Abstract
Background
Hybridomas that produce human monoclonal antibodies (HuMAbs) against Dengue virus (DV) had been prepared previously using peripheral blood lymphocytes from patients with DV during the acute and convalescent phases of a secondary infection. Anti-DV envelope glycoprotein (E) 99 clones, anti-DV premembrane protein (prM) 8 clones, and anti-DV nonstructural protein 1 (NS1) 4 clones were derived from four acute-phase patients, and anti-DV E 2 clones, anti-DV prM 2 clones, and anti-DV NS1 8 clones were derived from five convalescent-phase patients.
Methods and results
In the present study, we examined whether these clones cross-reacted with Japanese encephalitis virus (JEV), which belongs to the same virus family. Forty-six of the above-described 99 (46/99) anti-E, 0/8 anti-prM, and 2/4 anti-NS1 HuMAbs from acute-phase, and 0/2 anti-E, 0/2 anti-prM, and 5/8 anti-NS1 HuMAbs from convalescent-phase showed neutralizing activity against JEV. Thus, most of the anti-E and anti-NS1 (but not the anti-prM) antibodies cross-reacted with JEV and neutralized this virus. Interestingly, 3/46 anti-E HuMAbs derived from acute-phase patients and 3/5 anti-NS1 HuMAbs from convalescent-phase patients showed particularly high neutralizing activity against JEV. Consequently, the HuMAbs showing neutralization against JEV mostly consisted of two populations: one was HuMAbs recognizing DV E and showing neutralization activity against all four DV serotypes (complex-type) and the other was HuMAbs recognizing DV NS1 and showing subcomplex-type cross-reaction with DV.
Conclusion
Anti-DV E from acute phase (46/99) and anti-DV NS1 (7/12) indicate neutralizing activity against JEV. In particular, three of 46 anti-DV E clones from acute phase and three of five anti-NS1 clones from convalescent phase showed strong neutralizing activity against JEV.
Introduction
Dengue virus (DV) encodes capsid protein (C), premembrane protein (prM), and envelope glycoprotein (E), in addition to seven nonstructural proteins (NS).Citation1 There are four antigenically distinct serotypes (DV1–DV4), which share major antigens with each other and with other mosquito-borne and tick-borne flaviviruses, including Japanese encephalitis virus (JEV).Citation2–Citation8 DV and JEV are closely related, belonging to the same virus family, Flaviviridae. Both viruses are cocirculating in areas of Southeast Asia, including Thailand.Citation9 Indeed, vaccination rates against JEV in Thailand are high, at 84% in 1998 and 98% in 2008.Citation5
The immune response to a primary DV infection generates anti-DV neutralizing antibodies, which then protect against subsequent infection by the same serotype.Citation10 However, severe dengue infections often occur in patients who are secondarily infected with a different DV serotype.Citation10 The reason for this may be that the second virus uses preexisting anti-DV antibodies (raised during the primary infection) to gain entry to macrophages expressing Fc receptors, a process called antibody-dependent enhancement.Citation11,Citation12 Interestingly, most DV infections are asymptomatic,Citation13 even in individuals who are secondarily infected with a heterotypic DV.Citation14 However, in symptomatic cases, it can cause a wide spectrum, ranging from a mild illness, such as dengue fever, to severe illnesses, such as dengue hemorrhagic fever and dengue shock syndrome.Citation15
There have been several trials examining the clinical implications of prior exposure to JEV, or vaccination against JEV, which may increase the severity of subsequent DV infections. The results showed that neutralizing antibodies against JEV have both protective and detrimental effects upon subsequent DV infection.Citation8,Citation16–Citation20
Examination of the humoral immune status of DV-infected individuals, including dengue patients in the acute and convalescent phases of the secondary infection with heterotypic DV, may provide valuable information that will inform the development of anti-dengue vaccines. Previous reports showed that antibodies raised during primary infections were more type-specific, whereas those raised during secondary infections were more heterogeneous and wide-ranging in their ability to cross-react with heterotypes.Citation21,Citation22 Several groups have reported successful generation of hybridomas that produce anti-DV human monoclonal antibodies (HuMAbs),Citation22–Citation25 and all used peripheral blood mononuclear cells isolated from patients during the convalescent phases of primary and secondary infections. However, there are no reports of hybridomas being generated using peripheral blood mononuclear cells derived from the acute phase of a secondary DV infection. Information on the anti-DV antibodies derived from patients during the acute phase after secondary infection could be useful for understanding the mechanism(s) underlying dengue immunopathogenicity.
Recently, we reported the preparation of several hybridomas that secrete anti-DV HuMAbs by using peripheral blood mononuclear cells from dengue patients at the acute and convalescent phases of secondary infection with DV.Citation26,Citation27
The aim of the present study was to investigate whether these dengue patient-derived HuMAbs showed neutralizing activity against JEV. The results showed that two populations of HuMAbs, anti-E from acute-phase patients and anti-NS1 from convalescent-phase patients, showed neutralizing activity against JEV at high rates.
Materials and methods
Cell lines and viruses
Previously, 121 hybridomas were derived from dengue patients during the acute phase of a secondary DV infection and 15 were derived from patients during the convalescent phase.Citation26 For the present study, Vero cells were cultured in minimum essential medium supplemented with 10% fetal bovine serum and maintained in a 5% CO2 incubator at 37°C. The mosquito-derived cell line, C6/36, was cultured at 28°C in Leibovitz’s L-15 medium supplemented with 10% minimum essential medium and 0.3% tryptose phosphate broth. JEV (Nakayama strain) was cultured in C6/36 cells and the culture supernatants were used as viral stocks. Infectivity titers were estimated in Vero cells according to the number of focus-forming units, as previously described.Citation28
Immunofluorescence assay
Vero cells were plated in 96-well plates at a density of 2.5 × 104 per well and either mock-infected or infected with JEV. After incubation for 16 hours, the cells were fixed with 3.7% formaldehyde in phosphate-buffered saline and permeabilized with 1% Triton X-100 in phosphate-buffered saline. Undiluted hybridoma culture fluid was used as a source of HuMAbs. Bound antibodies were visualized using Alexa Fluor 488-conjugated anti-human or anti-mouse IgG secondary antibody (1:1,000, Invitrogen, Carlsbad CA, USA).
Viral neutralization assay
The viral neutralization assay was performed using undiluted hybridoma culture fluid as previously described.Citation29 The fluid or Dulbecco’s Modified Eagle’s Medium with 15% fetal bovine serum (as a negative control), was mixed with 100 focus-forming units of JEV (25 μL). After incubating for one hour, the mixture was used to infect Vero cells in a 96-well microplate. After inoculation at 37°C for 2 hours, 100 μL of minimum essential medium containing 3% fetal bovine serum was added to the wells. After a further overnight incubation at 37°C, the cells were fixed with 3.7% formaldehyde in phosphate-buffered saline and permeabilized with 1% Triton X-100 in phosphate-buffered saline. The plates were then stained with murine anti-E 4G2 monoclonal antibodyCitation30 at 4°C overnight for the immunofluorescence assay, as above. Bound antibodies were visualized using an Alexa Fluor 488-conjugated anti-mouse IgG (1:1,000; Invitrogen). All assays were performed in duplicate and the results were expressed as the mean. The viral neutralization activity of HuMAbs generated by the hybridoma clones was expressed as follows: −, <50%; +, 50% to <80%; or ++, ≥80% reduction of focus-forming units compared with the negative control.
Western blot analysis
The JEV-infected Vero cells were suspended in loading buffer containing 2-mercaptoethanol, electrophoresed in 10% sodium dodecyl sulfate polyacrylamide gels, then blotted onto polyvinylidene fluoride membranes. The blots were then incubated with undiluted hybridoma culture fluid at 4°C overnight, followed by incubation with horseradish peroxidase-conjugated anti-human IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for a further 2 hours at room temperature. The peroxidase reaction was visualized using ECL Plus Western Blotting Detection System (GE Healthcare UK Ltd, Little Chalfont, UK).
Results
Patient demographics
In total, peripheral blood mononuclear cell samples from nine secondarily infected dengue patients were used for anti-DV HuMAbs.Citation26 The peripheral blood mononuclear cells from D23, D32, and D33 (who had dengue fever), and D30 (who had dengue hemorrhagic fever) were obtained 6–8 days after the onset of fever (acute phase), and those from D25, D26, and D28 (who had dengue fever), and D22 and D27 (who had dengue hemorrhagic fever) were obtained between 12 and 15 days after the onset of fever (convalescent phase). The four acute-phase patients were all infected with DV2.
Cross-reactivity of HuMAbs with JEV
A previous study examined the serologic activity of the HuMAbs against all four serotypes using immunofluorescence and viral neutralization assays.Citation26 The present study went on to examine possible cross-reactions with JEV-infected cells by immunofluorescence. The results are summarized in . The results from a DV2-derived viral protein expression system showed that 99, eight, and four of the 121 HuMAbs derived from four acute-phase patients recognized DV E, prM, and NS1, respectively, whereas two, two, and eight of 15 HuMAbs derived from five convalescent-phase patients recognized DV E, prM, and NS1, respectively.Citation26 As summarized in , immunofluorescence analysis showed that 93 of the above-described 99 (93/99) anti-E clones, 1/8 anti-prM clones, and 1/4 anti-NS1 clones (anti-NS1 D30-2B1G5 also showed a weak reaction with E) derived from acute-phase patients were cross-reactive with JEV, whereas 2/2 anti-E clones and 0/8 anti-prM clones were cross-reactive with JEV, and 1/8 anti-NS1 clones were cross-reactive with JEV (anti-NS1 D25-2B11G11 also showed a weak reaction with E and prM, and anti-NS1 D25-4D4F10 also reacted weakly with prM).
Table 1 Patient disease status and the human monoclonal antibodies obtained in this study
Table 2 Cross-reactivity of Japanese encephalitis virus with human monoclonal antibodies derived from dengue patients by immunofluorescence
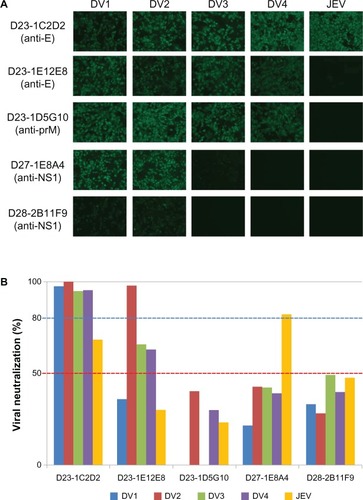
The results of the viral neutralization assay revealed that 46/99 anti-E, 0/8 anti-prM, and 2/4 anti-NS1 derived from acute-phase showed neutralizing activity against JEV, whereas 0/2 anti-E, 0/2 anti-prM, and 5/8 anti-NS1 derived from convalescent-phase showed neutralizing activity against JEV (). Interestingly, three anti-E HuMAbs derived from acute-phase and three anti-NS1 HuMAbs derived from convalescent-phase showed very high viral neutralization activity against JEV, as indicated by ++ () and clone numbers on the right side in parentheses (). There were no viral neutralization-positive clones among HuMAbs recognizing prM, irrespective of their origins (acute or convalescent phase). The characteristics of the individual HuMAbs are summarized in Table S1. Of the three clones that reacted with DV NS1 but also showed a weak cross-reaction with E and/or prM in the immunofluorescence with 293T cells transfected with individual DV2 viral genes,Citation26 D30-2B1G5 and D25-2B11G11, but not D25-4D4F10, showed neutralization against JEV.
Table 3 Cross-reactivity of Japanese encephalitis virus with human monoclonal antibodies derived from dengue patients as assessed by the viral neutralization assay
Most of the HuMAb clones showing a positive reaction with JEV in immunofluorescence recognized DV E and showed a complex-type cross-reaction with all four serotypes (91/96 clones [95%], ). Similarly, viral neutralization assay showed that 46/99 (46%) anti-E and 2/4 (50%) anti-NS1 HuMAbs from acute-phase showed neutralizing activity against JEV, as did 5/8 (63%) anti-NS1 HuMAbs from convalescent-phase (). Interestingly, 38/46 of the above anti-E (83%) were HuMAbs showing neutralization against all four serotypes (complex-type, ). On the other hand, five clones (one from acute-phase and four from convalescent-phase) of anti-NS1 HuMAbs were positive for viral neutralization activity against JEV, without viral neutralization activity against all four DV (<50% against all four serotypes); whereas two clones (one from acute-phase and one from convalescent-phase) of anti-NS1 HuMAbs were positive for viral neutralization activity against JEV, with subcomplex-type viral neutralization activity against DV2, DV3, and DV4.
shows the staining of Vero cells infected with serotypes DV1 to DV4 and Vero cells infected with JEV by several HuMAb clones (anti-E, anti-prM, or anti-NS1, ) and the percent reduction of viral replication in Vero cells incubated with the same HuMAb clones ().
Figure 1 Staining of Vero cells infected with serotypes DV1 to DV4 and of Vero cells infected with Japanese encephalitis virus using several human monoclonal antibody clones (anti-E, anti-prM, or anti-NS1). (A) Anti-DV E (D23-1C2D2 and D23-1E12E8), anti-DV prM (D23-1D5G10), and anti-DV NS1 (D27-1E8A4 and D28-2B11F9) clones were incubated with Vero cells infected with Dengue virus serotypes 1–4 or with Vero cells infected with Japanese encephalitis virus. (B) Percent reduction of viral replication in Vero cells incubated with the same human monoclonal antibody clones.
In this study, hybridoma culture fluids were prepared under the same conditions. These culture fluids from individual hybridoma cells are also positive against DV by immunofluorescence. In addition, the same lots of individual culture fluids were used throughout the experiments in this study, such as immunofluorescence (against both DV and JEV), viral neutralization (against both DV and JEV), and western blotting test. The IgG concentration of the HuMAbs was not adjusted in this study. Therefore, there are some risks that some antibody may have low immunofluorescence, viral neutralization, or western blot signal, because it may be produced from hybridoma at a low concentration/amount.
Cross-reactivity of HuMAbs with JEV as assessed by western blotting
The cross-reactivity of HuMAbs with JEV antigens was also examined by western blotting. The results are summarized in . More than half of the anti-DV E HuMAbs were also reactive with JEV E on western blots. Also, most of the anti-DV NS1 HuMAbs were reactive with JEV NS1. In contrast, all of the anti-DV prM HuMAbs were not reactive with JEV prM. Interestingly, three of the anti-DV E clones and two of the anti-DV NS1 clones that showed high neutralizing activity (++) against JEV showed no apparent reaction with JEV on the western blots.
Table 4 Reactivity of dengue patient-derived human monoclonal antibodies with Japanese encephalitis virus-infected cell lysate on western blots
Discussion
Overall, hybridomas previously generated from peripheral blood mononuclear cells isolated from dengue patients secondarily infected with DVCitation26,Citation27 were producers of HuMAbs cross-reactive with JEV-infected cells by immunofluorescence and showed high rates of neutralization against JEV by viral neutralization assay. The clones that cross-reacted with JEV and showed neutralizing activity against JEV were mainly classified into two groups: one contained clones that recognized DV E and were derived from acute-phase, and the other contained clones that recognized DV NS1 and were mostly derived from convalescent-phase. In contrast, none of the anti-DV prM clones showed neutralizing activity against JEV.
Of all the viruses that belong to the family Flaviviridae, JEV shows the closest antigenic relationship with West Nile virus, Murray Valley encephalitis virus, and St Louis encephalitis virus; JEV is much less antigenically related to DV.Citation8,Citation31 However, the present study showed that most of the anti-DV E antibodies from hybridomas generated from peripheral blood mononuclear cells from DV patients during the acute phase showed complex-type cross-reactions and neutralizing activity against all four DV serotypes; indeed, not only do these show strong viral neutralization activity against DV2 (which was replicating in these patients), they are also strongly cross-reactive with other DV serotypesCitation26 and with JEV. These results showed there are common antigenic sites between DV E and JEV E, although a low antigenic relationship exists between DV and JEV. This supports previous reports of flavivirus envelope glycoprotein cross-reactive epitopes analyzed by mouse monoclonal antibodies indicating that DV and JEV envelope proteins have sequence similarity and also share structure similarity in causing the cross-activity effects.Citation32,Citation33 In addition, anti-DV NS1 antibodies showing neutralizing activity against DV are 3/4 clones from acute-phase and 2/8 clones from convalescent-phase,Citation26 and 2/4 clones from acute-phase and 5/8 clones from convalescent-phase also showed neutralizing activity against JEV in the present study. There has been no report about cross-reactivity in flaviviruses. However, the NS1 gene shares a high degree of homology.Citation34 The results of the present study also showed that anti-prM HuMAbs (eight derived from acute-phase patients and two from convalescent-phase patients) had no neutralizing activity against JEV. This supports the findings of a previous study showing that anti-prM antibodies in sera from DV-infected or JEV-infected patients during the convalescent phase did not react with either DV or JEV on western blots.Citation35 The present study showed that most of the anti-DV E and anti-DV NS1 HuMAbs derived from dengue patients during the acute phase of a secondary infection (around one week after the onset of illness) neutralized JEV, but none of the anti-DV prM HuMAbs derived from acute-phase and convalescent-phase patients neutralized JEV; this is despite the finding that anti-prM antibody responses are amplified after a secondary infection.Citation36 A previous report described the generation of HuMAbs by transforming B cells derived from dengue patients (who were secondarily infected with DV) during the convalescent phase (15–24 days after defervescence). The results showed that 64% of anti-E, 7% of anti-NS1, and 3% of anti-prM antibodies were cross-reactive with JEV on western blots.Citation24
The E protein of flaviviruses is the principal antigen responsible for eliciting neutralizing antibody responses; however, neutralizing antibodies specific for the prM and NS1 proteins have also been detected.Citation37–Citation40 The anti-DV NS1 HuMAbs used in the present study were mostly derived from dengue patients during the convalescent phase of a secondary DV infection.Citation26 Several anti-NS1 HuMAbs showed strong neutralizing activity against JEV, although those antibodies showed no apparent reactions with JEV-infected cells on immunofluorescence analysis (). A previous paper also reported several cases that were negative by immunofluorescence (anti-JEV IgG IIFT, Euroimmune, Lübeck, Germany), but positive by plaque reduction neutralization test.Citation41 The present study examined three anti-NS1 clones that reacted with DV NS1, but also showed a weak cross-reaction with DV E and/or prM. Two of the three showed neutralizing activity against JEV. A previous study reported that all convalescent serum samples obtained from Japanese encephalitis patients contained anti-JEV NS1 IgG antibodies. Of these, 65% and 40% contained JEV NS1-specific IgM and IgA antibodies, respectively; also, these IgM and IgA antibodies did not cross-react with JEV.Citation42 The anti-NS1 clones used in the present study (four clones obtained from acute-phase patients and eight from convalescent-phase patients) were all IgG antibodies.Citation26
No information regarding the JEV infection or vaccination status of patients from whom the HuMAbs were derived was available; therefore, the possibility that the HuMAbs might have (at least in part) originated from memory immune cells that were initially primed with JEV antigens after a natural JEV infection or an anti-JEV vaccination cannot be ruled out. However, the acute phase of the disease in patients secondarily infected with heterotypic DV may be due to IgG antibodies induced by the secondary infection. Thus, it may be possible that some of the HuMAbs used in the present study could be derived from immune cells that had been primed after exposure to JEV.
Acknowledgments
The authors thank P Singhasivanon for valuable discussions, and P Sawanpanyalert and J Boon-Long for coordinating the project. This study was partly supported by Japan Science and Technology Agency/Japan International Cooperation Agency (SATREPS, 08080924), the Founding Research Center for Emerging and Re-emerging Infectious Diseases Program, which was launched through a project commissioned by the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the Thailand Research Fund through the Royal Golden Jubilee PhD Program and Mahidol University (Grant PHD/0260/2550, to CP).
Supplementary material
Table S1 Cross-reactivity of the individual dengue patient-derived human monoclonal antibodies with four dengue virus serotypes and with Japanese encephalitis virus
Disclosure
The authors report no conflicts of interest in this work.
References
- KuhnRJZhangWRossmannMGStructure of dengue virus: implications for flavivirus organization, maturation, and fusionCell2002108571772511893341
- InnisBLThirawuthVHemachudhaCIdentification of continuous epitopes of the envelope glycoprotein of dengue type 2 virusAm J Trop Med Hyg19894066766872472749
- CalisherCHKarabatsosNDalrympleJMAntigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antiseraJ Gen Virol198970Pt 137432543738
- MakinoYTadanoMSaitoMStudies on serological cross-reaction in sequential flavivirus infectionsMicrobiol Immunol199438129519557723688
- ChunsuttiwatSIssues Related to Integration of JE Vaccine into National EPI: Experience from ThailandBangkok, ThailandDepartment of Communicable Disease Control, Ministry of Public Health1998
- MartinDABiggerstaffBJAllenBJohnsonAJLanciottiRSRoehrigJTUse of immunoglobulin M cross-reactions in differential diagnosis of human flaviviral encephalitis infections in the United StatesClin Diagn Lab Immunol20029354454911986257
- OlsenSJSupawatKCampbellAPJapanese encephalitis virus remains an important cause of encephalitis in ThailandInt J Infect Dis20101410e888e89220674433
- MansfieldKLHortonDLJohnsonNFlavivirus-induced antibody cross-reactivityJ Gen Virol201192Pt 122821282921900425
- GrossmanRAGouldDJSmithTJJohnsenDOPantuwatanaSStudy of Japanese encephalitis virus in Chiangmai Valley, Thailand. I. Introduction and study designAm J Epidemiol19739821111204353435
- van der SchaarHMWilschutJCSmitJMRole of antibodies in controlling dengue virus infectionImmunobiology2009214761362919261353
- HalsteadSBO’RourkeEJDengue viruses and mononuclear hagocytes. I. Infection enhancement by non-neutralizing antibodyJ Exp Med19771461201217406347
- SangkawibhaNRojanasuphotSAhandrikSRisk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreakAm J Epidemiol198012056536696496446
- ReiterPYellow fever and dengue: a threat to Europe?Euro Surveill201015101950920403310
- GarcíaGSierraBPérezABAsymptomatic dengue infection in a Cuban population confirms the protective role of the RR variant of the FcgammaRIIa polymorphismAm J Trop Med Hyg20108261153115620519616
- HarrisEVideaEPérezLClinical, epidemiologic, and virologic features of dengue in the 1998 epidemic in NicaraguaAm J Trop Med Hyg2000631–251111357995
- SabinABResearch on dengue during World War IIAm J Trop Med Hyg195211305014903434
- HalsteadSBChowJMarchetteNJImmunological enhancement of dengue virus replicationNat New Biol1973243122242617319077
- HokeCHNisalakASangawhipaNProtection against Japanese encephalitis by inactivated vaccinesN Engl J Med1988319106086142842677
- GibbonsRVKalanaroojSJarmanRGAnalysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequencesAm J Trop Med Hyg200777591091317984352
- AndersonKBGibbonsRVThomasSJPre-existing Japanese encephalitis virus neutralizing antibodies and increased symptomatic dengue illness in a school-based cohort in ThailandPLoS Negl Trop Dis2011510e131121991398
- WahalaWMKrausAAHaymoreLBAccavitti-LoperMAde SilvaAMDengue virus neutralization by human immune sera: role of envelope protein domain III-reactive antibodyVirology2009392110311319631955
- BeltramelloMWilliamsKLSimmonsCPThe human immune response to dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activityCell Host Microbe20108327128320833378
- SchieffelinJSCostinJMNicholsonCONeutralizing and non-neutralizing monoclonal antibodies against dengue virus E protein derived from a naturally infected patientVirol J201072820132551
- DejnirattisaiWJumnainsongAOnsirisakulNCross-reacting antibodies enhance dengue virus infection in humansScience2010328597974574820448183
- de AlwisRBeltramelloMMesserWBIn-depth analysis of the antibody response of individuals exposed to primary dengue virus infectionPLoS Negl Trop Dis201156e118821713020
- SetthapramoteCSasakiTPuipromOHuman monoclonal antibodies to neutralize all dengue virus serotypes using lymphocytes from patients at acute phase of the secondary infectionBiochem Biophys Res Commun2012423486787222713454
- SasakiTSetthapramoteCKurosuTDengue virus neutralization and antibody-dependent enhancement activities of human monoclonal antibodies derived from dengue patients at acute phase of secondary infectionAntiviral Res201398342343123545366
- KurosuTKhamlertCPhanthanawiboonSIkutaKAnantapreechaSHighly efficient rescue of dengue virus using a co-culture system with mosquito/mammalian cellsBiochem Biophys Res Commun2010394239840420214880
- OkunoYIgarashiAFukaiKNeutralization tests for dengue and Japanese encephalitis viruses by the focus reduction method using peroxidase-anti-peroxidase stainingBiken J1978214137147383069
- FalconarAKIdentification of an epitope on the dengue virus membrane (M) protein defined by cross-protective monoclonal antibodies: design of an improved epitope sequence based on common determinants present in both envelope (E and M) proteinsArch Virol1999144122313233010664386
- Kimura-KurodaJYasuiKAntigenic comparison of envelope protein E between Japanese encephalitis virus and some other flaviviruses using monoclonal antibodiesJ Gen Virol198667Pt 12266326722432163
- HeinzFXStiasnyKFlaviviruses and their antigenic structureJ Clin Virol20125428929522999801
- CrillWDChangGJLocalization and characterization of flavivirus envelope glycoprotein cross-reactive epitopesJ Virol20047824139751398615564505
- MullerDAYoungPRThe flavivirus NS1 protein: molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarkerAntiviral Res201398219220823523765
- CardosaMJWangSMSumMSHTioPHAntibodies against prM protein distinguish between previous infection with dengue and Japanese encephalitis virusesBMC Microbiol20022912019028
- LaiCYTsaiWYLinSRAntibodies to envelope glycoprotein of dengue virus during the natural course of infection are predominantly cross-reactive and recognize epitopes containing highly conserved residues at the fusion loop of domain IIJ Virol200882136631664318448542
- PincusSMasonPWKonishiERecombinant vaccinia virus producing the prM and E proteins of yellow fever virus protects mice from lethal yellow fever encephalitisVirology199218712902971736531
- ColombageGHallRPavyMLobigsMDNA-based and alphavirus-vectored immunisation with prM and E proteins elicits long-lived and protective immunity against the flavivirus, Murray Valley encephalitis virusVirology199825011511639770429
- ShuPYChenLKChangSFDengue NS1-specific antibody responses: isotype distribution and serotyping in patients with dengue fever and dengue hemorrhagic feverJ Med Virol200062222423211002252
- ChungKMThompsonBSFremontDHDiamondMSAntibody recognition of cell surface-associated NS1 triggers Fc-gamma receptor-mediated phagocytosis and clearance of West Nile virus-infected cellsJ Virol200781179551955517582005
- LitzbaNKladeCSLedererSNiedrigMEvaluation of serological diagnostic test systems assessing the immune response to Japanese encephalitis vaccinationPLoS Negl Trop Dis2010411e88321103412
- ShuPYChenLKChangSFAntibody to the nonstructural protein NS1 of Japanese encephalitis virus: potential application of mAb-based indirect ELISA to differentiate infection from vaccinationVaccine20011913–141753176311166901