Abstract
Multiple sclerosis (MS) is a debilitating neurological disorder that affects nearly 2 million adults, mostly in the prime of their youth. An environmental trigger, such as a viral infection, is hypothesized to initiate the abnormal behavior of host immune cells: to attack and damage the myelin sheath surrounding the neurons of the central nervous system. While several other pathways and disease triggers are still being investigated, it is nonetheless clear that MS is a heterogeneous disease with multifactorial etiologies that works independently or synergistically to initiate the aberrant immune responses to myelin. Although there are still no definitive markers to diagnose the disease or to cure the disease per se, research on management of MS has improved many fold over the past decade. New disease-modifying therapeutics are poised to decrease immune inflammatory responses and consequently decelerate the progression of MS disease activity, reduce the exacerbations of MS symptoms, and stabilize the physical and mental status of individuals. In this review, we describe the mechanism of action, optimal dosing, drug administration, safety, and efficacy of the disease-modifying therapeutics that are currently approved for MS therapy. We also briefly touch upon the new drugs currently under investigation, and discuss the future of MS therapeutics.
Introduction
Multiple sclerosis (MS) is a complex and chronic demyelinating autoimmune neurological disorder that manifests through an interaction of environmental and genetic factors.Citation1–Citation5 The onset of MS occurs at an individual’s most productive years (20–40 years),Citation6,Citation7 and affects considerably more women than men.Citation8–Citation10 A long-term follow-up study of MS reports a steady rise in the incidence of MS, while the age at onset of disease symptoms has been continuously decreasing.Citation6 Nearly 2.5 million individuals worldwide (nearly one in every 400 individuals) are afflicted with MS, although experts consider this number to be an underestimation of true prevalence. MS is unquestionably a disabling disease that impairs both the physical function and cognitive ability of patients.Citation11 While their longevity is not severely compromised (reduction in life span by 6–7 years),Citation12 quality of life is significantly impacted, as individuals are plagued by MS-associated comorbidities, such as chronic pain, fatigue, depression, sleep disorders, spasticity, gait and coordination imbalances, migraines, sensory organ dysfunctions, and overall cognitive impairment. Since the description of MS by French neurologist Jean-Martin Charcot as a triad of symptoms (nystagmus, intention tremor, and slurred speech)Citation13 in 1868, research on the etiology, pathophysiology, and management of this disease has progressed dramatically, although a conclusive diagnostic marker or curative therapy still remains undefined.
MS diagnosis and subtype classification
Presently, a combination of paraclinical diagnostic investigations, including magnetic resonance imaging (MRI) assessment of brain-lesion dissemination in space and time, presence of oligoclonal bands in cerebrospinal fluid, delayed latencies in visual evoked potentials, and changes in retinal nerve fiber-layer thickness evaluated using optical coherence tomography alongside clinical symptoms, as recommended by the 2010 McDonald criteria, are used to guide MS diagnosis.Citation14–Citation16 While several potential biomarkers are being studied to ascertain their utility in diagnosing MS,Citation17,Citation18 none has yet been determined as clinically useful. Furthermore, discovery of markers to establish prognosis based on disease symptoms and treatment trajectories is also wanting.Citation19
A majority of MS patients (∼85%) experience symptomatic attacks between dormant states (“remission”), commonly referred to as the relapsing–remitting type (RRMS), which may initially present as a clinically isolated syndrome. This may segue, after a number of years, into secondary progressive MS, marked by fewer or no relapses and gradual neurological worsening with brain atrophy. Primary progressive MS presents with a continuous neurological worsening from the first onset of symptoms. Despite their similarity, studies have identified distinct pathological differencesCitation20 that could be translated to determine treatment decisions and predict the prognosis for patients based on the subtype presentation.Citation21,Citation22 Of note, primary and secondary progressive forms have generally been more resistant to anti-inflammatory therapies when compared to RRMS subtype.
Management of MS
MS therapeutics divides into primary disease treatment using immunomodulating agents, which will be discussed in detail, as well as specific symptom management (eg, spasticity, fatigue, depression, pain, etc), which will not be further addressed in this review.
Primary immunomodulatory therapeutics
The goal of mainstay therapies of MS is to reduce relapses and postpone progression of disability in patients.Citation23,Citation24 To this end, strategies adopted to treat MS are twofold: a short-term treatment to help reduce the accumulation of disease burden after an acute relapse, and a long-term, sustained treatment aimed at stabilizing the disease process.Citation25
Short-term treatment for acute relapse
In the initial stages of an MS relapse, individuals are generally treated with high doses (500–1,000 mg) of intravenous corticosteroids (eg, methylprednisolone) for a short period of 3–5 days. In rare cases, subcutaneous or intramuscular injections of adrenocorticotropic hormone (eg, HP Acthar® gel) are used, specifically for individuals who cannot tolerate or have poor response to intravenous prednisolone.Citation26–Citation28 These anti-inflammatory agents accelerate the process of recovery, and reduce duration of the relapse; however, they do not have any bearing on the occurrence of new relapses or on long-term disease progression.Citation27–Citation30
Long-term disease management
The fundamental pathogenesis of MS is characterized by two stages of disease development.Citation31 The inception of MS symptoms (clinical and paraclinical) and focal demyelination of neurons occur during the early inflammatory phase. The late neurodegenerative phase is characterized by further demyelination of neurons perpetrated by infiltrating macrophages, microglial cells, and lymphocytes that attack the endogenous myelin sheath proteins as antigens, leading to irreversible axonal loss.Citation32 Given the role played by lymphocytes in advancing MS, long-term disease management is largely directed towards suppressing the immune-inflammatory responses that promote demyelination and neuronal degradation in an effort to prevent any saltatory changes in the status quo of patients.Citation23,Citation24,Citation27,Citation33 Outcomes of MS treatments are evaluated based on a reduction in MS annualized relapse rate (ARR), stabilization, or regression in Expanded Disability Status Scale (EDSS) score, and unchanged brain and spinal cord MRI lesion burden.Citation34
Over the past decade, the disease-fighting armamentarium for MS has rapidly expanded with the discovery of new disease-modifying therapeutics (DMTs), which employ different mechanisms to slow or reverse inflammatory lesion formation. To date, regulatory agencies, such as the US Food and Drug Administration (FDA) and the European Medicines Agency, have approved nine different DMTs () to aid with modifying the disease course in MS patients (http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm). Emerging evidence suggests some DMTs may be able to stabilize and perhaps even improve neurological status; however, they are not capable of completely relieving all symptoms of MS.Citation23 Here, we briefly discuss the mechanism of action, optimal dosing, and efficacy of each of these DMTs.
Table 1 Dosing and side-effects of currently approved multiple sclerosis disease-modifying therapeutics
Interferons
Interferons (IFNs) are proteins that belong to the cytokine network and are involved with the regulation of immune response against microbial and viral antigens.Citation35 Their immunomodulatory properties were leveraged to develop the first DMTs for MS, namely, IFN-β1b (Betaseron®, Bayer HealthCare, Leverkusen, Germany; Extavia, Novartis, Basel, Switzerland), a fermented and purified recombinant protein produced in the bacterium Escherichia coli,Citation36 and two preparations of IFN-β1a (Avonex®, Biogen Idec Inc., Weston, MA, USA; Rebif®, Merck Serono, Geneva, Switzerland), which are recombinant human IFN proteins produced in mammalian cells in a glycosylated form.Citation27,Citation37
In vitro studies using human isogenic T cell lines and murine experimental autoimmune encephalomyelitis (EAE; a disease very similar to MS) models and in vivo studies on humans (clinical trials) have elucidated the mechanism of action of IFN-β to be as follows:Citation37–Citation39 IFN-β suppresses the proliferation of myelin-basic protein-specific T cells, reduces the production of proinflammatory cytokines (eg, IFN-γ), and induces anti-inflammatory cytokines, such as interleukin (IL)-10.Citation37,Citation39,Citation40 This results in a cytokine balance that protects neurons from demyelination by preventing the proliferation of T cells that are required for advancing the autoimmune process and inhibiting T cells from crossing the blood–brain barrier (BBB) and entering the central nervous system (CNS).Citation39,Citation40 Both IFN-β1b and IFN-β1a are equivalent with respect to their ability to reduce MS disease activity ( and ), and they reduce ARR by up to 30%, decrease formation of new or enlarging gadolinium-enhancing MRI lesions by 50%, and significantly lower the progression of EDSS scores.Citation27,Citation41–Citation44
Table 2 Efficacy of approved multiple sclerosis therapeutics: Phase III clinical trial results
Glatiramer acetate
Demyelination of neurons in MS is mediated by activation of helper T cells in response to a specific myelin-basic protein (MBP), which is one of the autoantigens in MS.Citation45,Citation46 Peptides of MBP bind to class II major histocompatibility complex (MHC II) molecules, which are then recognized by T cells as antigens and consequently destroyed.Citation47,Citation48 In 1995, Copolymer 1 (glatiramer acetate [GA]/Copaxone®; Teva Pharmaceuticals, Petach Tikva, Israel) was introduced as an alternate therapy to IFN-β. GA is a synthetic polymer of four amino acids (L-glutamate, L-lysine, L-alanine, and L-tyrosine) that mimics MBP, and hence competes with MBP antigens to bind with MHC II.Citation27,Citation49 Using human Epstein–Barr virus-transformed B cell lines, Fridkis-Hareli et alCitation50 demonstrated in vitro that GA binds to MHC-II molecules with high efficiency as well as at a fast rate. Thus, when the MHC-II molecules are blocked from binding to MBP, T cell responses are automatically diverted away from the myelin, resulting in neuronal protection.Citation49,Citation50 The proliferation of T cells is thus controlled by GA in a dose-dependent manner.Citation51 MHC-II molecules interact with CD4+ molecules that are present on the surface of helper T cells (Th1 and Th2) that produce proinflammatory cytokine (Th1-type cytokines: INF-γ) and anti-inflammatory cytokine (Th2-type cytokines: IL-10) molecules.Citation52 GA preferentially inhibits production of INF-γ, induces regulatory Th2-like T cell populations that cross-react with MBP, and produce anti-inflammatory cytokines, which in turn protects the myelin through a “bystander-suppression” mechanism.Citation51 GA is generally well tolerated and reduces ARR by 29%; however, it was unable to reduce disability progression significantly when compared to placebo ( and ).Citation53–Citation55
Given their safety profile, low toxicity, reasonable efficacy, and relative tolerability, IFN and GA are often prescribed as first-line MS therapies.Citation23,Citation27 While a meta-analysis study in 2004 indicated that GA was not useful in treating MS,Citation55 a more recent study substantiated its utility in treating relapses related to RRMS, but reiterated its limited impact on disability progression.Citation56
Natalizumab
In the early 1990s, Yednock et alCitation57 identified that monocytic cells selectively bound to inflamed blood vessels in the brain; the inflammation was caused by EAE. Using an EAE mouse model, they demonstrated that the selective adhesion of leukocytes to vascular cell adhesion molecule 1 (VCAM-1), a protein expressed on the surface of vascular endothelial cells in the brain and spinal cord,Citation58 is a critical step to gain entry into the CNS across the BBB. Over 95% of this adhesion was significantly inhibited by antibodies against the integrin molecule α4β1 (very late-activation antigen 4 [VLA-4]), a glycoprotein surface molecule found on all leukocytes except neutrophils.Citation57 Administration of the antibodies reduced the progression of inflammatory disease severity in MS patients (MRI lesions),Citation59,Citation60 and prevented the development of paralysis in animal studies.Citation61 Hence, it was hypothesized that monoclonal antibodies against VLA-4 could help with treating autoimmune inflammatory diseases such as MS by blocking the VCAM-1/VLA-4 interaction, and preventing infiltration of leukocytes across the BBB.Citation57,Citation58 In early 2000, natalizumab (Antegren; Elan, Dublin, Ireland, and Biogen Idec Inc.), an α4-integrin humanized monoclonal antibody to VLA-4, was developed.Citation62 Natalizumab specifically targets the T cellsCitation63 and inhibits the α4-integrin-mediated firm adhesion of T cells to the inflamed BBB by 70%, but does not interfere with the initial contact of T cells with the BBB, suggesting that the central mechanism of natalizumab action is prevention of T cell entry into the CNS.Citation64
After establishment of the relative safety profile in a Phase I clinical trial,Citation65 Phase II and Phase III double-blind placebo-controlled trials demonstrated its efficacy in reducing ARR, especially in patients with higher disease activity.Citation60,Citation66 The FDA subsequently approved natalizumab (Tysabri; Biogen Idec Inc.) as an MS monotherapy in 2004 (http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2004/125104_0000_ltr.pdf). However, the emergence of three cases of progressive multifocal leukoencephalopathy (PML) led to its withdrawal from the market for a brief period between February 2005 and June 2006.Citation67,Citation68 In 2006, the results of the Natalizumab Safety and Efficacy in Relapsing Remitting Multiple Sclerosis (AFFIRM) trial,Citation1 with a follow-up of over 2 years, elucidated the superiority of natalizumab in controlling progression of MS (), resulting in reinstatement of natalizumab as an MS therapeutic.Citation1,Citation69 Natalizumab therapy also significantly improves the overall quality of life of RRMS patients ().Citation70–Citation74 The Safety and Efficacy of Natalizumab in Combination with Interferon Beta-1a in Patients with Relapsing Remitting Multiple Sclerosis (SENTINEL) study further reiterated the efficacy of natalizumab as a combination therapy with IFN-β1a () than administration of IFN alone ().Citation75
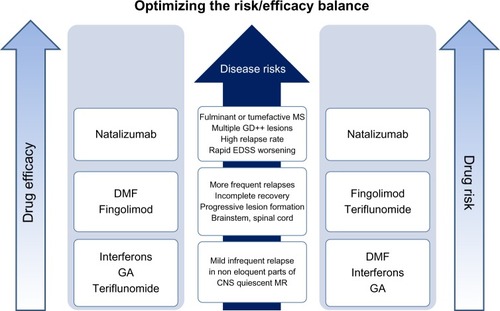
The principal limitation to natalizumab utilization has been PML, related to the mutation of the John Cunningham (JC) polyoma virus to a neurotrophic form. Postmarketing surveillance of natalizumab reported 377 incidences of PMLCitation76 across the world, and three principal factors have been identified to increase the risk of developing PML (therapy ≥24 months, history of immunosuppressant treatment, and JC-antibody positivity) in patients undergoing natalizumab therapy.Citation71,Citation76,Citation77 Further, recent studies show that higher-titer levels of JC virus antibody predispose to development of PML.Citation78 Less well defined is the possible contribution of excessively low CD62L (L-selectin) expression on CD4+ cell populationsCitation79 and the possible association between low body weight and increased PML risk, which are presently being studied extensively.Citation80 Although its superior efficacy in modulating disease activity and progression has made natalizumab a reliable therapy for MS, the risk-to-benefit ratio of the drug varies dramatically among patients, and hence demands a more personalized approach to utilization.
Dimethyl fumarate
Dimethyl fumarate (DMF; BG-12, Tecfidera®; Biogen Idec Inc.) is a methyl ester of fumaric acid approved by the FDA as an oral MS therapy on March 27, 2013 (www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm345528.htm). A well-known pathway to MS is oxidative stress brought about by the effector molecule peroxynitrite (reactive nitrogen species [RNS]).Citation81 Macrophages, upon localization in the glial cells, release proinflammatory cytokines and free radicals to aid with host immune protection.Citation82–Citation84 The antimicrobial properties of Nitric Oxide (NO) are well established,Citation85 but as a cytotoxic agent NO also leads to extensive host cellular damage. In inflamed regions, NO is released equivalently to the extent of inflammation: the more the inflammation, the more the NO released.Citation86,Citation87 NO reacts with other free radicals like superoxide to produce RNS,Citation82,Citation88 which in turn induces oxidative damage to the mitochondrial deoxyribonucleic acid, ultimately resulting in decreased adenosine triphosphate production.Citation89 Thus axonal transport, a process that requires adenosine triphosphate, and cellular respiration are impaired, leading to axonal degeneration and irreversible cell apoptosis.Citation89
Initial in vitro studies highlighted the detoxification and anti-inflammatory capabilities of DMF, which reduces with the production and release of inflammatory molecules, such as cytokines and NO, and elevates the production of detoxification enzymes such as reduced-form nicotinamide adenine dinucleotide phosphate quinone reductase 1 and/or glutathione.Citation83,Citation88,Citation90 Additional studies in EAE mouse models showed a DMF dose-dependent decrease in inflammatory cell infiltrates (composed of macrophages, microglial cells, and proinflammatory cytokines).Citation91 DMF also inhibits the expression of VCAM-1Citation92 and activates nuclear factor erythroid 2-related factor (Nrf2), a transcription factor with antioxidant properties. Nrf2-mediated antioxidative stress response reduces the free radicals, prevents synthesis of RNS, and thus protects the CNS from degeneration and axonal loss.Citation93 Thus, DMF preserves myelin integrity via two pathways: by down-regulating oxidative stress and corresponding cellular injury, as well as by inhibiting proinflammatory cytokines.Citation82,Citation93–Citation95
The first exploratory study of oral fumaric acid esters was performed in ten patients with RRMS in 2006.Citation96 Promising results from this study led to the expansion of clinical research to apply BG-12, a second-generation fumarate derivative as a potential oral therapeutic for RRMS.Citation97 Kappos et alCitation98 demonstrated the safety and efficacy of DMF, showing a 69% reduction in gadolinium-enhanced MRI (Gd-MRI) lesions and a 32% reduction in ARR when compared to placebo (). The Phase III trials DEFINE (Determination of the Efficacy and Safety of Oral Fumarate in Relapsing-Remitting MS)Citation99 and CONFIRM (Comparator and an Oral Fumarate in Relapsing-Remitting Multiple Sclerosis)Citation100 further elucidated the efficacy of DMF in reducing ARR by 53% and 44% compared to placebo or GA, respectively (), a decrease in Gd-MRI activity by 70%, and a decrease in disability progression of 38% (). In addition, the side effects of DMF were relatively benign, including gastrointestinal discomfort, flushing, decreased lymphocyte count, and elevated liver aminotransferase levels ().Citation99–Citation101 Although some formulations and metabolites of fumaric acid esters are known to cause PML,Citation102,Citation103 DMF by itself has been suggested as a safe drug with relatively low side effects.Citation104
Teriflunomide
Teriflunomide is the active metabolite of leflunomide, a chemical with known anti-inflammatory, anti-proliferative and immunosuppressive properties.Citation105 The utility of oral teriflunomide in treating MS was realized in 2006 through a Phase II clinical study reported by O’Connor et al,Citation106 which elucidated its immunomodulatory effects in decreasing MRI lesions and ARR in RRMS patients. Triggering of an immune response involves the proliferation of T cells and B cells to provide antigen-specific cell-mediated or humoral immunity, respectively. In order to activate the lymphocytes to undergo clonal expansion, adhesion of T cells to the antigen-presenting cells is a crucial step.Citation107 Teriflunomide primarily acts by interfering with the lymphocyte cell cycle and inhibiting proliferation. Lymphocyte mitosis requires an eightfold increase in the level of pyrimidine ribonucleotides (eg, ribonucleotide uridine monophosphate) during the interphase of the cell cycle. A key enzyme, dihydroorotate dehydrogenase (DHODH), is necessary for the de novo synthesis of these pyrimidine ribonucleotides, which in turn fulfills the metabolic needs that are necessary for clonal expansion of lymphocytes.Citation108,Citation109 By preventing the synthesis of DHODH, teriflunomide actively reduces the pyrimidine ribonucleotide levels, stalls mitosis and further lymphocyte proliferation, and thus protects neurons from autoimmune damage. Teriflunomide also acts by inhibiting protein tyrosine kinases, leading to decreased T cell proliferation, and by shifting the cytokine profile to prevent inflammation (ie, inhibiting synthesis of proinflammatory cytokines and promoting anti-inflammatory cytokines).Citation110 EAE animal models treated with teriflunomide showed a significant reduction in axonal damage by up to 96%, nonlatency or delay of motor-evoked potentials, and preservation of the anatomical integrity in both the ascending and descending tracts of the spinal cord, thus underscoring its direct effect on neuroprotection.Citation111,Citation112
The TEMSO (Teriflunomide Multiple Sclerosis Oral) trialCitation68 illustrated the efficacy of Aubagio® (Genzyme, Boston, MA, USA) as an MS DMT (), demonstrating nearly 31% reduction in ARR, a longer time to first relapse, approximately 20% decrease in disability progression, and a decrease in Gd-MRI lesion activity when compared to placebo. Recent animal studies have shown that teriflunomide can significantly improve motor function and decrease the probability of debilitating paralysis, suggesting that it might become one of the early treatment drugs for MS.Citation111 To this end, a Phase III clinical trial (TOPIC [Phase III Study with Teriflunomide Versus Placebo in Patients with First Clinical Symptom of Multiple Sclerosis]; ClinicalTrials.gov NCT00622700)Citation113 is ongoing, with an expected study completion date of August 2015, that will inform the utility of teriflunomide in early clinical treatment.
Fingolimod
Levels of T cells and B cells are regulated through a circulatory mechanism between the blood and appropriate secondary lymphoid organs (SLOs), and the homing of T cells from the blood to sites of inflammation in the CNS is crucial for MS pathogenesis. The MBP-activated T cells breach the BBB, reach the site of inflammation, become encephalitogenic effector cells, and initiate demyelination within the CNS.Citation114 An extracellular signaling molecule, sphingosine-1-phosphate (S1P), regulates the process of trafficking T cells and B cells from the lymph to the blood.Citation115 The S1P receptors, when activated, induce egress of T cells (naïve) from peripheral blood and sequester them within the SLOs, thus decreasing T cell levels in the blood.Citation106,Citation116–Citation118
FTY720 (2-amino-[2-{4-octylphenyl}ethyl]-1, 3-propanediol hydrochloride), a synthetic S1P analogue, immunomodulates the S1P receptors and revises the T cells’ migratory pathway (ie, prevents emigration of activated T cells from lymph nodes and sequesters them within SLOs).Citation118 This sequestration dramatically reduces the availability of T cells in the blood that can infiltrate the BBB and home in to the inflamed cells in the CNS. Thus, FTY720 (Fingolimod, Gilenya®; Novartis) effectively confers neuroprotection against demyelination.Citation119–Citation121 In murine models, FTY720 dramatically reduced the expression of the proinflammatory Th1-type cytokines due to the absence of T cell migrants to promote further inflammation.Citation122 In addition, FTY720 is also suggested to promote remyelination of neurons in the CNS via direct interaction with oligodendrocytes.Citation123
The FREEDOMS (FTY720 Research Evaluating Effects of Daily Oral Therapy in Multiple Sclerosis) studyCitation124 tested two doses (0.5 mg or 1.25 mg) of fingolimod or placebo taken once daily for 24 months. Results showed that patients treated with fingolimod had an approximately 70% decrease in MS disease activity and a stable EDSS score (), and about 50% of patients had no change in T2-weighted MRI lesions.Citation124 In a head-to-head comparison with IFN-β1a (TRANSFORMS [Trial Assessing Injectable Interferon Versus FTY720 Oral in Relapsing-Remitting Multiple Sclerosis]), RRMS patients on fingolimod on average had lower ARRs, significantly fewer new or enlarged T2-weighted MRI lesions, and a stable EDSS score ().Citation125 Despite its promising efficacy, fingolimod’s safety profile has been challenging, with occurrence of bradyarrhythmias that have caused deaths and elicited prolonged cardiac monitoring for first dosings.Citation126 Death of MS patients who were administered fingolimod in the presence of varicella zoster viral infection has also been reported.Citation127 A first case of PML in the absence of prior natalizumab therapy has also been identified.Citation128 Research reports on the safety and efficacy of fingolimod are still emerging, and additional data will further inform the risks versus benefits of this drug for MS therapy.
MS therapy: past, present, and future
Great strides have been made in the last 20 years in MS therapeutics, beginning with the initial INF-β-positive trials up through the recent approval of DMF. A number of newer agents are poised potentially to gain approval over the next few years, which we have briefly touched upon (). Incremental improvements in efficacy have been seen together with improved odds of disease stability with therapy and the potential for disease improvement with some agents.
Table 3 Disease-modifying therapies (DMTs) currently under review for use as multiple sclerosis therapies
As discussed earlier, most agents modify the disease course primarily through anti-inflammatory pathways, although the newest entrant (DMF) may well be efficacious in utilizing a novel antioxidant pathway. However, all these agents carry adverse side effects of varying degrees, and hence a thorough evaluation of the risk–benefit ratio for the individual patient is imperative prior to drug administration (). In MS, T cells are known to attack three different antigens: MBP, myelin oligodendrocyte glycoprotein, and proteolipid protein. The heterogeneity of MS disease is vast, and each patient’s antibody signature varies: the antigen epitopes that elicit antibody response differ among people, and so does the mechanism of “epitope spreading,” wherein autoreactive T cell activation is elicited by new epitopes secondary to the dominant epitope, either due to their physical proximity or molecular similarity to the dominant epitope, resulting in sequential self-damage. Currently, ways to personalize MS treatment by recognizing these individual epitopes and formulating corresponding antibodies are being explored. Remyelinating agents are actively under investigation, and may yield novel strategies to increase neuronal functionality and survival in the coming years. With preclinical animal trials showing superior efficacy in alleviating MS symptoms, ongoing human clinical trials are investigating the use of hematopoietic and mesenchymal stem cells for effective management of MS. While the goal of hematopoietic stem cell transplantation is refurbishing the aberrant T cell population with nonautoreactive T cells, mesenchymal stem cell transplantation can potentially promote neural restoration. MS therapeutics is now an area of rapid evolution, with broadening biological targets and ongoing improvement in efficacy.
Disclosure
Dr Foley has received personal compensation for consulting, speaking and scientific advisory boards from Genzyme, Biogen Idec Inc. and Teva. Dr Foley has also received research support from Biogen Idec Inc., Genzyme, Teva and Avanir. The other authors report no conflicts of interest in this work.
References
- PolmanCHO’ConnorPWHavrdovaEA randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosisN Engl J Med2006354989991016510744
- WilliamsRRigbyASAireyMRobinsonMFordHMultiple sclerosis: its epidemiological, genetic, and health care impactJ Epidemiol Community Health19954965635698596089
- CompstonAColesAMultiple sclerosisLancet200235993131221123111955556
- CompstonAColesAMultiple sclerosisLancet200837296481502151718970977
- KeeganBMNoseworthyJHMultiple sclerosisAnn Rev Medic200253285302
- Koch-HenriksenNThe Danish Multiple Sclerosis Registry: a 50-year follow-upMult Scler19995429329610467392
- MayrWTPittockSJMcClellandRLJorgensenNWNoseworthyJHRodriguezMIncidence and prevalence of multiple sclerosis in Olmsted County, Minnesota, 1985–2000Neurology200361101373137714638958
- HawkinsSAMcDonnellGVBenign multiple sclerosis? Clinical course, long term follow up, and assessment of prognostic factorsJ Neurol Neurosurg Psychiatry199967214815210406979
- WhitacreCCReingoldSCO’LooneyPAA gender gap in autoimmunity: Task Force on Gender, Multiple Sclerosis and AutoimmunityScience199928354061277127810084932
- OrtonSMHerreraBMYeeIMSex ratio of multiple sclerosis in Canada: a longitudinal studyLancet Neurol200651193293617052660
- KisterIChamotESalterARCutterGRBaconTEHerbertJDisability in multiple sclerosis: a reference for patients and cliniciansNeurology201380111018102423427319
- SadovnickADEbersGCWilsonRWPatyDWLife expectancy in patients attending multiple sclerosis clinicsNeurology19924259919941579256
- CharcotJMHistologie de la sclerose en plaquesGaz Hop Civ Mil Empire Ottoman186841554555
- PolmanCHReingoldSCBanwellBDiagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteriaAnn Neurol201169229230221387374
- StangelMFredriksonSMeinlEPetzoldAStuveOTumaniHThe utility of cerebrospinal fluid analysis in patients with multiple sclerosisNat Rev Neurol20139526727623528543
- KuenzBDeisenhammerFBergerTReindlMDiagnostic biomarkers in multiple sclerosisExpert Opinion Medical Diagn200712225233
- KatsavosSAnagnostouliMBiomarkers in multiple sclerosis: an up-to-date overviewMult Scler Int2013201334050823401777
- LeoneMABarizzoneNEspositoFAssociation of genetic markers with CSF oligoclonal bands in multiple sclerosis patientsPloS One201386e6440823785401
- RudickRAThe elusive biomarker for personalized medicine in multiple sclerosis: the search continuesNeurology201279649849922573625
- OberwahrenbrockTSchipplingSRingelsteinMRetinal damage in multiple sclerosis disease subtypes measured by high-resolution optical coherence tomographyMult Scler Int2012201253030522888431
- VukusicSConfavreuxCPrimary and secondary progressive multiple sclerosisJ Neurol Sci2003206215315512559503
- HagmanSRaunioMRossiMDastidarPElovaaraIDisease-associated inflammatory biomarker profiles in blood in different subtypes of multiple sclerosis: prospective clinical and MRI follow-up studyJ Neuroimmunol20112341–214114721397339
- FreedmanMSSelchenDArnoldDLTreatment optimization in MS: Canadian MS Working Group updated recommendationsCan J Neurol Sci201340330732323603165
- KieseierBCWiendlHHemmerBHartungHPTreatment and treatment trials in multiple sclerosisCurr Opin Neurol200720328629317495622
- SpainRICameronMHBourdetteDRecent developments in multiple sclerosis therapeuticsBMC Med200977419968863
- FilippiniGBrusaferriFSibleyWACorticosteroids or ACTH for acute exacerbations in multiple sclerosisCochrane Database Syst Rev20004CD00133111034713
- RudickRACohenJAWeinstock-GuttmanBKinkelRPRansohoffRMManagement of multiple sclerosisN Engl J Med199733722160416119371858
- BerkovichRTreatment of acute relapses in multiple sclerosisNeurotherapeutics20131019710523229226
- BurtonJMO’ConnorPWHoholMBeyeneJOral versus intravenous steroids for treatment of relapses in multiple sclerosisCochrane Database Syst Rev201212CD00692123235634
- MyhrKMMellgrenSICorticosteroids in the treatment of multiple sclerosisActa Neurologica Scand Suppl20091897380
- LassmannHPathology and disease mechanisms in different stages of multiple sclerosisJ Neurol Sci Epub622013
- BøLEsiriMEvangelouNKuhlmannTDemyelination and remyelination in multiple sclerosisDuncanIDFranklinRJMMyelin Repair and Neuroprotection in Multiple SclerosisNew YorkSpringer20132345
- CraytonHHeymanRARossmanHSA multimodal approach to managing the symptoms of multiple sclerosisNeurology20046311 Suppl 5S12S1815596731
- KurtzkeJFRating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS)Neurology19833311144414526685237
- Weinstock-GuttmanBRansohoffRMKinkelRPRudickRAThe interferons: biological effects, mechanisms of action, and use in multiple sclerosisAnn Neurol19953717157529476
- LinLBetaseronDev Biol Stand199896971049890522
- WeberFJanovskajaJPolakTPoserSRieckmannPEffect of interferon beta on human myelin basic protein-specific T-cell lines: comparison of IFNbeta-1a and IFNbeta-1bNeurology19995251069107110102432
- RudickRARansohoffRMLeeJCIn vivo effects of interferon beta-1a on immunosuppressive cytokines in multiple sclerosisNeurology1998505129413009595977
- YongVWChabotSStuveOWilliamsGInterferon beta in the treatment of multiple sclerosis: mechanisms of actionNeurology19985136826899748010
- KieseierBCThe mechanism of action of interferon-beta in relapsing multiple sclerosisCNS Drugs201125649150221649449
- PatyDWLiDKInterferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study GroupNeurology19934346626678469319
- [No authors listed]Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study GroupNeurology19934346556618469318
- JacobsLDCookfairDLRudickRAIntramuscular interferon beta-1a for disease progression in relapsing multiple sclerosisAnn Neurol19963932852948602746
- FernandezOAntiquedadAArbizuTTreatment of relapsing-remitting multiple sclerosis with natural interferon beta: a multicenter, randomized clinical trialMult Scler19951Suppl 1S67S699345404
- PonomarenkoNADurovaOMVorobievIIAutoantibodies to myelin basic protein catalyze site-specific degradation of their antigenProc Natl Acad Sci U S A2006103228128616387849
- PonomarenkoNADurovaOMVorobievIICatalytic activity of autoantibodies toward myelin basic protein correlates with the scores on the multiple sclerosis expanded disability status scaleImmunol Lett20061031455016297986
- ValliASetteAKapposLBinding of myelin basic protein peptides to human histocompatibility leukocyte antigen class II molecules and their recognition by T cells from multiple sclerosis patientsJ Clin Invest19939126166287679413
- OtaKMatsuiMMilfordELMackinGAWeinerHLHaflerDAT-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosisNature199034662801831871694970
- WolinskyJSCopolymer 1: a most reasonable alternative therapy for early relapsing-remitting multiple sclerosis with mild disabilityNeurology1995457124512477617175
- Fridkis-HareliMTeitelbaumDGurevichEDirect binding of myelin basic protein and synthetic copolymer 1 to class II major histocompatibility complex molecules on living antigen-presenting cells – specificity and promiscuityProc Natl Acad Sci U S A19949111487248767515181
- GranBTranquillLRChenMMechanisms of immunomodulation by glatiramer acetateNeurology200055111704171411113226
- BergerATh1 and Th2 responses: what are they?BMJ2000321725842410938051
- JohnsonKPBrooksBRCohenJACopolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study GroupNeurology1995457126812767617181
- KorczynADNisipeanuPSafety profile of copolymer 1: analysis of cumulative experience in the United States and IsraelJ Neurol19962434 Suppl 1S23S268965117
- MunariLLovatiRBoikoATherapy with glatiramer acetate for multiple sclerosisCochrane Database Syst Rev20041CD00467814974077
- La MantiaLMunariLMLovatiRGlatiramer acetate for multiple sclerosisCochrane Database Syst Rev20105CD00467820464733
- YednockTACannonCFritzLCSanchez-MadridFSteinmanLKarinNPrevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrinNature1992356636463661538783
- LegerOJYednockTATannerLHumanization of a mouse antibody against human alpha-4 integrin: a potential therapeutic for the treatment of multiple sclerosisHum Antibodies1997813169265500
- KentSJKarlikSJRiceGPHornerHCA monoclonal antibody to alpha 4-integrin reverses the MR-detectable signs of experimental allergic encephalomyelitis in the guinea pigJ Magn Reson Imaging1995555355408574037
- MillerDHKhanOASheremataWAA controlled trial of natalizumab for relapsing multiple sclerosisN Engl J Med20033481152312510038
- KentSJKarlikSJCannonCA monoclonal antibody to alpha 4 integrin suppresses and reverses active experimental allergic encephalomyelitisJ Neuroimmunol19955811107730443
- ElicesMJNatalizumab. Elan/BiogenCurr Opin Investig Drugs200341113541362
- BauerMBrakebuschCCoisneCBeta1 integrins differentially control extravasation of inflammatory cell subsets into the CNS during autoimmunityProc Natl Acad Sci U S A200910661920192519179279
- CoisneCMaoWEngelhardtBCutting edge: natalizumab blocks adhesion but not initial contact of human T cells to the blood-brain barrier in vivo in an animal model of multiple sclerosisJ Immunol2009182105909591319414741
- SheremataWAVollmerTLStoneLAWillmer-HulmeAJKollerMA safety and pharmacokinetic study of intravenous natalizumab in patients with MSNeurology19995251072107410102433
- O’ConnorPMillerDRiesterKRelapse rates and enhancing lesions in a phase II trial of natalizumab in multiple sclerosisMult Scler200511556857216193895
- SheremataWAMinagarAAlexanderJSVollmerTThe role of alpha-4 integrin in the aetiology of multiple sclerosis: current knowledge and therapeutic implicationsCNS Drugs2005191190992216268663
- O’ConnorPNatalizumab and the role of alpha 4-integrin antagonism in the treatment of multiple sclerosisExpert Opin Biol Ther20077112313617150024
- MillerDHSoonDFernandoKTMRI outcomes in a placebo-controlled trial of natalizumab in relapsing MSNeurology200768171390140117452584
- RudickRAMillerDHassSHealth-related quality of life in multiple sclerosis: effects of natalizumabAnn Neurol200762433534617696126
- BalcerLJGalettaSLCalabresiPANatalizumab reduces visual loss in patients with relapsing multiple sclerosisNeurology200768161299130417438220
- PhillipsJTGiovannoniGLublinFDSustained improvement in Expanded Disability Status Scale as a new efficacy measure of neurological change in multiple sclerosis: treatment effects with natalizumab in patients with relapsing multiple sclerosisMult Scler201117897097921421809
- Weinstock-GuttmanBGalettaSLGiovannoniGAdditional efficacy endpoints from pivotal natalizumab trials in relapsing-remitting MSJ Neurol2012259589890522008873
- CadavidDJurgensenSLeeSImpact of natalizumab on ambulatory improvement in secondary progressive and disabled relapsing-remitting multiple sclerosisPloS One201381e5329723308186
- RudickRAStuartWHCalabresiPANatalizumab plus interferon beta-1a for relapsing multiple sclerosisN Engl J Med322006354991192316510745
- Biogen IdecTysabri (natalizumab): benefit/risk update and PML risk stratification2013 Available from: http://www.slideshare.net/gavingiovannoni/natalizumanAccessed September 17, 2013
- BloomgrenGRichmanSHotermansCRisk of natalizumab-associated progressive multifocal leukoencephalopathyN Engl J Med2012366201870188022591293
- PlavinaTSubramanyamMBloomgrenGUse of JC virus antibody index to stratify risk of progressive multifocal leukoencephalopathy in natalizumab-treated patients with multiple sclerosisPoster presented at: 27th Annual Meeting of the CMSC and the Fifth Cooperative Meeting of the CMSC-ACTRIMSMay 29–June 1, 2013Orlando, FL
- SchwabNSchneider-HohendorfTPosevitzVL-selectin is a possible biomarker for individual PML risk in natalizumab-treated MS patientsNeurology2013811086587123925765
- FoleyJFNatalizumab related PML: an evolving risk stratification paradigmPoster presented at: American Academy of Neurology 2013 Annual MeetingMarch 16–23, 2013San Diego, CA
- GonsetteRENeurodegeneration in multiple sclerosis: the role of oxidative stress and excitotoxicityJ Neurol Sci20082741–2485318684473
- BrosnanCFCannellaBBattistiniLRaineCSCytokine localization in multiple sclerosis lesions: correlation with adhesion molecule expression and reactive nitrogen speciesNeurology1995456 Suppl 6S16S217540265
- WierinckxABrevéJMercierDSchultzbergMDrukarchBVan DamAMDetoxication enzyme inducers modify cytokine production in rat mixed glial cellsJ Neuroimmunol20051661–213214315993952
- MillerEWachowiczBMajsterekIAdvances in antioxidative therapy of multiple sclerosisCurrent medicinal chemistry Epub6252013
- BogdanCNitric oxide and the immune responseNat Immunol200121090791611577346
- NathanCShilohMUReactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogensProc Natl Acad Sci U S A200097168841884810922044
- HibbsJBJrTaintorRRVavrinZRachlinEMNitric oxide: a cytotoxic activated macrophage effector moleculeBiochem Biophys Res Commun1988157187943196352
- SmithKJKapoorRFeltsPADemyelination: the role of reactive oxygen and nitrogen speciesBrain Pathol19999169929989453
- SuKGBankerGBourdetteDForteMAxonal degeneration in multiple sclerosis: the mitochondrial hypothesisCurr Neurol Neurosci Rep20099541141719664372
- AlbrechtPBouchachiaIGoebelsNEffects of dimethyl fumarate on neuroprotection and immunomodulationJ Neuroinflammation2012916322769044
- SchillingSGoelzSLinkerRLuehderFGoldRFumaric acid esters are effective in chronic experimental autoimmune encephalomyelitis and suppress macrophage infiltrationClin Exp Immunol2006145110110716792679
- VandermeerenMJanssensSBorgersMGeysenJDimethylfumarate is an inhibitor of cytokine-induced E-selectin, VCAM-1, and ICAM-1 expression in human endothelial cellsBiochem Biophys Res Commun1997234119239168952
- LinkerRALeeDHRyanSFumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathwayBrain2011134367869221354971
- KohenRNyskaAOxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantificationToxicol Pathol200230662065012512863
- ScannevinRHChollateSJungMYFumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathwayJ Pharmacol Exp Ther2012341127428422267202
- SchimrigkSBruneNHellwigKOral fumaric acid esters for the treatment of active multiple sclerosis: an open-label, baseline-controlled pilot studyEur J Neurol200613660461016796584
- WakkeeMThioHBDrug evaluation: BG-12, an immunomodulatory dimethylfumarateCurr Opinion Investig Drugs2007811955962
- KapposLGoldRMillerDHEfficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb studyLancet200837296481463147218970976
- GoldRKapposLArnoldDLPlacebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosisN Engl J Med2012367121098110722992073
- FoxRJMillerDHPhillipsJTPlacebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosisN Engl J Med2012367121087109722992072
- HutchinsonMFoxRJMillerDHClinical efficacy of BG-12 (dimethyl fumarate) in patients with relapsing-remitting multiple sclerosis: subgroup analyses of the CONFIRM studyJournal of Neurology201326092286229623749293
- ErmisUWeisJSchulzJBPML in a patient treated with fumaric acidN Engl J Med2013368171657165823614603
- van OostenBWKillesteinJBarkhofFPolmanCHWattjesMPPML in a patient treated with dimethyl fumarate from a compounding pharmacyN Engl J Med2013368171658165923614604
- SweetserMTDawsonKTBozicCManufacturer’s response to case reports of PMLN Engl J Med2013368171659166123614605
- ZeydaMPoglitschMGeyereggerRDisruption of the interaction of T cells with antigen-presenting cells by the active leflunomide metabolite teriflunomide: involvement of impaired integrin activation and immunologic synapse formationArthritis Rheum20055292730273916142756
- O’ConnorPWLiDFreedmanMSA Phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapsesNeurology200666689490016567708
- WarnkeCMeyer zu HörsteGHartungHPStüveOKieseierBCReview of teriflunomide and its potential in the treatment of multiple sclerosisNeuropsychiatr Dis Treat2009533334019557143
- FoxRIHerrmannMLFrangouCGMechanism of action for leflunomide in rheumatoid arthritisClin Immunol199993319820810600330
- HerrmannMLSchleyerbachRKirschbaumBJLeflunomide: an immunomodulatory drug for the treatment of rheumatoid arthritis and other autoimmune diseasesImmunopharmacology2000472–327328910878294
- OhJO’ConnorPWTeriflunomide for the treatment of multiple sclerosisSemin Neurol2013331455523709212
- Iglesias-BregnaDHanakSJiZEffects of prophylactic and therapeutic teriflunomide in transcranial magnetic stimulation-induced motor-evoked potentials in the Dark Agouti rat model of experimental autoimmune encephalomyelitisJ Pharmacol Exp Ther Epub Jul26
- MerrillJEHanakSPuSFTeriflunomide reduces behavioral, electrophysiological, and histopathological deficits in the Dark Agouti rat model of experimental autoimmune encephalomyelitisJ Neurol200925618910319169851
- SanofiPhase III Study With Teriflunomide Versus Placebo in Patients With First Clinical Symptom of Multiple Sclerosis (TOPIC) Available from: http://clinicaltrials.gov/show/NCT00622700. Identifier: NCT00622700Accessed September 27, 2013
- OlssonTZhiWWHöjebergBAutoreactive T lymphocytes in multiple sclerosis determined by antigen-induced secretion of interferon-gammaJ Clin Invest19908639819851697609
- MandalaSHajduRBergstromJAlteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonistsScience2002296556634634911923495
- YoppACRandolphGJBrombergJSLeukotrienes, sphingolipids, and leukocyte traffickingJ Immunol2003171151012816975
- O’SullivanCDevKKThe structure and function of the S1P1 receptorTrends Pharmacol Sci201334740141223763867
- XieJHNomuraNKoprakSLQuackenbushEJForrestMJRosenHSphingosine-1-phosphate receptor agonism impairs the efficiency of the local immune response by altering trafficking of naive and antigen-activated CD4+ T cellsJ Immunol200317073662367012646631
- YanagawaYMasubuchiYChibaKFTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats, III. Increase in frequency of CD62L-positive T cells in Peyer’s patches by FTY720-induced lymphocyte homingImmunology19989545915949893050
- YanagawaYSugaharaKKataokaHKawaguchiTMasubuchiYChibaKFTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. II. FTY720 prolongs skin allograft survival by decreasing T cell infiltration into grafts but not cytokine production in vivoJ Immunol199816011549354999605152
- ChibaKYanagawaYMasubuchiYFTY720, a novel immuno-suppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homingJ Immunol199816010503750449590253
- FujinoMFuneshimaNKitazawaYAmelioration of experimental autoimmune encephalomyelitis in Lewis rats by FTY720 treatmentJ Pharmacol Exp Ther20033051707712649354
- MironVELudwinSKDarlingtonPJFingolimod (FTY720) enhances remyelination following demyelination of organotypic cerebellar slicesAm J Pathol201017662682269420413685
- KapposLRadueEWO’ConnorPA placebo-controlled trial of oral fingolimod in relapsing multiple sclerosisN Engl J Med2010362538740120089952
- CohenJABarkhofFComiGOral fingolimod or intramuscular interferon for relapsing multiple sclerosisN Engl J Med2010362540241520089954
- LindseyJWHaden-PinneriKMemonNBBujaLMSudden unexpected death on fingolimodMult Scler201218101507150822300970
- RatchfordJNCostelloKReichDSCalabresiPAVaricella-zoster virus encephalitis and vasculopathy in a patient treated with fingolimodNeurology201279192002200423035072
- RukovetsOFDA investigates PML in patient taking Gilenya2013 Available from: http://journals.lww.com/neurotodayonline/blog/breakingnews/pages/post.aspx?PostID=240Accessed September 17, 2013
- JacobsLDCookfairDLRudickRAIntramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG)Ann Neurol19963932852948602746
- [No authors listed]Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study GroupLancet19983529139149815049820297
- O’ConnorPWolinskyJSConfavreuxCRandomized trial of oral teriflunomide for relapsing multiple sclerosisN Engl J Med2011365141293130321991951
- CohenJAColesAJArnoldDLAlemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trialLancet201238098561819182823122652
- ComiGJefferyDKapposLPlacebo-controlled trial of oral laquinimod for multiple sclerosisN Engl J Med2012366111000100922417253
- GoldRGiovannoniGSelmajKDaclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trialLancet201338198842167217523562009
- KapposLLiDCalabresiPAOcrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo- controlled, multicentre trialLancet201137898051779178722047971