Abstract
Although anti-TNF drugs have changed the clinical course of rheumatoid arthritis (RA), survival rates and resistance-to-therapy data confirm that about 30% of RA patients fail to respond. The aim of this study was to evaluate the correlations between the development of antidrug antibodies, specific IgG4 antibodies against TNF inhibitors, and resistance to therapy in RA patients. This retrospective study involved 129 patients with established RA naïve to biological agents (98 females and 32 males, mean age 56.7±12.3 years, disease duration 6.3±1.2 years, baseline Disease Activity Score [DAS]-28 3.2–5.6) who received treatment with anti-TNF agents after the failure of conventional disease-modifying antirheumatic drugs (32 received infliximab [IFX], 58 etanercept [ETN], and 39 adalimumab [ADA]). After 6 months of treatment, the patients were classified as being in remission (DAS28 <2.6), having low disease activity (LDA; DAS28 2.6–3.2), or not responding (NR: DAS28 >3.2). The patients were also tested for serum antidrug antibodies and IgG4 antibodies against TNF inhibitors. After 24 weeks of treatment, 38% of the ETN-treated patients and 28% of those treated with ADA had injection-site reactions; the rate of systemic reactions in the IFX group was 25%. The differences among the three groups were not statistically significant (P=0.382; ETN versus ADA P=0.319). The percentages of patients with adverse events stratified by drug response were: LDA 8% and NR 18% in the ADA group; in remission 3%, LDA 22%, and NR 10% in the ETN group; and LDA 6% and NR 16% in the IFX group (P=0.051). The percentages of patients with antidrug antibodies were: ADA 33.3%, ETN 11.5%, and IFX 10.3% (P=0.025; ADA versus ETN P=0.015). The percentages of patients with IgG4 antibodies were: ADA 6%, ETN 13%, and IFX 26% (P=0.017; ADA versus ETN P=0.437). Associations between antidrug antibodies, specific IgG4 antibodies, and adverse reactions were not significant for any of the three drugs. IgG4 levels were higher in the ADA group than in the other two groups, and higher in the patients with worse DAS28 (NR) and in those experiencing adverse events. These data suggest a possible association between IgG4 levels and worse DAS28 (r2=5.8%, P=0.011). The presence of specific IgG4 antibodies against TNF blockers in patients with RA might affect the drugs’ activity. Patients with injection-site reactions and IgG4 against ETN may show a decreased response.
Introduction
Over the last 20 years, biological therapies (especially TNF inhibitors) have revolutionized the management of chronic inflammatory diseases, including rheumatoid arthritis (RA). Disease management has been dominated by the three TNF inhibitors infliximab (IFX), adalimumab (ADA), and etanercept (ETN), but despite an acceptable response rate of 60%–70%, a substantial proportion of patients fail to respond (primary failure) or experience significant side effects.Citation1 Some questions have also arisen concerning the safety of TNF inhibitors, because they can trigger immunization, induce rare type I and III hypersensitivity, and cause acute and delayed reactions.
There have been many reports of reactions in patients receiving intravenous IFX, a chimeric IgG1k anti-TNF agent,Citation2 and immunomediated side effects, such as cutaneous reactions, have been encountered during therapy with subcutaneous anti-TNF drugs. One recent paper described injection-site reactions in 29.3% of patients treated with ETN.Citation3 Adverse reactions to biological agents have been categorized into five types, including a complement-mediated reaction with immediate IgE or delayed IgG antibody formation.Citation4 The immunoglobulin IgG4 is an IgG subtype that has been described by some authors (particularly Parish in the 1970s)Citation5 as potentially causing transient sensitization that leads to signs and symptoms comparable with those induced by IgE-mediated reactions; this was initially termed IgG short-term sensitizing by Parish, because upon passive transfer to normal skin, the sensitivity persists for only 2–4 hours. IgG4 differs from IgE insofar as it present in amounts that are large enough to be detected by agglutination or precipitation assays, and its sensitizing activity is not destroyed by heat or (in most cases) chemical reducing agents.Citation4
All biological agents (whether of entirely human origin, chimeric, or “humanized”) can cause an immune response, leading to the formation of antidrug antibodies (ADAbs), which are also known as human antichimeric antibodies or human antihuman antibodies, depending on the nature of the drug. The generation of ADAbs is increasingly recognized as a mechanism explaining the failure of anti-TNF drugs in chronic inflammatory diseases. The lack of a clinical response in patients with ADAbs may be due to the formation of an immune complex between TNF inhibitors and ADAbs that suppresses the drug and restricts its therapeutic activity.Citation1
The aim of this study was to evaluate the correlations between the development of ADAbs and specific IgG4 antibodies against TNF inhibitors, adverse local and general hypersensitivity events, and resistance to therapy in RA patients.
Materials and methods
This retrospective study involved 129 patients with established RA naïve to biological agents (98 females and 32 males, mean age 56.7±12.3 years, disease duration 6.3±1.2 years, baseline Disease Activity Score [DAS]-28 scores 3.2–5.6) who received treatment with anti-TNF agents after the failure of conventional disease-modifying antirheumatic drugs: 32 (24.8%) received IFX, 58 (44.9%) ETN, and 39 (30.3%) ADA. shows their baseline characteristics.
Table 1 Characteristics of patients at baseline
After 6 months of treatment, the patients were classified as being in remission (DAS28 <2.6), having low disease activity (LDA; DAS28 2.6–3.2), or not responding (NR; DAS28 >3.2). During the 24 weeks of treatment, we also evaluated injection-site reactions in patients treated with ETN or ADA, and systemic reactions in those treated with IFX.
The patients were tested for serum ADAbs with an anti-TNFα-blocker enzyme-linked immunosorbent assay kit (Immundiagnostik, Milan, Italy) and IgG4 antibodies against TNF inhibitors (fluoroenzyme immunoassay kit for ImmunoCap® 250; Phadia, Uppsala, Sweden). ADAb (Immundiagnostik) titers higher than 0.44 OD for ETN, 0.18 OD for ADA, and 0.27 for IFX were considered positive. IgG4 antibodies against TNF inhibitors (ImmunoCap) higher than 5.9 mg arbitrary unit/liter for ETA, 19.5 mg A/L for ADA, and 8.6 mg A/L for IFX were considered positive.
Statistical analysis
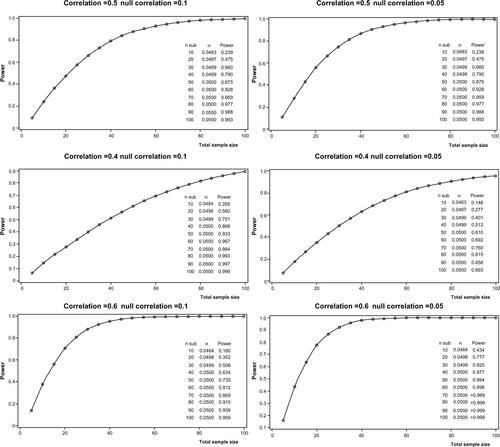
Power calculation was performed on correlation coefficients considering Fisher’s Z-transformation, with an α-value of 0.05. For our simulation, we considered the following scenarios: a correlation coefficient ranging from 0.4 to 0.6 and a lower 95% confidence limit of the correlation coefficient (null correlation) ranging from 0.05 to 0.1. In the more conservative scenario (rsp =0.4 and null correlation of 0.1), we calculated that more than 90 subjects inclusive of up to 10% of missing values may be considered sufficient to achieve a nominal power over 90% (Figure S1).
Statistics revised
Continuous variables were expressed as means ± standard deviation and categorical variables were described by percentages. The χ2 test was used to compare categorical variables among groups, and the Pearson correlation coefficient was used to investigate for relations between quantitative variables. An α-value of 0.05 was considered statistically significant, and all statistical tests were two-tailed. IgG4 levels were considered positive if superior to the following cutoff values: ETN 5.9, ADA 19.25, and IFX 8.6. ADAb levels were considered positive if superior to the following cutoff values: ETN 0.446, ADA 0.187, and IFX 0.275. Analyses were performed using Minitab16 software.
Results
After 24 weeks of treatment, 38% of the ETN-treated patients and 28% of those treated with ADA had injection-site reactions; the rate of systemic reactions in the IFX group was 25%. The differences were not statistically significant (P=0.382; ETN vs ADA P=0.319).
The percentages of patients with adverse events stratified by drug response were: LDA 8% and NR 18% in the ADA group; remission 3%, LDA 22%, and NR 10% in the ETN group; and LDA 6% and NR 16% in the IFX group (P=0.051). The percentages of patients with ADAbs were: ADA 33.3%, ETN 11.5%, and IFX 10.3% (P=0.025; ADA vs ETN P=0.015). The percentages of patients with IgG4 antibodies were: ADA 6%, ETN 13%, and IFX 26% (P=0.017; ADA vs ETN P=0.437). Associations between ADAbs, specific IgG4 antibodies and adverse reactions were not significant for any of the three drugs.
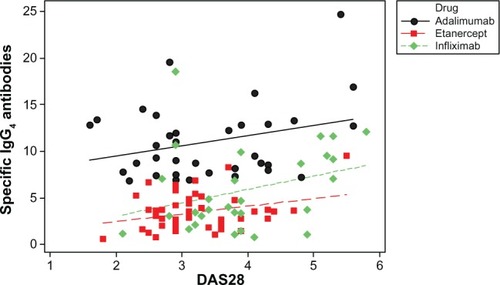
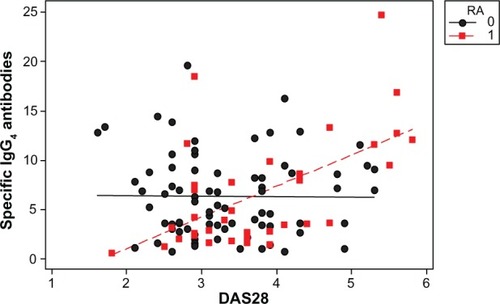
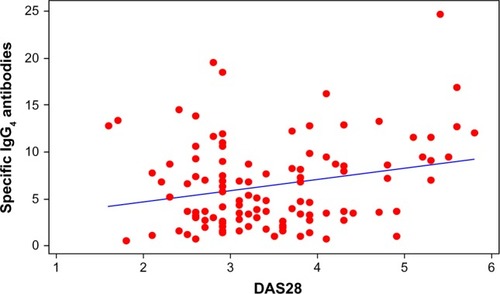
A scatter plot showed a positive correlation between IgG4 levels and worse DAS28, (0.241, r2=5.8%; P=0.011; ). IgG4 quantities were higher in the ADA group than in the other two groups (), but the correlation with DAS28 was only significant for the ETN group, (ADA 0.287, P=0.094; ETN 0.299, P=0.039; IFX 0.314, P=0.097). The correlation between ETN and DAS28 was consistent with the higher percentage of ETN patients positive for IgG4 (shown earlier) with respect to the ADA patients. There was a positive correlation between IgG4 levels (regardless of the drug group) in patients experiencing adverse events (, red line and dots; subjects having experienced adverse events correlating with DAS28 value =0.578, r2=33.5%; P=0.000).
Figure 1 Scatter plot of specific IgG4 antibodies versus DAS28.
Discussion
Our results suggest that IgG4 may play a role in the adverse reactions and therapeutic response to biological agents of patients with RA. The role of anti-ADA IgG4 was first detected by means of an immunoassay in 271 consecutive RA patients during 3 years of treatment: anti-ADA antibodies were detectable in 32%, and specific IgG4 antibodies in 29%. Although IgG4 is often considered to be harmless due to its lack of an effector function, the neutralization of ADA by specific IgG4 antibodies leads to a reduced clinical response.Citation6 In our 24-week study, we detected ADAbs in 33.3% of our ADA patients and IgG4 in only 6%.
Approximately 4% of the total IgG in the serum of Caucasian adults is IgG4,Citation7 which is considered an odd antibody insofar that it is the only IgG unable to activate complement and has a low affinity for Fcγ receptors.Citation8,Citation9 Furthermore, it is able to exchange half-molecules in vivo, thus leading to bispecific antibodies that do not cross-link and the consequent formation of small immune complexes.Citation10,Citation11 Because of its limited ability to trigger immunological effector functions and its tendency to form small immune complexes, it is thought that IgG4 has less effect on the clearance of antigens and plays a limited role in inflammation.
Although the percentage of serum IgG4 is generally low, antigen-specific IgG4 has been described as the main isotype produced in some immune responses. As early as the 1970s, it was shown that chronic antigen exposure to grass pollen and bee venom led to the predominant formation of IgG4,Citation12 and increased allergen-specific IgG4 is associated with a beneficial response to specific allergen immunotherapy in allergic patients.Citation13,Citation14 Chronic treatment with biological agents, such as factor VIII, IFNβ, or therapeutic monoclonal antibodies can be considered long-term exposure to protein antigens. There have been frequent reports of the formation of IgG antibodies against biological agents, some of which have been found to produce IgG4. Prolonged exposure to IFNβ can also lead to the development of IgG4 antibodies in patients with multiple sclerosis,Citation15 but although it has been shown that therapeutic monoclonal antibodies can give rise to the formation of IgG4, there is lack of long-term measurements.Citation16,Citation17 The observation that the proportion of IgG4 antibodies may diminish over time is somewhat unusual, and differs from what has been described in the various allergens.Citation18
Our results showed more injection-site reactions in the ETN group than in the ADA group (38% vs 28%), and the presence of anti-ETN IgG4 in 13% of the patients. Although it is not yet known what factors induce class switching to IgG4, it can be speculated that the immunological context of the patients and/or the intensity of treatment (dosing and frequency) may play a role. One of the reasons for the difference in IgG4 responses between ADA-treated and allergic patients may be that the former received 40 mg every 2 weeks, whereas the patients receiving allergen-specific immunotherapy received 20 μg of allergen twice a week. The frequency of treatment could also explain the increase in anti-ETN IgG4.
Our findings indicate a correlation between adverse drug reactions and a poor response, and suggest that anti-ETN IgG4 may have a decreased response to ETN therapy. Other studies have shown that the formation of anti-ADA antibodies is related to lower functional drug levels and a diminished clinical response.Citation19,Citation20 A large proportion of these antibodies are IgG4 antibodies, which suggests IgG4 is not simply an innocent bystander in this case.Citation21 Although IgG4antibodies cannot activate complement and have a low affinity for Fc receptors, they may still be able to compete with drug-induced TNFα binding to drugs, thus leading to a clinical nonresponse.
We detected anti-ADA antibodies more frequently than anti-ETN antibodies (33% vs 11.5%), whereas the rate of detection of IgG4 anti-ETN was 13%, which may predict a lower response to ETN therapy in patients with injection-site reactions. One limitation of this study is that it did not measure the serum levels of the three drugs. Our study confirms that patients with injection-site reactions and anti-ETN IgG4 may not respond to ETN therapy.
Author contributions
MB and FA designed the study and drafted the paper. MM, MI, and FM performed the laboratory analysis. FLG and MB recruited the patients. PSP commented on and critically edited the paper. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The study was supported by the Rheumatology Unit of the Nuovo Ospedale S Giovanni di Dio, Florence, Italy.
Supplementary material
Figure S1 Power calculations.
Notes: Power calculation reporting power (1-beta) vs sample size (number of pairs) under the assumption of a correlation coefficient ranging from 0.4 to 0.6 (moderate weak to moderate strong) with a null correlation of 0.1 and 0.05 for the left and the right panels respectively.
Disclosure
The authors report no conflicts of interest in this work
References
- VincentFBMorandEFMurphyKMackayFMarietteXMarcelliCAntidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspectiveAnn Rheum Dis20137216517823178294
- CheifetzASmedleyMMartinSThe incidence and management of infusion reactions to infliximab: a large center experienceAm J Gastroenterol2003981315132412818276
- DoreRKMathewsSSchechtmanJThe immunogenicity, safety, and efficacy of etanercept liquid administered once weekly in patients with rheumatoid arthritisClin Exp Rheumatol200725404617417989
- PichlerWJCampiPAdverse side effects to biological agentsPichlerWJDrug HypersensitivityBaselKarger2007160174
- ParishWEShort term anaphylactic IgG antibodies in human seraLancet1970192 (7673):591–592.
- van SchouwenburgPAKrieckaertCLNurmohamedMIgG4 production against adalimumab during long term treatment of RA patientsJ Clin Immunol2012321000100622622790
- AalberseRCvan der GaagRvan LeeuwenLJSerologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted responseJ Immunol19831307227266600252
- TaoMHCanfieldSMMorrisonSLThe differential ability of human IgG1 and IgG4 to activate complement is determined by the COOH-terminal sequence of the CH2 domainJ Exp Med1991173102510282007852
- NirulaAGlaserSMKalledSLTaylorFRWhat is IgG4? A review of the biology of a unique immunoglobulin subtypeCurr Opin Rheumatol20112311912421124094
- Van Der ZeeJSVan SwietenPAalberseRCSerologic aspects of IgG4 antibodies. II. IgG4 antibodies form small, nonprecipitating immune complexes due to functional monovalencyJ Immunol1986137356635713782791
- van der NeutKMSchuurmanJLosenMAnti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchangeScience20073171554155717872445
- van der GiessenMHomanWLvan KernbeekGAalberseRCDiegesPHSubclass typing of IgG antibodies formed by grass pollen-allergic patients during immunotherapyInt Arch Allergy Appl Immunol197650625640773842
- GehlharKSchlaakMBeckerWBufeAMonitoring allergen immunotherapy of pollen-allergic patients: the ratio of allergen-specific IgG4 to IgG1 correlates with clinical outcomeClin Exp Allergy19992949750610202364
- Nouri-AriaKTWachholzPAFrancisJNGrass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocks IgG activityJ Immunol20041723252325914978133
- DeisenhammerFReindlMBergerTImmunoglobulin subclasses in patients with neutralizing and non-neutralizing antibodies against IFN-β1bJ Interferon Cytokine Res20012116717111331039
- SvensonMGeborekPSaxneTBendtzenKMonitoring patients treated with anti-TNF-alpha biopharmaceuticals: assessing serum infliximab and anti-infliximab antibodiesRheumatology (Oxford)2007461828183418032541
- WoutersDStapelSVisMHuman antichimeric antibodies to infliximab and infusion-related allergic reactions in patients with rheumatoid arthritisAllergy Clin Immunol Int20072198200
- AalberseRCStapelSOSchuurmanJRispensTImmunoglobulin G4: an odd antibodyClin Exp Allergy20093946947719222496
- van KuijkAWde GrootMStapelSODijkmansBAWolbinkGJTakPPRelationship between the clinical response to adalimumab treatment and serum levels of adalimumab and anti-adalimumab antibodies in patients with psoriatic arthritisAnn Rheum Dis20106962462520223840
- BarteldsGMWijbrandtsCANurmohamedMTClinical response to adalimumab: relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritisAnn Rheum Dis20076692192617301106
- BarteldsGMKrieckaertCLNurmohamedMTDevelopment of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-upJAMA20113051460146821486979