and chromosomal segregation.Citation35 One of the best-known mechanisms of APC is the regulation of the Wnt/b-catenin signaling pathway.Citation36 A mutation in APC in mouse intestinal epithelial cells can decrease the level of E-cadherin at the cell membrane and association between catenin and E-cadherin,Citation37 thus affecting cell adhesion. Mutations in APC are usually deletion, insertion, or frameshift, which introduce premature stop codons and lead to the production of truncated APC proteins that lack normal function and have tumorigenic properties.Citation38 APC is lost in a variety of cancers, including breast, colorectal, and prostate cancers, and its loss leads to decreased overall survival in patients with non-small cell lung cancer and breast cancer.Citation39 This implies that APC- deficient patients have a worse prognosis than APC-competent patients. In recent years, it has been found that APC gene mutation status can be used as a predictor of poor prognosis in patients with stage III colorectal cancer.Citation40 However, the regulatory mechanism of APC in glioma remains unclear.
Although the role and mechanics of GAS5 in glioma have been reported, whether miR-135b-5p is involved in the development and progression of glioma and whether GAS5 can target and regulate miR-135b-5p to meditate the progression of glioma have not been studied. In our study, we researched the biological functions and expression characteristics of miR-135b-5p and GAS5 in glioma cells and tissues, and also explored the regulatory function of GAS5 on miR-135b-5p, further proving that GAS5 can inhibit the progression of glioma by reducing miR-135b-5p and facilitating the expression of APC.
Materials and Methods
Cell Culture and Tissue Collection
Twenty-two glioma tissues and paired corresponding normal tissues were collected from the Third Affiliated Hospital of Chongqing Medical University. Tissue samples were conserved in 2 mL cryotubes in liquid nitrogen for 1 hour and then transferred to −80°C. The human glioma cell lines T98, LN229, A172, and U251 and the normal human astrocyte cell line (NHA) were acquired from the China Center for Type Culture Collection (Wuhan, China). Glioma cell lines were cultivated in high glucose DMEM containing 10% FBS (Moregate, Australia), and NHAs were grown in AM medium (Astrocyte Medium, ScienCell, USA) at 37°C and 5% CO2.
Cell Transfection
GAS5 overexpression plasmid, sh-GAS5, miR-135b-5p inhibitor, miR-135b-5p mimic, APC overexpression plasmid, and their controls were purchased from Gene Pharma (Shanghai, China). The above plasmids and controls were transfected into T98 or A172 cells using Lipofectamine 3000 (Thermo, USA).
Reverse Transcription‑Quantitative (RT‑qPCR)
Total RNA from cell lines and tissues was extracted using a TRIzol reagent kit (Invitrogen, USA) according to the manufacturer’s instructions. The primers were designed and provided by Sangon Biotech, Shanghai. qRT‑PCR was performed using SYBR Green I (Takara, Japan). The miRNA and mRNA expression levels were normalized against U6 and GAPDH, respectively.Citation31,Citation41–44 Reaction conditions for qRT‑PCR were as follows: 95°C 30s, 55°C 30s, and 72°C 90s, for a total of 40 cycles. All samples were repeated three times, and the primers used in this study are listed in .
Table 1 The Primer Sequences Used in Study
Cell Counting Kit-8 Assay
Cells in the logarithmic phase of growth (2000 cells/well) were cultured on 96-well plates filled with 150 μL complete medium for 1 day. After different treatments, CCK-8 solution (10 μL, Sigma, Germany) was added and cultured for 4 h at 37°C. After that, the optical density of each well at 450 nm was detected.
Wound Healing Assay
First, cells were seeded into 6-well plates. A line was drawn in the cell surface with a pipette tip (200 μL) when the cells reached 70–85% confluence. After 24 h, the scratch width was measured to assess wound healing, photographs were taken, and ImageJ was used to evaluate the gap distance.
Transwell Assay
The polycarbonate membrane bourdon chamber in the transwell apparatus was used to detect cell infiltration. A total of 5 × 104 cells were plated in the upper chambers, while 100 μL of DMEM with 10% FBS was added to the lower chambers. After 24 h of incubation at 37°C, a cotton swab was used to remove the cells on the top of the membrane, and then the cells were fixed with 4% paraformaldehyde followed by staining in crystal violet. Finally, the cells were observed and photographed under an inverted microscope (×100).
Dual-Luciferase Reporter Gene Experiment
The mutant (MUT) or wild-type (WT) binding sequence between GAS5 and miR-135b-3p or APC 3’-UTR and miR-135b-3p was linearized with restriction enzymes and cloned and inserted into the pmirGLO dual luciferase vector. These reporters were cotransfected with miR-135b-5p mimics or mimics-NC using Lipofectamine 3000 reagent. Then, after 48 h, a Dual luciferase Reporter Assay System kit (Promega) was used to measure luciferase activity. The experiment was conducted three times in total.
Western Blot
Cells were lysed with RIPA containing protease inhibitor PMSF (MCE, USA) to lyse cells. Total protein lysates were quantified according to the BCA kit (MCE, USA). Equal amounts of proteins mixed with SDS‒PAGE loading buffer were transferred onto PVDF membranes. The membrane was then blocked with 5% nonfat milk and incubated with the primary antibody (1:1000) overnight at 4°C. Then, the secondary antibody (1:10,000) was added after washing, and another 1 h was needed for incubation. The experiment was conducted three times in total.
Statistical Analysis
Data were expressed as the mean ± standard deviation of three repeated experiments. All data were analyzed using GraphPad Prism 8.0. Student’s t-test was performed to evaluate the differences between the two groups. Multiple comparisons were conducted with 1-way ANOVA, followed by Tukey post hoc test. P<0.05 was considered statistically significant.
Results
GAS5 Was Abnormally Decreased in Glioma Tissues and Cells and Related to a Low Survival Rate
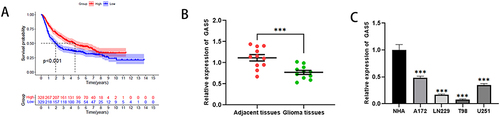
Data analysis using CGGA (http://www.cgga.org.cn/) database indicated that patients with high expression of GAS5 had a significantly higher survival rate (). To examine the level of GAS5 in glioma tissues and cells, quantitative PCR was used. The data indicated that GAS5 expression in glioma tissues and cells was markedly decreased () indicating that GAS5 may be a potential factor promoting glioma deterioration.
Figure 1 Reduced GAS5 in glioma tissues is related to low survival rates. (A) Correlation between GAS5 expression and overall survival was significantly higher in patients with high expression of GAS5. (B) The mRNA expression level of GAS5 in glioma tissues and adjacent tissues. (C) The mRNA expression level of GAS5 in normal glial and glioma cells. *** means P < 0.001.
Up-Regulation of GAS5 Suppressed the Metastasis, Invasiveness, and Proliferation of Glioma Cells
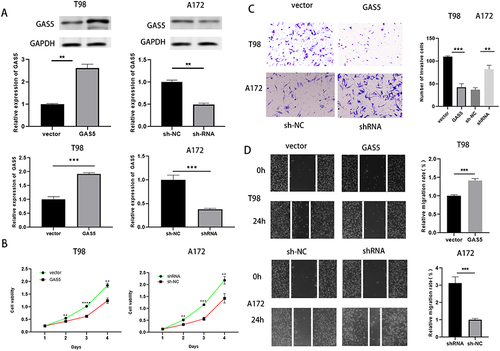
Firstly, GAS5 overexpression vector and shRNA vector were constructed to investigate the biological role of GAS5 in glioma. After transfection, Western blot and RT‑qPCR verified that the overexpression and knockdown effects of the vectors were satisfactory in T98 and A172 cells, respectively (). Subsequently, CCK-8 and transwell experiment showed that the overexpression of GAS5 in T98 cells reduced cell proliferation, migration, and invasion, while the knockdown of GAS5 in A172 cells showed the opposite phenomenon (). In conclusion, up-regulation of GAS5 inhibited the proliferation, migration, and invasion of glioma cells.
Figure 2 GAS5 suppressed the invasion, propagation, and migration of glioma cells. (A) Western blot and qRT-PCR were implied to detect the overexpression and knockdown efficiency of GAS5. (B, C, D)CCK-8, scratch test (magnification, x40) and transwell assay (magnification, x100) were applied to detect cell proliferation, invasion and migration ability after overexpression or knockdown of GAS5. ** means P < 0.01, *** means P < 0.001.
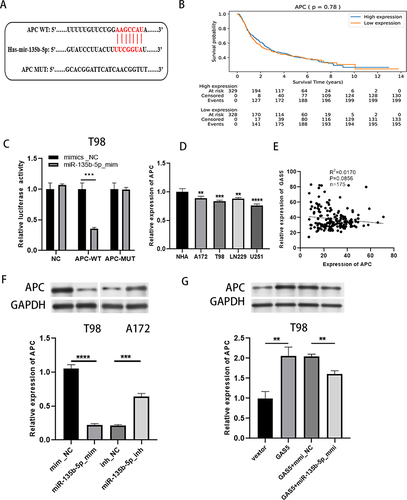
GAS5 Acted as a Molecular Sponge for miR-135b-5p
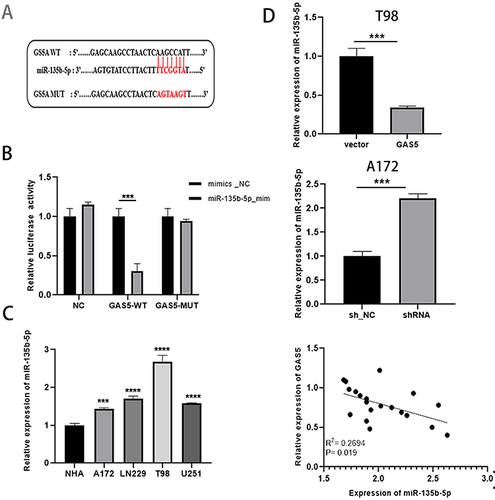
The miRNA related to lncRNA GAS5 was searched in StarBase v2.0, and referring to literature reports,Citation32 we found that GAS5 had a complementary sequence to miR-135b-5p (). We subsequently performed a dual luciferase validation experiment. Compared with the control, the miR-135b-5p mimic obviously weakened the luciferase activity of WT-GAS5 but not that of GAS5-MUT in A172 cells (). RT‑qPCR results showed that compared with the adjacent tissues and NHA cells, miR-135b-5p in glioma tissues and cells increased significantly (). Additionally, the overexpression and knockdown of GAS5 triggered the up-regulation and declined of miR-135b-5p and there was a negative correlation between them (). These results illustrated that miR-135b-5p was a target of GAS5, which is a natural sponge of miR-135b-5p.
Figure 3 GAS5 acted as a molecular sponge for miR-135b-5p. (A) Starbase was used to predict the target sites between GAS5 and miR-135b-5p. (B) Dual luciferase reporter assay was used to verify the binding of GAS5 to miR-135b-5p. (C) qRT-PCR was taken to detect the expression of miR-135b-5p in glioma cells and NHA. (D) The expression of miR-135b-5p was determined by qRT-PCR after GAS5 overexpression and knockdown, and there was a negative correlation between them. *** means P < 0.001, **** means P < 0.0001.
GAS5 Regulated Cell Proliferation, Migration, and Invasion by Sponging miR-135b-5p
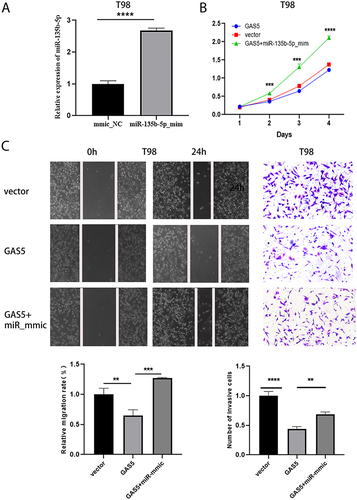
To further investigate the role of GAS5 and miR-135b-5p in glioma progression, we constructed a functional gain model of miR-135b-5p. By analyzing the RT‑qPCR results, overexpression effects were significant (). We then performed reverted experiments to explore the regulation of GAS5 on miR-135b-5p. The result showed that the cell viability of GAS5+miR-135b-5p_mmic group was clearly higher contrasted to GAS5 group (). The cotransfection of GAS5 and miR-135b-5p_mmic rescued GAS5-induced invasion and migration inhibition of T98 cells (). Taken together, GAS5 regulated the evolution of glioma cells dependent on sponging miR-135b-5p.
Figure 4 GAS5 regulated cell proliferation, migration and invasion by sponging miR-135b-5p. (A) qRT-PCR was used to detect the transfection efficiency of miR-135b-5p. (B, C) CCK-8, scratch test (magnification, x40) and transwell assay (magnification, x100) were applied to detect cell proliferation, invasion and migration ability after transfection with vector, GAS5, GAS5+ miR-135b-5p_mmic. ** means P < 0.01, *** means P < 0.001, **** means P < 0.0001.
MiR-135b-5p Targeted APC and GAS5 Regulated APC Expression by Targeting miR-135b-5p
Through a literature review and database prediction,Citation19 we found that 3ʹUTR of APC has a binding target for miR-135b-5p in sequence (). Moreover, there was no correlation between APC expression level and the overall survival rate of glioma patients (). We performed a dual luciferase assay to verify whether APC could combine with miR-135b-5p. Compared with mmic_NC, transfection of miR-135b-5p_mmic significantly inhibited the luciferase activity of APC-WT group, but not APC-MUT group (), suggesting the interaction between miR-135b-5p and APC. Whereafter, APC expression was clearly decreased in glioma tissues and cells compared with adjacent tissues and NHA cells, respectively (). Data analysis obtained from TCGA database showed that there was no significant correlation between GAS5 and APC expression levels in glioma samples (). Western blot result showed that APC was down-regulated after overexpression of miR-135b-5p and increased after the knockdown of miR-135b-5p (). Furthermore, the overexpression of GAS5 elevated APC, while co-transfection of GAS5 and miR-135b-5p_mmic attenuated the effect (). In conclusion, APC was a target of miR-135b-5p and GAS5 indirectly promoted the expression of APC by sponging miR-135b-5p.
Figure 5 MiR-135b-5p targeted APC and GAS5 regulated APC expression by targeting miR-135b-5p. (A) Starbase was taken to predict the biding sites between APC and miR-135b-5p. (B) CCGA database analysis the relationship between APC expression level and survival rate of glioma patients. (C) Dual luciferase reporter gene assay was implied to exam the interaction between miR-135b-5p and APC. (D) The expression of APC in glioma cells and NHA was detected by qRT-PCR. (E) Relationship between GAS5 and APC expression levels in glioma samples from TCGA database. (F) Western blot was taken to detect the APC expression after increase or inhibition of miR-135b-5p. (G) The expression of APC was detected by western blot after transfected with GAS5, GAS5+ miR-135b-5p_mmic or controls in T98 cells. ** means P < 0.01, *** means P < 0.001, **** means P < 0.0001.
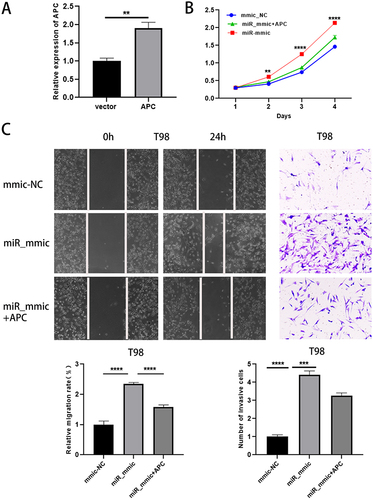
Increasement of APC Attenuated the Effects of miR-135b-5p Overexpression on Glioma Cells
The expression level of APC was detected after transfecting the overexpression plasmid of APC into T98 cells, and the RT‑qPCR result was satisfactory (). Subsequently, CCK-8, scratch test and transwell assay showed that overexpression of miR-135b-5p could promote cell proliferation, migration, and invasion, but cotransfection of APC could attenuate this effect ().
Figure 6 Increasement of APC attenuated the effects of miR-135b-5p overexpression on glioma cells. (A) The efficiency of APC overexpression was detected by qRT-PCR. (B, C) CCK-8, scratch test (magnification, x40) and transwell assay (magnification, x100) were performed to detect the proliferation, migration and invasion ability after transfection with mmic_NC, miR_mmic, miR_mmic+APC in T98 cells. ** means P < 0.01, *** means P < 0.001, **** means P < 0.0001.
Discussion
As reported by the World Health Organization (WHO), glioma is one of the most common primary brain tumors in adults.Citation45 Epigenetic alterations, including DNA methylation and posttranscriptional modifications, play important functions in the etiology and biology of gliomas.Citation46 In recent years, molecular markers of glioma, such as O6-methylguanine-DNA methyltransferase (MGMT), phosphatase and tensin homolog (PTEN), and isocitrate dehydrogenase (IDH), have become increasingly important in predicting treatment outcome, prognosis, and diagnosis.Citation47 Tumor targeted therapy is a new treatment strategy for glioma that uses drugs or other substances to identify and destroy cancer cells without compromising the survival and overall survival of normal healthy cells.Citation48 Pathways involved in tumor growth, invasion, and angiogenesis processes represent major processes in glioma.Citation49 Multi-target kinase inhibitors or single-target kinase inhibitors combined with multiple signaling pathways can improve the therapeutic effect.Citation49 Additionally, the prognosis for high-grade gliomas is not satisfying. Uncovering the underlying mechanisms may help to explore specific therapeutic strategies.Citation50 GAS5, a well-known lncRNA that functions as a cancer suppressor, was inhibited in multiple tumors, including lung cancer, gliomas, gastric cancer, prostate cancer, etc.Citation20 Chen L et al demonstrated that GAS5 overexpression sensitizes A549 cells to radiotherapy through regulating miR-21/PTEN/Akt axis.Citation51 Another report proved that LncRNA GAS5 modulates the progression of non-small cell lung cancer through repressing miR-221-3p and up-regulating IRF2.Citation52 Currently, numerous studies generally agree that in glioma cells, GAS5 is expressed at low levels; additionally, downregulation of GAS5 can act as a predictive warning factor for poor prognosis when glioma is of low grade.Citation53–55 Consistent with these statements, the impacts of GAS5 on suppressing cell migration, migration, and invasion of glioma cells were exhibited in our current study. We also confirmed that GAS5 was associated with the survival rate of glioma patients.
Duan et al found that miR-498 promoted the proliferation, migration, and invasion of prostate cancer cells and reduced radiosensitivity by targeting PTEN.Citation56 Accumulated reports have shown that numerous abnormally expressed miRNAs were discovered in gliomas through RNA sequencing, proving that miRNAs may be associated with the formation and deterioration of gliomas.Citation37,Citation38 It has been certified that lncRNA often rely on acting as sponges for miRNAs to play a role in cancers.Citation57,Citation58 Accumulated reports have shown that numerous abnormally expressed miRNAs were discovered in gliomas through RNA sequencing, proving that miRNAs may be associated with the formation and deterioration of gliomas.Citation37,Citation38 For example, GAS5 can regulate numerous pathological and physiological processes in cells by targeting numerous miRNA molecules.Citation59 In lung cancer, GAS5 can sponge miR-205 and regulate PTEN expression to affect cell proliferation and metastasis.Citation60 Chen et al showed that GAS5, through promoting the expression of miR-424, suppressed the progression of glioma cells and restrained xenograft growth in vivo.Citation61 In the present work, we verified that miR-135b-5p was the downstream target of GAS5, and there is a negative correlation. This finding has not been previously reported. It has been proven that up-regulation of miR-135b-5p promotes cancer metastasis in lung cancer, neck squamous, and head cell carcinoma.Citation62,Citation63 We found that the expression level of miR-135b-5p in glioma cells was significantly higher than that in NHA cells. However, its expression data in clinical samples could not be found in multiple databases, which limited the validation of some of our findings. Besides, miR-135b can inhibit the development of tumors and reverse drug resistance.Citation64–66 Though miR-135b-5p has been found to modulate gene expression in various cancers, the role in glioma has not been reported. This work provided a certain basis for its cancer-promoting effect. We demonstrated that the expression of miR-135b-5p was increased in glioma tissues and cells, and it facilitated the malignant phenotypes of glioma cells. Moreover, overexpression of miR-135b-5p reversed the effect of GAS5 on glioma cells. Based on these results, we proposed that miR-135b-5p was an oncomiR in glioma, and the effect of GAS5 were obtained by sponging miR-135b-5p.
MiRNAs play an important regulatory role in the occurrence and development of tumors by targeting the 3‘-untranslated region (UTR) of messenger RNA (mRNA). Wang et al demonstrated that miR-135b-5p overexpression reduces chemoresistance via directly degrading the mRNA of upstream regulator of integrin subunit alpha 2 (ITGA2) in gastric cancer.Citation67 Duan et al found that miR-498 promoted the proliferation, migration, and invasion of prostate cancer cells and reduced radiosensitivity by targeting PTEN.Citation56 Li et al proposed that miR-135b-5p expression downregulates PPM1E to activate AMPK signaling, which inhibits LPS-induced TNF-α production via suppression of ROS production and NF-κB activation.Citation68 Jin et al have confirmed that miR-135b can affect tumor metastasis through the Wnt pathway.Citation69 β-catenin is a positive regulator of Wnt pathway, while APC is an important negative regulator, which has been confirmed by some studies.Citation70–72 APC lost by upregulation of miR-135 contributes to the development of colorectal, breast, and gastric cancers.Citation30,Citation73,Citation74 In glioma cells, we found that APC was the target gene of miR-135b-5p, and APC decreased with the overexpression of miR-135b-5p. This regulatory mechanism has also been validated in diffuse large B cell lymphoma.Citation44 In addition, we certified that overexpression of GAS5 led to an increase in APC, but this effect was reversed by miR-135b-5p_mmic. Meanwhile, TCGA data analysis showed that there was no significant correlation between the expression levels of GAS5 and APC in glioma clinical samples, and there was no significant correlation between APC and glioma patient survival after analyzing the CGGA database. These results are not what we expected. It is possible that the expression of APC is related to other factors, which needs further study. In conclusion, GAS5 indirectly regulates APC through sponging miR-135b-5p.
This study has some limitations. Firstly, although APC is generally considered a negative regulator of the Wnt pathway, we did not explore Wnt/β-contenin pathway changes. Because APC is a multi-domain protein, it contains binding sites for many proteins, including the Wnt/Wg pathway components β-catenin and axin, microtubules, the cytoskeletal regulators IQGAP1 and EB1, and the Rac guanine-nucleotide-exchange factor (GEF) Asef1. It has been proposed that mutations in APC contribute to cancer development through processes other than Wnt signaling. We were discussing and designing the validation of regulatory mechanisms independent of the WNT pathway, which would be a huge job, so we decided to do it separately. Secondly, there is a lack of in vivo experimental validation, which is also an important work that we intend to study separately at a later stage. Last but not least, the regulatory functions of GAS5 in glioma cells, such as chemoresistance, apoptosis, cell cycle progression, and immune evasion, need to be further studied.
Conclusion
In summary, we unraveled the molecular mechanism of GAS5 affecting glioma progression by sponging miR-135b-5p and regulating APC through a suite of in vitro experiments, which is of great significance for elucidating the mechanism of glioma development. In addition, this study provided a reference for the prognosis evaluation of glioma patients and new ideas and directions for clinical multi-target treatment of glioma.
Abbreviations
GAS5, Long noncoding RNA growth arrest-specific 5; APC, Adenomatous polyposis coli; GAPDH, Glycine-3-proline dehydrogenase; CGGA, Chinese Glioma Genome Atlas.
Ethics Approval and Consent for Participation and Publication
The research was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of The Third Affiliated Hospital of Chongqing Medical University (ethics number: KLYS (2021) 80). All 22 patients signed informed consent for participation and publication.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We would like to thank the authors for their contributions and the support of The Third Affiliated Hospital of Chongqing Medical University.
Additional information
Funding
References
- Weller M, Wick W, Aldape K. et al. Glioma. Nat Rev Dis Primers. 2015;1(1):15017. doi:10.1038/nrdp.2015.17
- Chen R, Smith-Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics. 2017;14(2):284–297. doi:10.1007/s13311-017-0519-x
- Zhu XP, Pan SA, Chu Z, Zhou YX, Han DQ, Han D-Q. LncRNA GAS5 regulates epithelial-mesenchymal transition and viability of glioma cells by targeting microRNA-106b and regulating PTEN expression. Neurosci Res. 2020;170:32–40. doi:10.1016/j.neures.2020.08.009
- Le VH, Minh TNT, Kha QH, Le NQK. A transfer learning approach on MRI-based radiomics signature for overall survival prediction of low-grade and high-grade gliomas. Med Biol Eng Comput. 2023;61(10):2699–2712. doi:10.1007/s11517-023-02875-2
- Bondy ML, Scheurer ME, Malmer B, et al. Brain tumor epidemiology: consensus from the brain tumor epidemiology consortium. Cancer. 2008;113(S7):1953–1968. doi:10.1002/cncr.23741
- Connelly JM, Malkin MG. Environmental risk factors for brain tumors. Curr Neurol Neurosci Rep. 2007;7(3):208–214. doi:10.1007/s11910-007-0032-4
- Ostrom QT, Barnholtz-Sloan JS. Current state of our knowledge on brain tumor epidemiology. Curr Neurol Neurosci Rep. 2011;11(3):329–335. doi:10.1007/s11910-011-0189-8
- Shete S, Hosking FJ, Robertson LB, et al. Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet. 2009;41(8):899–904. doi:10.1038/ng.407
- Wrensch M, Jenkins RB, Chang JS, et al. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat Genet. 2009;41(8):905–908. doi:10.1038/ng.408
- Walsh KM, Codd V, Smirnov IV, et al. Variants near TERT and TERC influencing telomere length are associated with high-grade glioma risk. Nat Genet. 2014;46(7):731–735. doi:10.1038/ng.3004
- Rajaraman P, Melin BS, Wang Z, et al. Genome-wide association study of glioma and meta-analysis. Hum Genet. 2012;131(12):1877–1888. doi:10.1007/s00439-012-1212-0
- Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi:10.1007/s00401-007-0243-4
- Li X, Guan J, Jiang Z, Cheng S, Wang Z. Microglial exosome miR-7239-3p promotes glioma progression by regulating circadian genes. Bulle Neurosci. 2021;37(4):497–510. doi:10.1007/s12264-020-00626-z
- Nabors LB, Portnow J, Ammirati M, et al. NCCN guidelines insights: central nervous system cancers, version 1.2017. J Natl Compr Canc Netw. 2017;15(11):1331–1345. doi:10.6004/jnccn.2017.0166
- Ghantasala S, Gollapalli K, Epari S, Moiyadi A, Srivastava S. Glioma tumor proteomics: clinically useful protein biomarkers and future perspectives. Expert Rev Proteomics. 2020;17(3):221–232. doi:10.1080/14789450.2020.1731310
- Didier M, Kinan DA, Andre N, Ivan B, Antonin M. Long noncoding RNAs as new architects in cancer epigenetics, prognostic biomarkers, and potential therapeutic targets. Biomed Res Int. 2015;2015:320214. doi:10.1155/2015/320214
- Liu B, Wu S, Ma J, et al. lncrna gas5 reverses emt and tumor stem cell- mediated gemcitabine resistance and metastasis by targeting mir-221/socs3 in pancreatic cancer. Mol Ther Nucleic Acids. 2018;13:472–482. doi:10.1016/j.omtn.2018.09.026
- Yue Q, Zhao C, Wang Y, et al. Downregulation of growth arrest-specific transcript 5 alleviates palmitic acid-induced myocardial inflammatory injury through the miR26a/HMGB1/NFκB axis. Molecu Med rep. 2018;18(6):5742–5750. doi:10.3892/mmr.2018.9593
- Dai SP, Jin J, Li WM. Diagnostic efficacy of long non-coding RNA in lung cancer: a systematic review and meta-analysis. Postgrad Med J. 2018;94(1116):578–587. doi:10.1136/postgradmedj-2018-135862
- Yang X, Xie Z, Lei X, Gan R. Long noncoding RNA GAS5 in human cancer (Review). Oncol Lett. 2020;20(3):2587–2594. doi:10.3892/ol.2020.11809
- Juan G, Yueping W, Xuedong W, et al. Effect of the LncRNA GAS5-MiR-23a-ATG3 axis in regulating autophagy in patients with breast cancer. Cell Physiol Biochem. 2018;48(1):194–207. doi:10.1159/000491718
- Liu J, Chen M, Ma L, Dang X, Du G. LncRNA GAS5 suppresses the proliferation and invasion of osteosarcoma cells via the miR-23a-3p/PTEN/PI3K/AKT pathway. Cell Transplan. 2020;29:1182–1188. doi:10.1177/0963689720953093
- Bahubeshi A, Tischkowitz M, Foulkes WD. miRNA processing and human cancer: DICER1 cuts the mustard. Sci trans med. 2011;3(111):111ps146. doi:10.1126/scitranslmed.3002493
- Chou CH, Shrestha S, Yang CD, et al. miRTarBase update 2018: a resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 2018;46(D1):D296–D302. doi:10.1093/nar/gkx1067
- Võsa U, Vooder TN, Kolde R, et al. Identification of MiR-374a as a prognostic marker for survival in patients with early-stage nonsmall cell lung cancer. Genes Chromosomes Cancer. 2011;50(10):812–822. doi:10.1002/gcc.20902
- Zhang Z, Li J, Huang Y. Upregulated miR-1258 regulates cell cycle and inhibits cell proliferation by directly targeting E2F8 in CRC. Cell Proliferation. 2018;51(6):e12505. doi:10.1111/cpr.12505
- Robertis MD, Poeta ML, Signori E, et al. Current understanding and clinical utility of miRNAs regulation of colon cancer stem cells. Semi Cancer Biol. 2018;53:232–247. doi:10.1016/j.semcancer.2018.08.008
- Akcakaya P, Ekelund S, Kolosenko I. Ak�akaya. miR-185 and miR-133b deregulation is associated with overall survival and metastasis in colorectal cancer. Int j Oncol. 2011;39(2):311–318. doi:10.3892/ijo.2011.1043
- Wen SY, Lin Y, Yu YQ, Cao SJ, Zhang ZG. miR-506 acts as a tumor suppressor by directly targeting the hedgehog pathway transcription factor Gli3 in human cervical cancer. Oncogene. 2014;34(6):717–725. doi:10.1038/onc.2014.9
- Nagel R, le Sage C, Diosdado B, et al. Regulation of the adenomatous polyposis coli gene by the miR-135 family in colorectal cancer. Cancer Res. 2008;68(14):5795–5802. doi:10.1158/0008-5472.CAN-08-0951
- Chen Z, Gao Y, Gao S, Song D, Feng Y. MiR-135b-5p promotes viability, proliferation, migration and invasion of gastric cancer cells by targeting Krüppel-like factor 4 (KLF4). Arch Med Sci. 2020;16(1):167–176. doi:10.5114/aoms.2019.87761
- Li L, Wang A, Cai M, Tong M, Chen F, Huang L. Identification of stool miR-135b-5p as a non-invasive diagnostic biomarker in later tumor stage of colorectal cancer. Life Sci. 2020;260:118417. doi:10.1016/j.lfs.2020.118417
- Bodmer WF, Bailey CJ, Bodmer J, et al. Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature. 1987;328(6131):614–616. doi:10.1038/328614a0
- Kinzler KW, Nilbert MC, Su LK, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253(5020):661–665. doi:10.1126/science.1651562
- Senda T, Shimomura A, Iizuka-Kogo A. Adenomatous polyposis coli (APC) tumor suppressor gene as a multifunctional gene. Anat Sci Int. 2005;80(3):121–131. doi:10.1111/j.1447-073x.2005.00106.x
- Stefanski CD, Prosperi JR. Wnt-independent and wnt-dependent effects of APC loss on the chemotherapeutic response. Int J Mol Sci. 2020;21(21):7844. doi:10.3390/ijms21217844
- Carothers AM, Melstrom KA, Mueller JD, Weyant MJ, Bertagnolli MM. Progressive changes in adherens junction structure during intestinal adenoma formation in APC mutant mice. J Biol Chem. 2001;276(42):39094–39102. doi:10.1074/jbc.M103450200
- Hankey W, Frankel WL, Groden J. Functions of the APC tumor suppressor protein dependent and independent of canonical WNT signaling: implications for therapeutic targeting. Cancer Metastasis Rev. 2018;37(1):159–172. doi:10.1007/s10555-017-9725-6
- Aoki K, Taketo MM. Adenomatous polyposis coli (APC): a multi-functional tumor suppressor gene. J Cell Sci. 2007;120(19):3327–3335. doi:10.1242/jcs.03485
- van den Broek E, Krijgsman O, Sie D, et al. Genomic profiling of stage II and III colon cancers reveals APC mutations to be associated with survival in stage III colon cancer patients. Oncotarget. 2016;7(45):73876–73887. doi:10.18632/oncotarget.12510
- Zhang X, Zhang X. MicroRNA-135b-5p regulates trophoblast cell function by targeting phosphoinositide-3-kinase regulatory subunit 2 in preeclampsia. Bioengineered. 2022;13(5):12338–12349. doi:10.1080/21655979.2022.2073655
- Zhang XH, Xin ZM. MiR-135b-5p inhibits the progression of malignant melanoma cells by targeting RBX1. Eur Rev Med Pharmacol Sci. 2020;24(3):1309–1315. doi:10.26355/eurrev_202002_20188
- Liu D, Jin Y, Wu J, Zhu H, Ye D. MiR-135b-5p is an oncogene in pancreatic cancer to regulate GPRC5A expression by targeting transcription factor KLF4. Cell Death Discov. 2022;8(1):23–34. doi:10.1038/s41420-022-00814-y
- Zhao CC, Jiao Y, Zhang YY, et al. Lnc SMAD5-AS1 as ceRNA inhibit proliferation of diffuse large B cell lymphoma via Wnt/β-catenin pathway by sponging miR-135b-5p to elevate expression of APC. Cell Death Dis. 2019;10(4):252–266. doi:10.1038/s41419-019-1479-3
- Gusyatiner O, Hegi ME. Glioma epigenetics: from subclassification to novel treatment options. Semi Cancer Biol. 2018;51:50–58. doi:10.1016/j.semcancer.2017.11.010
- Ostrom QT, Luc B, Davis FG, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro-Oncology. 2014;16(7):896–913. doi:10.1093/neuonc/nou087
- Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23(8):1231–1251. doi:10.1093/neuonc/noab106
- Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. doi:10.1001/jama.2017.18718
- Sathornsumetee S, Reardon DA, Desjardins A, Quinn JA, Vredenburgh JJ, Rich JN. Molecularly targeted therapy for malignant glioma. Cancer. 2007;110(1):13–24. doi:10.1002/cncr.22741
- Zhu XP, Pan SA, Chu Z, Zhou YX, Huang YK, Han DQ. LncRNA GAS5 regulates epithelial-mesenchymal transition and viability of glioma cells by targeting microRNA-106b and regulating PTEN expression. Neurosci Res. 2021;170:32–40.
- Chen L, Ren P, Zhang Y, Gong B, Yu D, Sun X. Long non‑coding RNA GAS5 increases the radiosensitivity of A549 cells through interaction with the miR‑21/PTEN/Akt axis. Oncol Rep. 2020;43(3):897–907. doi:10.3892/or.2020.7467
- Ma J, Miao H, Zhang H, et al. LncRNA GAS5 modulates the progression of non-small cell lung cancer through repressing miR-221-3p and up-regulating IRF2. Diagn Pathol. 2021;16(1):46–54. doi:10.1186/s13000-021-01108-0
- Wang H, Wang D, Shen Y, et al. GAS5 attenuates the malignant progression of glioma stem-like cells by promoting E-cadherin. Cancer Genet Ther. 2023;30(3):450. doi:10.1038/s41417-022-00566-y
- Zhao X, Wang P, Liu J, et al. Gas5 Exerts Tumor-suppressive functions in human glioma cells by targeting miR-222. Mol Ther. 2015;23(12):1899–1911. doi:10.1038/mt.2015.170
- Wang Y, Xin S, Zhang K, Shi R, Bao X. Low GAS5 levels as a predictor of poor survival in patients with lower-grade gliomas. J Oncol. 2019;2019:1–15.
- Duan XM, Liu XN, Li YX, et al. MicroRNA-498 promotes proliferation, migration, and invasion of prostate cancer cells and decreases radiation sensitivity by targeting PTEN. Kaohsiung J Med Sci. 2019;35(11):659–671. doi:10.1002/kjm2.12108
- Su P, Mu S, Wang Z. Long noncoding RNA SNHG16 promotes osteosarcoma cells migration and invasion via sponging miRNA-340. DNA Cell Biol. 2019;38(2):170–175. doi:10.1089/dna.2018.4424
- Peng W, Deng W, Zhang J, Pei G, Rong Q, Zhu S. Long noncoding RNA ANCR suppresses bone formation of periodontal ligament stem cells via sponging miRNA-758. Biochem Biophys Res Commun. 2018;503(2):815–821. doi:10.1016/j.bbrc.2018.06.081
- Rezaei O, Tamizkar KH, Sharifi G, Taheri M, Ghafouri-Fard S. Emerging Role of Long Non-Coding RNAs in the Pathobiology of Glioblastoma. Front Oncol. 2021;10:625884. doi:10.3389/fonc.2020.625884
- Dong L, Li G, Li Y, Zhu Z. Upregulation of long noncoding RNA GAS5 inhibits lung cancer cell proliferation and metastasis via miR-205/PTEN axis. Med Sci Monit. 2019;25:2311–2319. doi:10.12659/MSM.912581
- Jin C, Zhao J, Zhang ZP, Wu M, Liu JP. Long non-coding RNA GAS5, by up-regulating PRC2 and targeting the promoter methylation of miR-424, suppresses multiple malignant phenotypes of glioma. J Neuro-oncol. 2020;148(3):529–543. doi:10.1007/s11060-020-03544-2
- Lin CW, Chang YL, Chang YC, et al. MicroRNA-135b promotes lung cancer metastasis by regulating multiple targets in the Hippo pathway and LZTS1. Nat Commun. 2013;4(1):1877–1890. doi:10.1038/ncomms2876
- Zhang L, Sun ZJ, Bian Y, Kulkarni AB. MicroRNA-135b acts as a tumor promoter by targeting the hypoxia-inducible factor pathway in genetically defined mouse model of head and neck squamous cell carcinoma. Cancer Lett. 2013;331(2):230–238. doi:10.1016/j.canlet.2013.01.003
- Wang N, Tao L, Zhong H, et al. miR-135b inhibits tumour metastasis in prostate cancer by targeting STAT6. Oncol Lett. 2016;11(1):543–550. doi:10.3892/ol.2015.3970
- Su W, Mo Y, Wu F, et al. miR-135b reverses chemoresistance of non-small cell lung cancer cells by downregulation of FZD1. Biomed Pharmacother. 2016;84:123–129. doi:10.1016/j.biopha.2016.09.027
- Lulli V, Buccarelli M, Martini M, et al. miR-135b suppresses tumorigenesis in glioblastoma stem-like cells impairing proliferation, migration and self-renewal. Oncotarget. 2015;6(35):37241–37256. doi:10.18632/oncotarget.5925
- Wang Q, Cao T, Guo K, et al. Regulation of integrin subunit alpha 2 by miR-135b-5p modulates chemoresistance in gastric cancer. Front Oncol. 2020;10:308–319. doi:10.3389/fonc.2020.00308
- Li P, Fan JB, Gao Y, et al. miR-135b-5p inhibits LPS-induced TNFα production via silencing AMPK phosphatase Ppm1e. Oncotarget. 2016;7(47):77978–77986. doi:10.18632/oncotarget.12866
- Jin H, Luo S, Wang Y, et al. miR-135b stimulates osteosarcoma recurrence and lung metastasis via notch and wnt/β-catenin signaling. Mol Ther Nucleic Acids. 2017;8:111–122. doi:10.1016/j.omtn.2017.06.008
- Xu M, Liu X, Xu Y, Zhu S, Gao Y. Co‑expression of Axin and APC gene fragments inhibits colorectal cancer cell growth via regulation of the wnt signaling pathway. Mol Med Rep. 2017;16(4):3783–3790. doi:10.3892/mmr.2017.7049
- Schweigert A, Fischer C, Mayr D, von Schweinitz D, Kappler R, Hubertus J. Activation of the Wnt/β-catenin pathway is common in Wilms tumor, but rarely through β-catenin mutation and APC promoter methylation. Pediatr Surg Int. 2016;32(12):1141–1146. doi:10.1007/s00383-016-3970-6
- Lee E, Salic A, Krüger R, Heinrich R, Kirschner MW. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1(1):E10–E26. doi:10.1371/journal.pbio.0000010
- Jiang D, Zhou B, Xiong Y, Cai H. [Corrigendum] miR‑135 regulated breast cancer proliferation and epithelial‑mesenchymal transition acts by the Wnt/β‑catenin signaling pathway. Int J Mol Med. 2022;50(2):105–107. doi:10.3892/ijmm.2022.5161
- Wang LP, Ma XQ, Cai JC. Clinicopathological significance and function of miR-135b in the occurrence and development of gastric cancer. Zhonghua Yi Xue Za Zhi. 2012;92(46):3269–3273.
